Abstract
A series of 2′,3′-dideoxy-2′,2′-difluoro-4′-azanucleosides of both pyrimidine and purine nucleobases were synthesized in an efficient manner starting from commercially available L-pyroglutamic acid via glycosylation of difluorinated pyrrolidine derivative 15. Several 4′-azanucleosides were prepared as a separable mixture of α- and β-anomers. The 6-chloropurine analogue was obtained as a mixture of N7 and N9 regioisomers and their structures were identified based on NOESY and HMBC spectral data. Among the 4′-azanucleosides tested as HIV-1 inhibitors in primary human lymphocytes, four compounds showed modest activity and the 5-fluorouracil analogue (18d) was found to be the most active compound (EC50 = 36.9 μM) in this series. None of the compounds synthesized in this study demonstrated anti-HCV activity.
Keywords: Azanucleosides, Synthesis, Anti-HIV-1, Anti-HCV, L-Pyroglutamic acid
1. Introduction
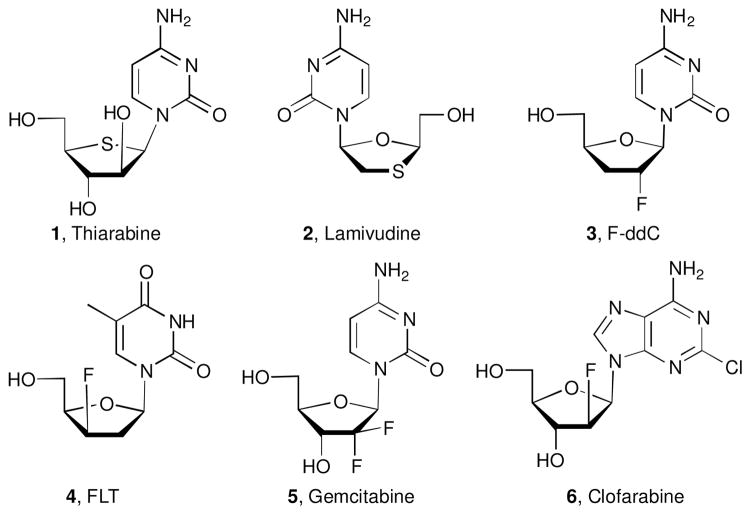
Considerable research efforts have been concentrated to prepare chemically modified nucleoside derivatives as effective anticancer1 and antiviral agents.2 Modifications in the carbohydrate moiety of nucleosides have resulted in improved biological properties.3 In this regard, heteronucleosides, wherein the ring oxygen in the carbohydrate moiety is replaced by sulfur,4 nitrogen,5 and more recently selenium,6 have received much attention for their therapeutic applications. Among the bioactive thionucleosides, two examples are shown in Figure 1: thiarabine (4′-thioaracytosine, 1) is currently in clinical trials as a potent antitumor agent7 and lamivudine (L-2′,3′-dideoxy-3′-thiacytidine, 2), in which the 4′-oxygen is present but a sulfur atom was placed at the 3′-position. Lamivudine was approved by the FDA in 1995 for the treatment of HIV infection.8 More recently, 4′-azanucleosides and 4′-selenonucleosides have shown significant anti-HCV9 and anticancer6a activities, respectively. The synthesis of heterocyclic modified nucleosides has been recently reviewed.10 Another sugar modification that improves the biological properties of some nucleoside analogues is the introduction of fluorine,11 a common functionality used in drug discovery efforts.12
Figure 1.
Selected structures of bioactive nucleosides.
The presence of fluorine in the carbohydrate moiety of nucleoside offers stabilization of the glycosidic bond. This increases the resistance to metabolic degradation while improving the lipophilicity to cross lipid membranes more effectively. Another important feature that arises from a structure activity relationship (SAR) analysis is the lack of a 3′-hydroxyl group in many of the nucleoside analogues that show anti-HIV activity. It is well known that the incorporation of 3′-deoxynucleosides in a viral DNA prevents chain elongation and terminates cell growth.13
Among fluorinated nucleosides with antiviral activity, representative examples are FddC (2′,3′-dideoxy-2′-fluorocytosine, 3)14 and FLT (3′-fluoro-3′-deoxythymidine, 4)15 which inhibit the HIV reverse transcriptase. In addition, there are two nucleosides fluorinated at the 2′-position of the sugar moiety approved by the FDA for the treatment of cancer: (i) gemcitabine (2′-deoxy-2′,2′-difluorocytidine, 5), a potent drug against ovarian,16 pancreatic,17 and breast18 cancers, and (ii) clofarabine (2-chloro-2′-deoxy-2′-fluoroarabinoadenosine, 6) which is used clinically for the treatment of leukemia in children.19
Although fluorine substitution in nucleosides and 4′-thionucleosides11a,20 has been extensively studied, few examples of fluorinated 4′-azanucleosides have been described and even less biological properties have been reported. Qiu and Qing21 carried out the preparation of pyrimidine 2′- and 3′-fluoromethyl-4′-azanucleosides, representing the only examples of fluorinated 4′-azanucleosides described to-date. To the best of our knowledge there are no other examples of 4′-azanucleosides in which the 2′,2′-difluoro substituent is directly attached to the pyrrolidine moiety.
Consequently, studies on the synthesis and biological activity of these nucleoside derivatives are worth pursuing. On the basis of the above considerations and our ongoing interest in the preparation and biological evaluation of nucleoside analogues,22 herein, we report the synthesis and antiviral evaluation of a series of 2′,3′-dideoxy-2′,2′-difluoro-4′-azanucleosides.
2. Results and discussion
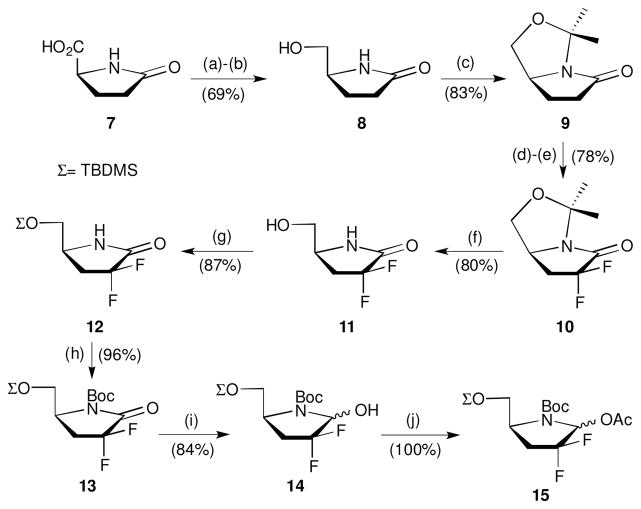
The synthesis of difluorinated pyrrolidine 15 as a substrate for the glycosylation reaction is outlined in Scheme 1. Commercially available L-pyroglutamic acid (7) possesses the correct configuration to furnish 4′-azanucleosides, which mimics the D-configuration of naturally occurring nucleosides. Conversion to L-pyroglutaminol (8) was accomplished in two steps via formation of the corresponding methylester from 7, followed by reduction with NaBH4.23 Protection with 2,2-dimethoxypropane afforded the bicyclic lactam 9. Electrophilic difluorination was achieved by the procedure described by Coward and Konas.24 Treatment of compound 9 with LDA followed by N-fluorodibenzenesulfonimide (NFSI) at −78 °C generated a 1.2:1.0 mixture of diastereomers, which was then subjected to the same reaction conditions again to furnish difluorinated product 10 in high overall yield. The acid hydrolysis of hemiaminal ether 10 with AcOH/MeCN/H2O mixture afforded difluorinated L-pyroglutaminol (11) in 80% yield. Protection of the resulting hydroxyl group of 11 with TBDMSCl yielded the silylated compound 12, which was then treated with Boc2O under basic conditions to obtain the protected product 13 in high yield. Reduction of lactam 13 using LiBEt3H in anhydrous THF provided 14 as a 1.9:1 mixture of anomers. Further reaction of 14 with Ac2O gave 15 in quantitative yield, which was used for glycosylation reactions with silylated nucleobases.
