Abstract
Early events responsible of tumor growth in patients with a normal immune system are poorly understood. Here, we discuss, in the context of human melanoma, the Prehn hypothesis according to which a weak antitumor immune response may be required for tumor growth before weakly or non-immunogenic tumor cell subpopulations are selected by the immune system.
Keywords: cancer immunosurveillance, early tumor growth, immune escape, melanoma, microenvironment, tumor antigens
Introduction
According to the original theory of immunosurveillance of Burnet and Thomas, tumor cells develop and/or grow better in immunosuppressed/immunoprotected microenvironments (e.g., severely immunodeficient mice, immunoprivileged sites, chronic immunosuppressed organ recipients), where they cannot be targeted by the (missing or crippled) immune system.1 Thus, antitumor immune reactions are important for limiting tumor growth since the earliest steps of tumorigenesis. However, the necessity to explain why, even in normal subjects, tumors may grow apparently unrestricted, has generated the hypothesis of the three “E”s, for “Elimination,” “Equilibrium” and “Escape.” This hypothesis incorporates the original immunosurveillance theory and explains the frequent observation that tumors often become clinically evident years after their molecular origin, i.e., when the equilibrium between antitumor immune reactions and tumor growth is broken and tumor cells can grow unrestricted.2
Thus, at the end of this confrontation (immune response vs. tumor growth), the immune response allows for (when it doesn’t favor) the outgrowth of a subpopulation of tumor cells that (1) show a downregulation of tumor-associated antigens (TAAs)/HLA complexes, (2) constitutively lack TAA expression3 and/or (3) release factors immunosuppressive factors such as transforming growth factor β (TGFβ), interleukin (IL)-10 and indoleamine2,3-dioxygenase (IDO).4
Similar, or even more effective, factors helping early neoplastic cells to survive, grow and invade, can be provided by the immune system itself, as suggested by Richmond Prehn four decades ago5 and discussed more recently by the same author, who proposed that an immune response might even be required for tumor growth.6 This may occur even in the presence of pre-malignant tumor cells that, depending on the causative factor (e.g., chemical carcinogens, activation of oncogenes), express immunogenic TAAs, as these may stimulate only weak immune reactions that facilitate, instead of impairing, tumor growth.3,7,8 Although this process differs from the immunoediting, i.e., the selection and growth of subpopulations of immunoresistant tumor cells,2 the underlying mechanisms remain to be elucidated.
Melanoma as a Paradigm
In this perspective, we will use human melanoma as a paradigm to recapitulate the bio-molecular events that characterize tumor growth and progression in its microenvironment and to assess whether the Prehn hypothesis of immune system-mediated stimulation of early tumor growth is supported or not by available data.
One should take into consideration that different molecular subtypes of melanoma exist,9 which may show a different antigenic profile depending on several factors, including the number and quality of genetic alterations along with the ability of TAAs to generate melanoma-specific T and/or B-cell epitopes. For example, BRAF mutations (which are frequent in melanoma cells but found also in premalignant lesions like atypical nevi),10 can lead to the synthesis of T-cell epitopes that are recognized as TAAs by the immune system.11
The first, weak immune reaction against a small number of early appearing melanoma cells can only be caused by the recognition of mutated TAAs by T and/or B cells, but such a recognition results in the stimulation—rather than in the inhibition—of tumor cell growth, due to the immune/ inflammatory reactions that these TAAs evoke in the tumor microenvironment. In addition to mutation-derived TAAs, melanoma is known to express normal, melanocytic lineage-related antigens (e.g., gp100, tyrosinase, MART-1) that are usually not recognized by the immune system in the early phases of tumor progression owing to some form of tolerance.12 However, if early tumor cells start to proliferate driven by inflammatory,13 immunological14 and/or angiogenetic15 signals released in the microenvironment, they will reach a threshold population size that is necessary to break tolerance and to trigger an adaptive, but usually ineffective, T-cell response against self antigens.12
Primary melanoma development
Most melanomas are caused by exposure to UVB, and this may have different effects that complement each other in allowing the neoplastic transformation of melanocytes and their early growth driven by an inflammatory-like reaction that may involve several factors.16 In fact, UVB can damage the melanocyte DNA, thus causing hundreds of mutations, including in genes controlling cell cycle progression and signal transduction pathways (e.g., TP53, NRAS, BRAF, PTEN), DNA repair and invasiveness (i.e., genes that regulate adhesion, migration and angiogenesis).17 However, UVB can also weaken both the innate and adaptive immune system by promoting the release of immunosuppressive IL-10 by Langerhans cells18,19 and/or by favoring the infiltration of interferon γ (IFNγ)-producing macrophages endowed with immunosuppressive and pro-angiogenic activities.20 These early changes occurring in the melanoma microenvironment result in a lowered recognition by the immune system of transformed melanocytes in the stimulation of their growth. This may allow the melanoma precursor cells to grow horizontally, thus invading the epidermal layers despite the potential expression of TAAs generated by UV rays.