Scheme 1.
Synthesis of 15. Reagents and conditions: (a) 1 equiv SOCl2, MeOH, r.t., 4 h; (b) 2 equiv NaBH4, EtOH, r.t., 14 h; (c) 2,2-dimethoxypropane (solvent), 0.03 equiv CSA, reflux, 2 h; (d) 1.4 equiv iPr2NH, 1.2 equiv nBuLi, 1.4 equiv NFSI, THF, −78 °C; (e) 1.4 equiv iPr2NH, 1.2 equiv nBuLi, 1.4 equiv NFSI, THF, −78 °C; (f) AcOH/MeCN/H2O (14:3:3), 90 °C, 14 h; (g) 1.3 equiv TBDMSCl, 1.3 equiv imidazole, 0.1 equiv DMAP, CH2Cl2, r.t., 30 min; (h) 2 equiv Boc2 O, 1.3 equiv Et3N, 1.1 equiv DMAP, CH2Cl2, r.t., 30 min; (i) 1.2 equiv LiEt3BH, THF, −78 °C, 1 h; (j) 15 equiv Ac2O, 30 equiv Et3N, CH2Cl2, r.t., 30 min.
2.1. Synthesis of pyrimidine 4′-azanucleosides
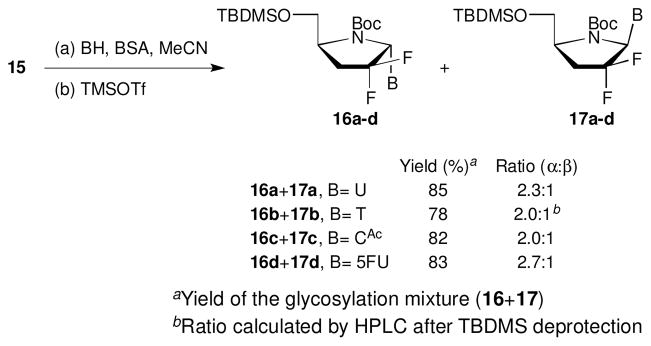
Glycosylation of 15 under Vorbrüggen’s conditions25 with various pyrimidine heterocyclic bases gave α/β mixtures of 4′-azanucleosides in high overall yields (78–85%). The ratio of α/β-anomers was determined by HPLC-MS of the crude reaction mixture (Scheme 2). The poor resolution of the 1H NMR spectra for the TBDMS protecting 4′-azanucleosides hindered the determination of α/β-ratio. Thus, to assign the stereochemistry of the glycosylation products, TBDMS protecting group was removed and the configuration of the anomeric carbon was established by NOESY experiments showing the α-anomer as the major product. This was probably due to the steric hindrance of the bulky silyl protecting group. Similar results were also reported during glycosylation of a Boc-protected proline with pyrimidine base.21 Correlations between H1′ and H4′ as well as H5′ and H6 were clearly observed in the β-anomers, while correlations between H4′ and H6 of the corresponding nucleobase appeared in the α-anomers.
Scheme 2.
Glycosylation of 15 with pyrimidines. Reagents and conditions: (a) 1 equiv 15, 4 equiv base (BH= uracil, thymine, N4-acetylcytosine, and 5-fluorouracil), 6 equiv BSA, MeCN, 80 °C, 1 h; (b) 2.7 equiv TMSOTf, 0–80 °C, 30 min, 78–85% over two steps.
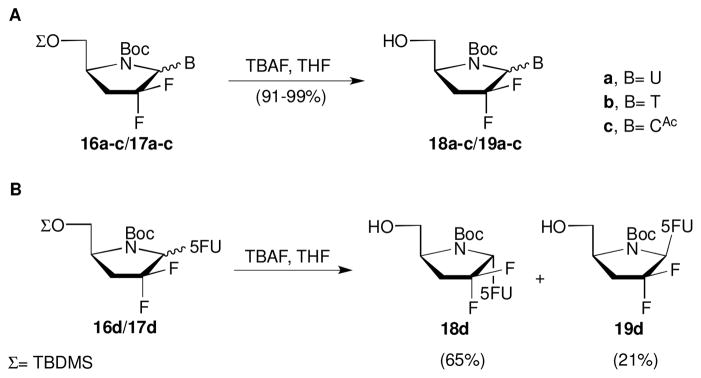
After glycosylation with silylated uracil, the resulting mixture of anomers 16a/17a had different Rf values, and the products were easily separable by column chromatography. However the other nucleosides 16b-d/17b-d were isolated as inseparable α/β-anomeric products. Removal of the TBDMS protecting group was accomplished under standard conditions by treating the separated pure anomers (16a and 17a) or the anomeric mixtures 16b-c/17b-c with TBAF in THF to afford 4′-azanucleosides 18/19 in excellent yields (Panel A, Scheme 3). It is noteworthy that after deblocking the TBDMS, the anomers 18d/19d had different polarity and were separable by silica gel column chromatography (Panel B, Scheme 3). After the two step procedure of glycosylation–deprotection, four anomers were isolated as pure compounds (18a, 19a, 18d and 19d) while 18b-c/19b-c were isolated as non separable mixture of α/β-anomeric products.
Scheme 3.
Removal of TBDMS protecting group. Reagents and conditions: 1.5 equiv TBAF, THF, 0–25 °C, 1 h.
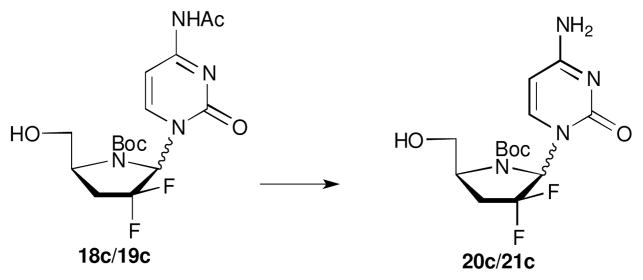
Next, removal of the acetyl protecting group from the cytidine derivatives 18c/19c was accomplished by treatment with ammonia affording an anomeric mixture of 20c/21c (Scheme 4). The deprotection of Boc group from the uracil derivative 18a was attempted with 2 equiv of TFA in CH2Cl2. However, only free uracil was isolated from the reaction mixture showing that despite the stabilization of the glycosidic bond by the two fluorine atoms, the unprotected 4′-azanucleosides are still prone to acid-mediated degradation. Additionally, it has been recently shown that the potent antiviral activity of a series of azanucleoside analogues was not compromised despite the presence of the Boc protecting group.9
Scheme 4.
Synthesis of 20c/21c. Reagents and conditions: NH3 sat-MeOH, r.t., 1 h, 82%.
2.2. Synthesis of purine 4′-azanucleosides
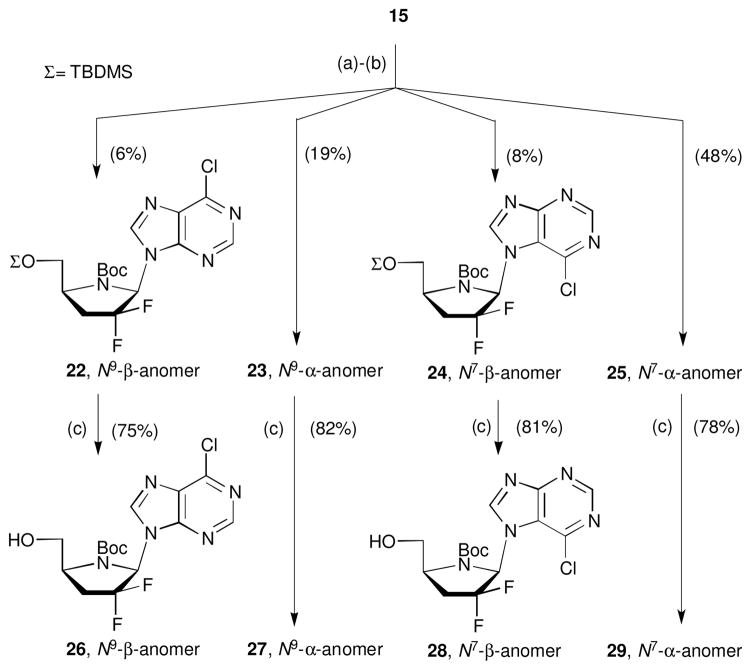
The success with the preparation of the pyrimidine nucleosides together with the few examples of purine 4′-azanucleosides described in the literature26 encouraged us to try the coupling of 15 with purine nucleobases. It is well know that Vorbrüggen coupling25 of silylated purine nucleobases typically results in N7/N9 isomeric mixtures. In addition, since two anomers may result from each glycosylated regioisomer, four compounds may be present in the crude reaction mixture. As expected, after the coupling of 15 with silylated 6-chloropurine, the TLC of the crude reaction mixture showed the presence of four products. Mass spectrometry analysis indicated the same formula weight for all four products. Interestingly, for this particular case, four possible products had sufficient difference in the Rf values to be separated by silica gel column chromatography (Scheme 5).
Scheme 5.
Glycosylation of 15 with 6-chloropurine. Reagents and conditions: (a) 1 equiv 15, 4 equiv 6-chloropurine, 6 equiv BSA, MeCN, 80 °C, 1 h; (b) 2.7 equiv TMSOTf, 0–80 °C, 30 min; (c) 1.5 equiv TBAF, THF, r.t., 1 h.
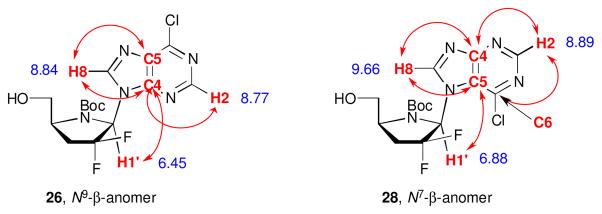
To identify the structure of glycosylated products, it was necessary to remove the TBDMS protecting group (TBAF in THF). The structure of 26-29 was established by 2D NMR spectroscopy. NOESY experiments assisted in the assignment of the stereochemical configuration and HMBC experiments allowed us to determine the attachment of 6-chloropurine via N7 or N9 to the pyrrolidine moiety. In the case of nucleoside 26, H2 and H8 of the 6-chloropurine moiety together with H1′ showed correlation (3JCH) with C4 of the nucleobase (Figure 2). Correlation of these three hydrogen atoms with the same carbon can only be possible in the N9 regioisomer. In the case of compound 28 the expected correlations for an N7 isomer were observed, allowing confirmation of the structure. The same correlations patterns were also observed in the corresponding α-anomers. In addition, the structures were also confirmed by comparing the UV maxima with previously reported data (see experimental section).
Figure 2.
HMBC correlations of N9 and N7 regioisomers. In red are correlations mentioned in the text. The 1H NMR values (δ ppm) are in blue.
The ratio of the four products obtained after glycosylation reaction, was determined by HPLC-MS data on the crude reaction mixture of protected nucleosides 22-25 (22:23:24:25, 8:27:12:53). As seen before for the pyrimidine nucleosides, the α-anomers were also the major products with the purine series. Interestingly, despite of higher reaction temperature (80 °C) employed during the glycosylation step, the N7 product was dominant. This observation, which is unusual for glycosylation of 6-chloropurine base, is likely due to the sterically demanding structure of 15.
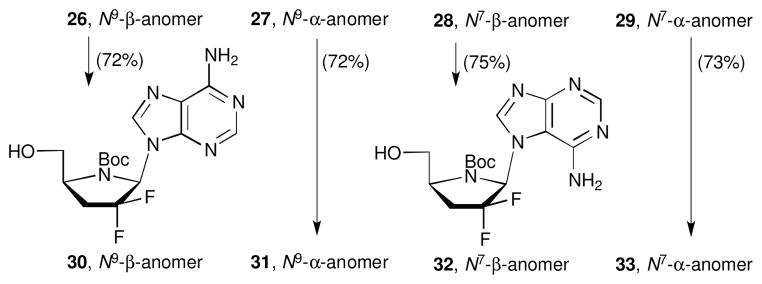
The treatment of 6-chloropurine analogues 26-29 with ammonia furnished the corresponding adenine derivatives 30-33 (Scheme 6) in good yield.
Scheme 6.
Synthesis of adenine 4′-azanucleosides 30-33. Reagents and conditions: NH3 sat-MeOH, 100 °C, 3 h.
3. Biological evaluation
3.1. Antiviral assays
All azanucleosides were tested against HIV-1LAI using 3′-azido-3′-deoxythymidine (AZT, zidovudine) as a reference in an assay with human peripheral blood mononuclear (PBM) cells. We opted for including all products in the screen to maximize the database despite of the fact that some were isolated as mixture of anomeric products.