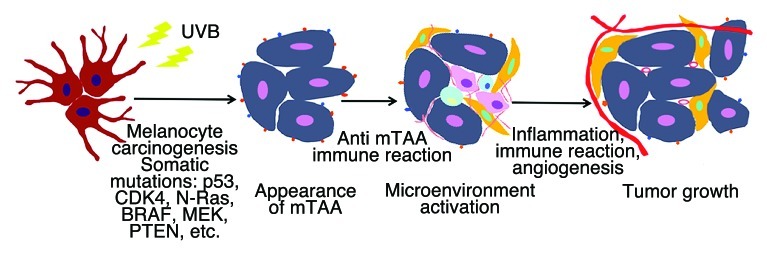
Thus, our hypothesis is that the melanoma precursor cells generated by UVB exposure express TAAs that can be recognized by B cells8 and/or T cells.21 IgG antibodies and their Fcγ receptors (expressed by macrophages, dendritic cells and mast cells) play a key role in recruiting B-regulatory cells to neoplastic lesions.8,22,23 Infiltrating immune cells (in particular, Type 2 tumor-associated macrophages, TAMs) stimulate the development of new blood vessels, which in turn feed nutrients to cancer cells and hence stimulate tumor progression (Fig. 1).
Figure 1. The UVB-induced transformation of melanocytes causes the expression of mutated tumor-associated antigens (mTAA), hence triggering an inflammatory, immune and/or pro-angiogenic response that favors early tumor growth.
Moreover, melanoma precursor cells may be sensed as a small, recently-generated population of normal or pseudo-normal melanocytes, which need to expand to exert their physiological, anti-UV barrier function. Thus, it is possible that stromal cells (e.g., keratinocytes, endothelial cells and/or fibroblasts) might send a proliferative, cytokine-mediated signal to early melanocytes that mimic normal cells that are supposed to replicate.
At some stage, a change in the genetic program of melanocytes occurs24 involving the disruption of adhesion molecules25 and the activation of the vascular endothelial growth factor (VEGF) system,26 which allows for the switch from a proliferative to an invasive activity. Transformed melanocytes thus initiate a vertical (nodal) journey to invade the reticular dermis. This process, however, can alarm the immune system, owing to the overexpression of normal, differentiation-associated antigens (e.g., MART1, gp100, tyrosinase), which can be recognized by T cells. A chronic inflammatory reaction ensues, resulting in the local recruitment of several immune cells including T cell, natural killer (NK) cells and TAMs. A nice example of the ability of melanoma cells to modify the local microenvironment has been recently reported by Schields et al.,27 who showed that melanoma can re-organize its stromal microenvironment into a structure similar to lymphoid tissues, which allows for the recruitment of immune suppressive cells (Tregs, MDSCs) that promote tumor progression by expressing chemokines such as CCL21 and CXCL13.