A summary of the data expressed as the effective concentration required to inhibit viral replication by 50% (EC50) and 90% (EC90) is shown in the Table 1. The 5-fluorouracil analogues 18d (EC50 = 36.9 μM), 19d (EC50 = 44.5 μM) together with the 6-chloro purine derivatives 27 (EC50 = 64.5 μM) and 29 (EC50 = 92.3 μM) showed modest activity when compared with the AZT as a control. It is noteworthy that the α-anomers demonstrated better activities in comparison to their β-counterparts. Also, it is of interest to observe modest activity despite the fact that all products were protected with the Boc group.
Table 1.
Effect of analogues against HIV-1LAI in human peripheral blood mononuclear (PBM) cells
| Analogue | Base | Anti-HIV-1 activity in PBM cellsa | Cytotoxicity (IC50, μM) b | |||
|---|---|---|---|---|---|---|
|
| ||||||
| EC50, μM | EC90, μM | PBM cells | CEM cells | VERO cells | ||
| AZT | β-T | 0.0017 | 0.0027 | >100 | 14.3 | 56.0 |
| 18a | α-U | >100 | >100 | >100 | >100 | >100 |
| 19a | β-U | >100 | >100 | 81.4 | >100 | 44.4 |
| 18b/19b | α/β-T | >100 | >100 | >100 | >100 | >100 |
| 18c/19c | α/β-C | >100 | >100 | >100 | >100 | >100 |
| 18d | α-5-F-U | 36.9 | 75.6 | 30.5 | >100 | >100 |
| 19d | β-5-F-U | 44.5 | 90.8 | 28.2 | >100 | >100 |
| 26 | N9-β-6-Cl-Pu | >100 | >100 | >100 | 57.1 | >100 |
| 27 | N9-α-6-Cl-Pu | 64.5 | >100 | 58.1 | 38.1 | >100 |
| 28 | N7-β-6-Cl-Pu | >100 | >100 | >100 | >100 | >100 |
| 29 | N7-α-6-Cl-Pu | 92.3 | >100 | 59.5 | 55.4 | >100 |
| 30 | N9-β-A | >100 | >100 | >100 | >100 | >100 |
| 31 | N9-α-A | >100 | >100 | >100 | >100 | >100 |
| 32 | N7-β-A | >100 | >100 | >100 | >100 | >100 |
| 33 | N7-α-A | >100 | >100 | >100 | >100 | >100 |
3.2. Cytotoxicity assays
All compounds were evaluated for their potential cytotoxicity in uninfected phytohemagglutinin stimulated human PBM cells, in lymphocytic CEM cells, and Vero (African green monkey Kidney) cells. The majority of compounds did not show any toxicity except the β-uracil analogue 19a in Vero cells, 19a, 18d, 19d, 27 and 29 in PBM cells, and 26, 27 and 29 in CEM cells.
3.3. HCV Replicon assays28
All compounds were tested at 10 μM in an HCV replicon assay using 2′-C-Me-Cytidine as the positive control. No anti-HCV activity was observed (data not shown).
4. Conclusions
In summary, we have developed an efficient synthesis of novel 2′,3′-dideoxy-2′,2′-difluoro-4′-azanucleosides both as pyrimidine and purine analogues. A high yielding sequence of electrophilic difluorination of L-pyroglutamic acid followed by the coupling of protected pyrrolidine 15 as the glycosyl donor with four pyrimidine and one purine nucleobase was established. The pyrimidine 4′-azanucleosides were obtained as mixtures of α- and β-anomeric products, increasing the breadth of novel nucleoside analogues available for biological screening. The α-anomers were obtained as major products during glycosylation. After glycosylation and TBDMS deprotection, the anomeric mixtures of U (18a/19a) and 5-F-U (18d/19d) analogues could be easily separated by silica gel column chromatography. However, the anomeric mixtures of T (18b/19b) and C (18c/19c) analogues could not be separated by silica chromatography. Gratifyingly, glycosylation with 6-chloropurine afforded a separable mixture of four nucleosides arising from the formation of N7/N9 glycosylated regioisomers and the corresponding α/β-anomers. These isomers were characterized based on 2D NMR spectroscopy, and constitute the only examples of fluorinated purine 4′-azanucleosides described to date. Further reaction with ammonia of each isomer furnished a direct route for the purine nucleosides 30-33. All fluorinated 4′-azanucleosides synthesized were tested as inhibitors of HIV-1 in PBM cells. The α-5-F-U analogue 18d was found to be the most active compound (EC50 = 36.9 μM) in this series. These compounds did not exhibit anti-HCV activity in a hepatitis C replicon assay probably due to the lack of a 3′-hydroxyl group or mimic for the moiety. The limited examples of fluorinated 4′-azanucleosides described in the literature and the interesting activity found in some of the nucleosides described in this work, warrants further studies with this new class of compounds.
5. Experimental section
All reagents were bought from Aldrich and Acros at highest commercial quality and used without further purification. All non-aqueous reactions were carried out under anhydrous conditions in dry, freshly distilled solvents. THF and CH2Cl2 were purified by passage through a bed of activated alumina. Reactions were monitored by TLC carried out on 0.25 mm E. Merck silica gel plates (60F-254) using UV light as visualizing agent and/or acidic aqueous permanganate. Flash chromatography was performed using silica gel 60 (230–400 mesh). LC-ESI-MS analyses were carried out in a chromatogram with UV detector at 254 nm using Agilent Poroshell column 120 SB, C18, or Mediterranea column (250 × 45 mm) flow 1 mL min−1 r.t. gradient MeCN-H2O as eluent. Melting points were taken on samples in open capillary tubes and are uncorrected. 1H, 13C NMR, and DEPT were obtained using Varian Mercury and/or Bruker 300.13, 400.13 or 600.13 MHz for 1H, and 75.5, 100.61 MHz or 150.92 for 13C. The same spectrometers were used for the acquisition of 1H-1H homonuclear (COSY and NOESY) and 1H-13C heteronuclear (HSQC and HMBC) correlations. Optical rotations were recorded on a Jasco P-1010 polarimeter and values are reported as follows: [α]λT (c: g/100 mL, solvent). High resolution mass spectra (HRMS) were recorded on a VG 7070 HS mass spectrometer under electron spray ionization (ESI) conditions. The tert-butyldimethyl silyl protecting group is abbreviated below as TBDMS.