Since at this stage the immune response is based on the recognition of normal differentiation antigens, the immune system will first recognize such antigens and then activate mechanisms that allow an abnormal autoimmune response to be rapidly switched off, in order to protect the body from its own activity. Such a control is mediated by antigen-specific Tregs and may result in the stimulation, rather than in the inhibition, of melanoma growth. It is only when the tumor mass reaches a considerable size that self TAAs, released in high amounts by dying/apoptotic melanoma cells, can break tolerance and generate a T-cell response. Such a response, however, is of limited efficacy due to the lack of terminally differentiated CD8+ T cells.12 In vaccination settings, providing self antigens (e.g., MART1) together with external danger signals like CpG oligonucleotides can increase the strength of the antitumor T-cell response.28 Interestingly, targeting FAP+ fibroblasts may help to get rid of their immunosuppressive function, which is mainly mediated by the stromal cell-derived factor 1 (SDF1/CXCL12).29
A new form of maternal/embryonic relationship?
The relationship between melanoma cells and the host immune system can also be viewed within the framework provided by the interaction of the maternal immune system and the growth of allogeneic or syngeneic embryos. The former, like the tumor, needs to survive and grow in a potentially hostile environment and, therefore, must protect itself from the activity of the maternal immune system. This is likely to occur not only via the downregulation of foreign HLA molecules at the maternal-embryo interface (cytotrophoblasts), and by the release of soluble factors like HLA-G,30 but also via the establishment of a weak cellular immune response that may confer a growth advantage and eventually result in embryo growth. In fact, allogeneic murine embryos seem to elicit an inflammatory/NK-mediated immune reaction at the site of implantation, which allows them to grow better than syngeneic embryos, which do not elicit inflammatory reactions.31 Likewise, an early reaction of the immune system against tumor cells (e.g., the recruitment of macrophages and/or NK cells)20 may help or even be necessary for tumor growth.
Potential Role of Cancer Stem Cells
Cancer stem cells (CSCs) are thought to constitute a minor population of self-renewing tumor cells that sustain tumor maintenance and growth.32 We have recently found that glioblastoma33 and colorectal CSCs (unpublished data) are quite resistant to cytotoxic immune responses, which they neutralize by that downregulation of MHC Class I molecules, the lack of expression of TAAs (e.g., gp100, MAGE, MART1),34 the release of immunosuppressive factors like IDO (unpublished data) and/or the induction of Tregs.35
The presence of CSCs has been demonstrated even in melanoma.36,37 However, at variance with other tumors, melanoma CSCs appear to be quite frequent38 and express molecules that are not fully accepted as markers of stemness, with the possible exception of CD271,34,37,39,40 casting doubts on the true essence of these cells.
Such CSCs provide signals for the differentiation and growth of neoplastic cells and for their own self-renewal. These proliferation signals may either either constituted by factors released by CSC themselves (autocrine loops) or by inflammatory/immune factors (e.g., IL-10, IL-13) locally produced upon recognition of melanoma (CSC and non-CSC) cells by the immune system. As we proposed above, an early immune response may follow the recognition of mutated TAAs expressed by early CSCs and non-CSCs. However, due to lack of target TAAs and/or MHC Class I molecules on CSCs,33,34,41 this population may be spared while non-CSC that do express MHC/TAA complexes may be eliminated by immune effector mechanisms.33
Immunization (Vaccination) Worsening the Clinical Outcome in Melanoma
A support for Prehn hypothesis of the immune system-mediated growth of early tumor cells may come from recent Phase II-III clinical trials testing immunotherapy in melanoma patients. In fact, in some of these studies, patients receiving antitumor vaccines quite unexpectedly showed a worse outcome as compared with patients in the control arm.42 It should be noted that these patients had a limited (or apparently no) tumor burden (disease-free, adjuvant setting), thus mimicking the early phases of tumor growth when a few, clinically silent cells are present. One explanation for this unexpected result lies in the use of high doses of granulocyte macrophage colony-stimulating factor (GM-CSF) as an adjuvant, since it was not considered that high-dose GM-CSF in some of these protocols43,44 can be immunosuppressive.45 However, a tendency for increased tumor growth in immunized patients was observed even in trials using no GM-CSF.46 In such an instance, the proliferative stimulus may have derived from the recruitment of B cells secreting tumor necrosis factor α (TNFα), as described above.