Synthesis of (S)-Pyroglutaminol (8)
To a cooled solution of L-pyroglutamic acid (7) (6.0 g, 41.9 mmol) in dry MeOH (80 mL), was added SOCl2 (4.9 g, 41.9 mmol) dropwise with magnetic stirring at room temperature for 2 h. The mixture was concentrated under vacuum to give the methyl ester as clear oil (5.2 g, 80%). This oil (33.2 mmol) was poured in a flask, dissolved in dry EtOH (80 mL) and NaBH4 (2.54 g, 67.0 mmol) was added portionwise. After stirring at room temperature for 2 h, the mixture was acidified with concentrated HCl to pH 1. Solvents were removed under vacuum and the residue subjected to flash chromatography (15% MeOH, CH2Cl2) to afford 8 (3.3 g 28.5 mmol). The synthesis of 8 has been previously described.23
Synthesis of (5S)-2,2-dimethyl-8-oxo-1-aza-3-oxa-bicyclo[3.3.0]octane (9)
A mixture of compound 8 (3.3 g, 28.7 mmol), CSA (0.68 mmol, 158 mg) and 2,2-dimethoxypropane (DMP; 12 mL) was refluxed for 2 h. The volatiles components (DMP, MeOH) were removed in vacuo. Fresh DMP was added, and the mixture again refluxed for 2 h. This process was repeated a total of three times. After the final evaporation the residue was subjected to flash chromatography (50% AcOEt/hexane) and then distilled under vacuo to afford 9 as a colorless oil (3.67 g, 83%). The synthesis of 9 has been previously described.24
Synthesis of (5S)-2,2-dimethyl-7,7-difluoro-8-oxo-1-aza-3-oxa-bicyclo[3.3.0]octane (10)
Diisopropyl amine (2.3 mL, 16.5 mmol) was added to dry THF with magnetic stirring and the solution was cooled to −78 °C. N-buthylitium (3.7 mL, 13.9 mmol) was added slowly and the mixture was stirred for 1 h. A solution of 9 (1.8 g, 11.6 mmol) in THF (9 mL) was added slowly. The mixture was stirred for 1 h at −78 °C before the addition of a solution of N-fluorodibenzenesulfonimide (NFSI; 5.19 g, 16.5 mmol) in THF (18 mL), the solution was again stirred for 45 min and then quenched by the addition of saturated NH4Cl. THF was removed under vacuo and the residue extracted with AcOEt and water. The combined organic layers were dried over Na2SO4, filtered and evaporated. The residue was purified by flash chromatography (20–50% AcOEt-hexane) to give the monofluorinated lactam (1.84 g, 92%). The same fluorination procedure was carried out using the previous monofluorinated lactam (1.84 g, 10.6 mmol) as a substrate to get the difluorinated compound 10 (1.72 g, 85%) as a pale yellow oil. The synthesis of 10 has been previously described.24
Synthesis of (5S)-3,3-difluoro-5-hydroxymethyl-2-pyrrolidinone (11)
Compound 10 (1.65 g, 8.37 mmol) was stirred in a mixture of acetic acid, acetonitrile and water (14:3:3, v/v) (20 mL). The solution was heated at 90 °C for 14 h. After evaporation of solvents, the residue was purified by flash chromatography (10% MeOH/CH2Cl2) to yield pure 11 as a white solid (1.04 g, 80%). The synthesis of 11 has been previously described.24
Synthesis of (5S)-5-(tert-butyldimethylsilyloxymethyl)-3,3-difluoro-2-pyrrolidinone (12)
To a solution of 11 (960 mg, 6.39 mmol) in dry CH2Cl2 (40 mL) at 0 °C was added imidazole (566 mg, 8.31 mmol), DMAP (0.63 mmol, 78 mg) and TBDMSCl (1.25 g, 8.31 mmol). The reaction was stirred at room temperature for 30 min, quenched by the addition of water (300 μL) and extracted with CH2Cl2. The combined organic layers were dried over Na2SO4, solvents were evaporated and the residue subjected to flash chromatography (15% AcOEt/hexane) to afford 12 (1.8 g, 87%) as viscous oil. Rf: (20% AcOEt/hexane):0.23. [α]D20 = +35 (c 0.5, CH2Cl2). 1H NMR (CDCl3, 400.13 MHz): δ 0.05 (s, 6H, Me2Si), 0.87 (s, 9H, tBu), 2.29 (m, 1H, H3), 2.55 (m, 1H, H-3), 3.54 (dd, 1H, CH2O, JHH 6.2 Hz, JHH 10.3 Hz), 3.67 (dd, 1H, CH2O, JHH 4.4 Hz, JHH 10.4 Hz), 3.80 (m, 1H, H-5), 7.67 (br s, 1H, NH). 13C NMR (CDCl3, 100.61 MHz): δ 5.4 (2CH3, tBu), 18.4 (1C, tBu), 26.0 (3CH3, tBu), 33.1 (t, C-4, JCF 22.1 Hz), 50.7 (C-5), 65.1 (CH2O), 118.0 (t, C-3, JCF 249.5 Hz), 166.7 (t, C=O, JCF 31.2 Hz). HRMS (ESI+) calcd for C11H21F2NO2SiNa [M+Na]+ 288.1202, found 288.1203.
Synthesis of (5S)-5-(tert-butyldimethylsilyloxymethyl)-N-tert-butyloxycarbonyl-3,3-difluoro-2-pyrrolidinone (13)
To a solution of 12 (854 mg, 3.21 mmol), in dry CH2Cl2 (28 mL), was added Et3N, (0.6mL, 4.17 mmol), DMAP (427 mg, 3.53 mmol) and Boc2O (1.41, 6.42 mmol). The reaction was stirred at room temperature for 30 min. Solvents were concentrated to dryness and the residue purified by column chromatography (10% AcOEt/hexane) to give 13 (1.13 g, 96%). Rf (20% AcOEt/hexane): 0.53. [α]D20 = −51 (c 0.5, CH2Cl2). 1H NMR (CDCl3, 400.13 MHz): δ 0.02 (s, 6H, Me2Si), 0.85 (s, 9H, tBu), 1.53 (s, 9H, tBu), 2.48 (m, 2H, H-4), 3.70 (dd, 1H, CH2O, JHH 2.5 Hz, JHH 10.3 Hz), 3.83 (dd, 1H, CH2O, JHH 5.1 Hz, JHH 10.3 Hz), 4.22 (m, 1H, H-5). 13C NMR (CDCl3, 100.61 MHz): δ 5.7 (tBu), 18.4 (tBu), 26.0 + 27.8 (tBu + Boc), 31.0 (t, C-4, JCF 22.1 Hz), 53.4 (C-5), 62.3 (CH2O), 84.7 (Boc), 116.6 (t, C-3, JCF 251.0 Hz), 149.2 (C=O), 166.7 (t, C=O, JCF 32.2 Hz). MS (ESI+, m/z) 382 [(M+NH4)+ 100%]; 388 [(M+Na)+ 10%]. HRMS (ESI+) calcd for C16H29F2NO4SiNa [M+Na]+ 388.1726, found 388.1727.
Synthesis of (5S)-5-(tert-butyldimethylsilyloxymethyl)-N-tert-butyloxycarbonyl-3,3-difluoro-2-hydroxy-pyrrolidine (14) (mixture of anomers)
To a solution of 13 (368 mg, 1.0 mmol) in anhydrous THF (9.3 mL) was slowly added dropwise LiEt3BH (504 μL, 0.5 mmol) at −78 °C under argon atmosphere. The reaction mixture was stirred 1 h, then additional LiEt3BH (706 μL, 0.70 mmol) was added. After 1 h, the reaction was quenched with water (4 mL) and the organic solvent was evaporated. The aqueous phase was extracted with CH2Cl2 dried over Na2SO4, filtered and concentrated under vacuum. The resulting residue was purified by flash chromography (30% AcOEt/hexane) to afford a inseparable mixture of anomers 14 (311 mg, 84%) in 4:1 ratio. Rf (20% AcOEt/hexane): 0.51. Major isomer 1H NMR (CDCl3, 400.13 MHz): δ 0.11 (s, 6H, Me2Si), 0.91 (9H, tBu), 1.48 (s, 9H, Boc), 2.45 (m, 2H), 3.39 (d, 1H, CH2O, JHH 10.2 Hz), 3.83 (d, 1H, CH2O, JHH 10.0 Hz), 4.07 (d, 1H, H-5, JHH 10.0 Hz), 5.16 (t, 1H, H-2, JHF 10.2 Hz). Minor isomer 1H NMR (CDCl3, 400.13 MHz): δ 0.11 (s, 6H, tBu), 0.91 (9H, tBu), 1.48 (s, 9H, Boc), 2.45 (m, 2H), 3.51 (d, 1H, CH2O, JHH 9.8 Hz), 3.69 (m, 1H, CH2O), 4.21 (m, 1H, H-5), 5.16 (m, 1H, H-2). MS (ESI+, m/z) 368 [(M+H)+ 10%]; 390 [(M+Na)+ 100%]. HRMS (ESI+) calcd for C16H31F2NO4SiNa [M+Na]+ 390.1883, found 388.1885.
Synthesis of (5S)-2-acetyloxy-5-(tert-butyldimethylsilyloxymethyl)-N-tert-butyloxycarbonyl-3,3-difluoro-pyrrolidine (15) (mixture of anomers)
To a solution of anomers 14 (300 mg) in CH2Cl2 (10 mL) was added Et3N (3.4 mL, 2.5 mmol), Ac2O (1.2 mL, 12.0 mmol), and DMAP (catalytic). The solution was allowed to stir for 30 min. Solvents were evaporated and the residue purified by flash column chromatography (20% AcOEt/hexane) to afford 15 (332 mg, 100%). The presence of TBDMS protecting complicates the analysis of the NMR spectra. Rf (20% AcOEt/hexane): 0.64. 1H NMR (CDCl3, 400.13 MHz): δ 0.11 (s, 6H, Me2Si), 0.95 (9H, tBu), 1.47 (s, 9H, Boc), 2.09 (m, 2H), 3.77 (br s, 1H), 4.01 (br s, 2H), 6.49 (br s, 1H). MS (ESI+, m/z) 409 [(M+H)+ 10%]; 432 [(M+Na)+ 100%]. HRMS (ESI+) calcd for C18H33F2NO5SiNa [M+Na]+ 432.1988, found 432.2003.
General procedure for glycosylation of fluorinated pyrrolidine 15 with pyrimidine bases followed by deprotection. Synthesis of nucleosides 18a-d/19a-d
To a stirred solution of 15 (0.3 mmol, 122 mg) and the different bases (1.2 mmol) in dry MeCN was added BSA (0.59 mL, 1.8 mmol). The reaction mixture was stirred at 80 °C for 1 h. This solution was cooled to 0 °C and TMSOTf (0.17 mL, 0.83 mmol) was added dropwise. The solution was heated at 80 °C for 1 h. The reaction was quenched by the addition of Et3N (0.2 mL). Solvents were evaporated and the resulting residue purified by column chromatography (20–50% AcOEt/hexane) to afford the protected 4′-azanucleosides (16a-d/17a-d) (78–85%). Then stirred solutions of the nucleoside derivatives in THF (4 mL) were treated with 1.0 M solutions of TBAF in THF (1.5 equiv). Reactions were quenched with water, and after evaporation of solvents the residues purified by flash chromatography (2–5% MeOH/CH2Cl2) to afford the pure 4′-azanucleosides (18a-d/19a-d) as white solids (90–99%).
1-[(2S,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-uracil (18a)
61% yield after glycosylation and 99% yield for TBDMS deprotection. Rf (5% MeOH/CH2Cl2): 0.19. mp: 82–85 °C. [α]D20 = +18 (c 1.0, CH2Cl2). 1H NMR (CDCl3. 600.15 MHz, 328 K): δ 1.41 (s, 9H, Boc), 2.55 (m, 2H, H-4), 3.71 (dd, 1H, CH2O, JHH 6.2 Hz, JHH 11.1 Hz), 3.91 (s, 1H, CH2O), 4.36 (s, 1H, H-5), 5.75 (s, 1H, H-5B), 6.35 (br s, H-2), 7.09 (1H, H-6B), 9.55 (br s, 1H, NH). 13C NMR (CDCl3. 150.92 MHz, 328 K): δ 28.1 (Boc), 35.0 (C-4), 58.1 (C-5), 63.7 (CH2O), 70.6 (m, C-2), 83.4 (Boc), 101.6 (C-5B), 124.6 (C-3), 138.1 (C-6B), 150.2 (C-2B), 153.0 (C=O), 162.9 (C-4B). HRMS (ESI+) calcd for C14H19F2N3O5Na [M+Na]+ 370.1185, found 370.1186.
1-[(2R,5S)-N-tert-butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-uracil (19a)
24% yield after glycosylation and 98% yield for TBDMS deprotection. Rf (5% MeOH/CH2Cl2): 0.21. mp: 73–75 °C. [α]D20 = −54 (c 0.5, CH2Cl2). 1H NMR (acetone-d6, 300.13 MHz): δ 1.42 (s, 9H, Boc), 2.63 (m, 2H, H-4), 3.74 (ddd, 1H, CH2O, JHH 2.4 Hz, JHH 4.2 Hz, JHH 11.4 Hz), 4.10 (tt, 1H, H-5, JHH 2.7 Hz, JHH 8.1 Hz), 4.31 (m, 1H, CH2O), 4.64 (t, 1H, OH, JHH 4.4 Hz), 5.63 (d, 1H, H-5B, JHH 8.1 Hz), 6.36 (d, 1H, H-2, JHH 13.8 Hz), 8.33 (d, 1H, H-6B, JHH 8.1 Hz), 10.22 (br s, 1H, NH). 13C NMR (acetone-d6, 75.5 MHz): δ 27.3 (Boc), 33.1 (t, C-4, JCF 22.6 Hz), 54.1 (C-5), 57.1 (CH2O), 71.8 (m, C-2), 81.5 (Boc), 101.6 (C-5B), 124.5 (t, C-3, JCF 251.8 Hz), 140.1 (C-6B), 150.7 (C-2B), 153.6 (C=O), 162.6 (C-4B). MS (ESI+, m/z) 348 [(M+H)+ 100%]; 370 [(M+Na)+ 35%]. HRMS (ESI+) calcd for C14H19F2N3O5Na [M+Na]+ 370.1185, found 370.1190.