Conclusions
The findings discussed in this perspective may support and explain the hypothesis put forward by Richmond T. Prehn several years ago, and suggest a model in which T and B lymphocytes, the release of chemokines in the tumor microenvironment, antibody reactions and activating FcγRs are involved in the chronic inflammatory program that promote early melanoma cell growth by the combination of different mechanisms. An indirect support for such a conclusion comes from a recent epidemiology study showing that the reduction of inflammatory reactions by chronic (5 y) intake of acetylsalicilic acid and other non-steroid anti-inflammatory drugs (NSADs) is associated with a significant reduced risk of melanoma development.47 These conclusions suggest that an early interaction between melanoma cells and other cells of the tumor microenvironment should be interrupted to limit tumor growth before an effective immune response can be mounted. Therefore, in our opinion, the elimination, equilibrium, escape theory (E, E, E) should be integrated by a first step of stimulation, as S, E, E, E.
Acknowledgments
The authors’ work was supported by Grants of the Alliance Against Cancer (Rome) and the Italian Association for Cancer Research (AIRC, Milano).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21455
References
- 1.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 3.Parmiani G, Sensi ML, Castelli C, Rivoltini L, Anichini A. T-cell response to unique and shared antigens and vaccination of cancer patients. Cancer Immun. 2002;2:6. [PubMed] [Google Scholar]
- 4.Rivoltini L, Canese P, Huber V, Iero M, Pilla L, Valenti R, et al. Escape strategies and reasons for failure in the interaction between tumour cells and the immune system: how can we tilt the balance towards immune-mediated cancer control? Expert Opin Biol Ther. 2005;5:463–76. doi: 10.1517/14712598.5.4.463. [DOI] [PubMed] [Google Scholar]
- 5.Prehn RT. The immune reaction as a stimulator of tumor growth. Science. 1972;176:170–1. doi: 10.1126/science.176.4031.170. [DOI] [PubMed] [Google Scholar]
- 6.Prehn RT. The initial immune reaction to a new tumor antigen is always stimulatory and probably necessary for the tumor’s growth. Clin Dev Immunol. 2010;2010:ID851728. doi: 10.1155/2010/851728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Outzen HC. Development of carcinogen-induced skin tumors in mice with varied states of immune capacity. Int J Cancer. 1980;26:87–92. doi: 10.1002/ijc.2910260114. [DOI] [PubMed] [Google Scholar]
- 8.Willimsky G, Czéh M, Loddenkemper C, Gellermann J, Schmidt K, Wust P, et al. Immunogenicity of premalignant lesions is the primary cause of general cytotoxic T lymphocyte unresponsiveness. J Exp Med. 2008;205:1687–700. doi: 10.1084/jem.20072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 10.Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–8. [PubMed] [Google Scholar]
- 11.Sharkey MS, Lizée G, Gonzales MI, Patel S, Topalian SL. CD4(+) T-cell recognition of mutated B-RAF in melanoma patients harboring the V599E mutation. Cancer Res. 2004;64:1595–9. doi: 10.1158/0008-5472.CAN-03-3231. [DOI] [PubMed] [Google Scholar]
- 12.Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, et al. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63:2535–45. [PubMed] [Google Scholar]
- 13.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Stewart T, Henderson R, Grayson H, Opelz G. Reduced incidence of rectal cancer, compared to gastric and colonic cancer, in a population of 73,076 men and women chronically immunosuppressed. Clin Cancer Res. 1997;3:51–5. [PubMed] [Google Scholar]
- 15.Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest. 2008;118:3660–70. doi: 10.1172/JCI36157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72:2473–80. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191–6. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer Res. 1994;54:6102–5. [PubMed] [Google Scholar]
- 19.Beissert S, Schwarz T. Ultraviolet-induced immunosuppression: implications for photocarcinogenesis. Cancer Treat Res. 2009;146:109–21. doi: 10.1007/978-0-387-78574-5_10. [DOI] [PubMed] [Google Scholar]