1-[(2S,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-thymine (18b) and 1-[(2R,5S)-N-tert-butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-thymine (19b)
78% yield after glycosylation and 98% yield for TBDMS deprotection. Rf (5% MeOH/CH2Cl2): 0.22. α-isomer (18b) 1H NMR (CDCl3, 400.13 MHz): δ 1.38 (s, 9H, Boc), 1.92 (s, 3H, CH3), 2.54 (m, 2H, H-4), 3.23 (s, 1H, OH), 3.71 (s, 1H, CH2O), 4.07 (s, 1H, CH2O), 4.35 (s, 1H, H-5), 6.35 (br s, H-2), 6.83 (1H, H-6B), 9.32 (br s, 1H, NH). β-isomer (19b) δ 1.42 (s, 9H, Boc), 1.90 (s, CH3), 2.54 (m, 2H, H-4), 3.15 (br s, 1H, OH), 3.77 (dd, 1H, CH2O, JHH 2.8 Hz, JHH 11.2 Hz), 4.10 (q, 1H, H-5, JHH 4.0Hz), 4.26 (d, 1H, CH2O, JHH 11.2 Hz), 6.18 (d, 1H, H-2, JHF 13.2 Hz), 7.66 (s, 1H, H-6B), 9.24 (br s, 1H, NH). MS (ESI+, m/z) 362 [(M+H)+ 25%]; 384 [(M+Na)+ 100%]. HRMS (ESI+) calcd for C15H21F2N3O5Na [M+Na]+ 384.1341, found 384.1349.
N4-acety1-1-[(2S,5S)-N-tert-butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]cytosine (18c) and N4-acety1-1-[(2R,5S)-N-tert-butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-cytosine (19c)
82% yield after glycosylation and 91% yield for TBDMS deprotection. Rf (70% AcOEt/hexane): 0.11. α-isomer (18c): 1H NMR (MeOH-d4, 300.13 MHz): δ 1.33 (s, 9H, Boc), 2.21 (s, 3H, CH3), 2.61 (m, 2H, H-4), 3.58 (t, 1H, CH2O, JHH 8.7 Hz), 3.91 (s, 1H, CH2O), 4.33 (m, 1H, H-5), 6.45 (d, 1H, H-2, JHF 12.9 Hz), 7.46 (s, 1H, H-5), 7.92 (s, 1H, H-5). β-isomer (19c): δ 1.41 (s, 9H, Boc), 2.20 (s, 3H, CH3), 2.62 (m, 2H, H-4), 3.66 (d, 1H, CH2O, JHH 11.4 Hz), 4.07 (s, 1H, H-5), 4.30 (s, 1H, CH2O), 6.47 (d, 1H, H-2, JHF 12.9 Hz), 7.41 (d, 1H, H-5B, JHF 7.5 Hz), 8.76 (d, 1H, H-6B, JHF 7.5 Hz). HRMS (ESI+) calcd for C15H22F2N4O5Na [M+Na]+ 411.1450, found 411.1467.
1-[(2S,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-5-fluorouracil (18d)
83% overall yield (α+β) after glycosylation and 65% yield for TBDMS deprotection (pure α-anomer). Rf (5% MeOH/CH2Cl2): 0.24. mp: 61–63 °C. [α]D20 = +57 (c 0.5, CH2Cl2). 1H NMR (acetone-d6, 300.13 MHz): δ 1.39 (rotamers, 9H, Boc), 2.75 (m, 2H, H-4), 3.55 (t, 1H, CH2O, JHH 6.2 Hz), 3.98 (d, 1H, CH2O, JHH 9.6 Hz), 4.43 (s, 1H, H-4), 6.30 (d, 1H, JHF 9.6 Hz), 7.84 (s, 1H, H-6). 13C NMR (acetone-d6, 75.5 MHz): δ 27.3 (Boc), 33.1 (t, C-4, JCF 23.4 Hz), 54.1 (C-5), 60.7 (CH2O), 70.9 (m, C-2), 81.5 (Boc), 123.2 (d, C-6B, JCF 36.2 Hz), 124.5 (t, C-3, JCF 249.8 Hz), 142.0 (d, C-5B, JCF 249.1 Hz), 149.2 (C-2B), 156.2, 156.6 (C-4+C=O). HRMS (ESI+) calcd for C15H18F3N3O5Na [M+Na]+ 388.1091, found 388.1108.
1-[(2R,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-5-fluorouracil (19d)
83% overall yield (α+β) after glycosylation and 27% yield for TBDMS deprotection (pure β-anomer). Rf (5% MeOH/CH2Cl2): 0.29. mp: 82–84 °C. [α]D20 = −57 (c 0.5, CH2Cl2). 1H NMR (acetone-d6, 300.13 MHz): δ 1.44 (s, 9H, Boc), 2.75 (m, 2H, H-4), 3.72 (dd, 1H, CH2O, JHH 1.5 Hz, JHH 11.4 Hz), 4.12 (tt, 1H, H-5, JHH 2.1 Hz, JHH 8.4 Hz), 4.43 (tt, 1H, CH2O, JHH 2.7 Hz, JHH 11.4 Hz), 6.33 (d, 1H, H-2, JHF 13.8 Hz), 8.79 (d, 1H, H-6, JHF 7.5 Hz). 13C NMR (acetone-d6, 125.61 MHz): δ 27.3 (Boc), 33.1 (t, C-4, JCF 23.4 Hz), 54.1 (C-5), 57.1 (CH2O), 71.8 (m, C-2), 81.5 (Boc), 124.4 (d, C-6B, JCF 36.2 Hz), 124.5 (t, C-3, JCF 251.8 Hz), 142.0 (d, C-5B, JCF 231.8 Hz), 149.3 (C-2B), 156.3, 156.7 (C-4 + C=O). HRMS (ESI+) calcd for C15H18F3N3O5Na [M+Na]+ 388.1091, found 388.1085.
1-[(2S,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-cytosine (20c) and 1-[(2R,5S)-N-tert-butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-cytosine (21c)
The mixture of anomers 18c/19c (44 mg, 0.11 mmol) was dissolved in a saturated solution of ammonia in MeOH (3 mL). The reaction was stirred for 2 h at room temperature for 2 h. MeOH was evaporated and the residue subjected to flash chromatography 1–5% MeOH/CH2Cl2 to afford the mixture of anomers 20c/21c (32 mg, 82%) as a white solid. Rf (5% MeOH/CH2Cl2): 0.38. α-isomer (20c) 1H NMR (MeOH-d4, 300.13 MHz): δ 1.39 (rotamers, 9H, Boc), 2.59 (m, 2H, H-4), 3.52 (t, 1H, CH2O, JHH 8.7 Hz), 3.92 (s, 1H, CH2O), 4.32 (m, 1H, H-5), 5.96 (s, 1H, H-5), 6.45 (d, 1H, H-2, JHF 13.5 Hz), 7.45 (s, 1H, H-6). β-isomer (21c) δ 1.39 (rotamers, 9H, Boc), 2.59 (m, 2H, H-4), 3.67 (dd, 1H, CH2O, JHH 2.1 Hz, JHH 11.4 Hz), 4.05 (tt, 1H, H-5, JHH 2.6 Hz, JHH 7.4), 4.26 (ddd, 1H, CH2O, JHH 2.3 Hz, JHH 11.4, JHH 11.6), 5.91 (d, 1H, H-5B, JHH 7.5 Hz), 6.40 (d, H-2, JHF 13.5 Hz), 8.29 (d, 1H, H-6B, JHH 9.1 Hz). HRMS (ESI+) calcd for C14H20F2N4O4Na [M+Na]+ 369.1345, found 369.1348.