- 20.Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-γ links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–53. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zorn E, Hercend T. A natural cytotoxic T cell response in a spontaneously regressing human melanoma targets a neoantigen resulting from a somatic point mutation. Eur J Immunol. 1999;29:592–601. doi: 10.1002/(SICI)1521-4141(199902)29:02<592::AID-IMMU592>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–34. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–16. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoek KS, Eichhoff OM, Schlegel NC, Döbbeling U, Kobert N, Schaerer L, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–6. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 25.Vallacchi V, Daniotti M, Ratti F, Di Stasi D, Deho P, De Filippo A, et al. CCN3/nephroblastoma overexpressed matricellular protein regulates integrin expression, adhesion, and dissemination in melanoma. Cancer Res. 2008;68:715–23. doi: 10.1158/0008-5472.CAN-07-2103. [DOI] [PubMed] [Google Scholar]
- 26.Bayer-Garner IB, Hough AJ, Jr., Smoller BR. Vascular endothelial growth factor expression in malignant melanoma: prognostic versus diagnostic usefulness. Mod Pathol. 1999;12:770–4. [PubMed] [Google Scholar]
- 27.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328:749–52. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 28.Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–46. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 30.Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, et al. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci U S A. 1998;95:4510–5. doi: 10.1073/pnas.95.8.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loke YW, King A. Immunology of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:827–37. doi: 10.1053/beog.2000.0122. [DOI] [PubMed] [Google Scholar]
- 32.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res. 2010;16:800–13. doi: 10.1158/1078-0432.CCR-09-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–7. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei J, Barr J, Kong L-Y, Wang Y, Wu A, Sharma AK, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461–73. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 37.Perego M, Deho P, Tragni G, Carbone A, Della Mina P, Villa A, et al. Heterogeneous phenotype of human melanoma cells with in vitro and in vivo features of tumor-initiating cells. J Invest Dermatol. 2010;130:1877–86. doi: 10.1038/jid.2010.69. [DOI] [PubMed] [Google Scholar]
- 38.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Held MA, Curley DP, Dankort D, McMahon M, Muthusamy V, Bosenberg MW. Characterization of melanoma cells capable of propagating tumors from a single cell. Cancer Res. 2010;70:388–97. doi: 10.1158/0008-5472.CAN-09-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 41.Schatton T, Schütte U, Frank NY, Zhan Q, Hoerning A, Robles SC, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eggermont AMM. Immunostimulation versus immunosuppression after multiple vaccinations: the woes of therapeutic vaccine development. Clin Cancer Res. 2009;15:6745–7. doi: 10.1158/1078-0432.CCR-09-2377. [DOI] [PubMed] [Google Scholar]
- 43.Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res. 2009;15:7029–35. doi: 10.1158/1078-0432.CCR-09-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slingluff CL, Jr., Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–44. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–32. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 46.Cancervax Corp. Communication, press release, April 2005.
- 47.Curiel-Lewandrowski C, Nijsten T, Gomez ML, Hollestein LM, Atkins MB, Stern RS. Long-term use of nonsteroidal anti-inflammatory drugs decreases the risk of cutaneous melanoma: results of a United States case-control study. J Invest Dermatol. 2011;131:1460–8. doi: 10.1038/jid.2011.58. [DOI] [PubMed] [Google Scholar]