General procedure for the synthesis of purine nucleosides (26-29)
Similar procedure as the described for the synthesis of nucleosides 18a-d/19a-d. After glycosylation and solvents evaporation the residue was purified by column chromatography (20% AcOEt/hexane) to afford the silyl protected isomeric nucleosides 22-25. Each separated 4′-azanucleoside was treated with 1.0 M solutions of TBAF in THF (1.5 equiv) to give pure 26-29 as white solids (75–82%).
9-[(2R,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-6-chloropurine (26)
6% yield after glycosylation and 75% yield for TBDMS deprotection. Rf (10% MeOH/CH2Cl2): 0.52. 1H NMR (CDCl3, 400.13 MHz): δ 1.29 (9H, Boc), 2.64 (m, 1H, H-4), 2.99 (m, 1H, H-4), 3.84 (dd, 1H, CH2O, JHH 2.8 Hz, JHH 11.6 Hz), 4.24 (t, 1H, H-5, JHH 8.0 Hz), 4.46 (d, 1H, JHH 11.6 Hz), 6.45 (d, 1H, H-2, JHF 12.8 Hz), 8.77 (s, 1H, H-2B), 8.84 (s, 1H, H-8B). 13C NMR (CDCl3, 100.61 MHz): δ 27.9 (Boc), 33.5 (t, C-4, JCF 23.2 Hz), 57.7 (C-5), 61.6 (CH2O), 72.2 (m, C-2), 83.3 (Boc), 124.5 (t, C-3, JCF 225.9 Hz), 131.71 (C-5B), 144.2 (C-8B), 151.4 (C-4B), 151.6 (C-6B), 152.1 (C-2B), 153.8 (C=O). HRMS (ESI+) calcd for C15H18ClF2N5O3Na [M+Na]+ 412.0958, found 412.0969.
9-[(2S,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-6-chloropurine (27)
19% yield after glycosylation and 82% yield for TBDMS deprotection. Rf (10% MeOH/CH2Cl2): 0.52. mp: 64–66 °C. [α]D20 = −12 (c 0.5, CH2Cl2). UV λmax (MeOH) 265 nm (6866 M−1 cm−1). 1H NMR (CDCl3, 300.13 MHz): δ 1.22 (rotamers, 9H, Boc), 2.67 (m, 1H, H-4), 3.17 (m, 1H, H-4), 3.79 (dd, 1H, CH2O, JHH 5.7 Hz, JHH 10.5 Hz), 4.05 (dd, 1H, CH2O, JHH 5.7 Hz, JHH 11.1 Hz), 4.65 (s, 1H, H-4), 6.18 (d, 1H, H-2, JHF 10.5 Hz), 8.15 (s, 1H, H-2), 8.77 (s, 1H, H-8). 13C NMR (CDCl3, 75.5 MHz): δ 27.9 (Boc), 33.5 (t, C-4, JCF 22.7 Hz), 58.9 (C-5), 64.5 (CH2O), 72.5 (m, C-2), 83.2 (Boc), 124.6 (t, C-3, JCF 254.4 Hz), 132.0 (C-5B), 144.4 (C-8B), 150.9 (C-4B), 151.7 (C-6B), 152.3 (C-2B), 153.2 (C=O). HRMS (ESI+) calcd for C15H18ClF2N5O3Na [M+Na]+ 412.0958, found 412.0963.
7-[(2R,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-6-chloropurine (28)
8% yield after glycosylation and 81% yield for TBDMS deprotection. Rf (10% MeOH/CH2Cl2): 0.50. 1H NMR (CDCl3, 400.13 MHz): δ 1.31 (9H, Boc), 2.55 (td, 1H, H-4, J 7.2, J 15.2), 2.89 (m, 1H, H-4), 3.85 (dd, 1H, CH2O, JHH 2.0 Hz, JHH 11.6 Hz), 4.16 (t, 1H, H-5, JHH 6.2 Hz), 4.69 (d, 1H, JHH 11.6 Hz), 6.88 (d, 1H, H-2, JHF 12.0 Hz), 8.77 (s, 1H, H-2B), 8.84 (s, 1H, H-8B). 13C NMR (CDCl3, 100.61 MHz): δ 27.9 (Boc), 33.5 (t, C-4, JCF 23.6 Hz), 57.4 (C-5), 60.2 (CH2O), 72.2 (m, C-2), 83.3 (Boc), 122.5 (C-5B), 123.3 (t, C-3, JCF 254.5 Hz), 143.1 (C-4B), 147.4 (C-8B), 152.7 (C-2B), 153.5 (C=O), 161.8 (C-6B). HRMS (ESI+) calcd for C15H18ClF2N5O3Na [M+Na]+ 412.0958, found 412.0943.
7-[(2S,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-6-chloropurine (29)
48% yield after glycosylation and 78% yield for TBDMS deprotection. Rf (10% MeOH/CH2Cl2): 0.47. mp: 76–78 °C. [α]D20 = +9 (c 0.5, CH2Cl2). UV λmax (MeOH) 270 nm (6616 M−1 cm−1). 1H NMR (CDCl3, 400.13 MHz): δ 1.23 (rotamers, 9H, Boc), 2.67 (m, 2H, H-4), 3.28 (br s, 1H, OH), 3.79 (m, 1H, CH2O), 4.11 (m, 1H, CH2O), 451 (s, 1H, H-4), 6.83 (d, 1H, H-2, JHF 9.2 Hz), 8.30 (s, 1H, H-8), 8.91 (s, 1H, H-2). 13C NMR (CDCl3, 75.5 MHz): δ 27.7 (Boc), 33.7 (t, C-4, JCF 22.7 Hz), 57.6 (C-5), 63.0 (CH2O), 72.4 (m, C-2), 83.7 (Boc), 122.7 (C-5B), 124.2 (t, C-3, JCF 254.4 Hz), 143.2 (C-4B), 144.8 (C-8B), 152.5 (C=O), 152.8 (C-2B), 161.8 (C-6B). HRMS (ESI+) calcd for C15H18ClF2N5O3Na [M+Na]+ 412.0958, found 412.0970.
General procedure for the synthesis of adenine nucleosides (30-33)
4′-Azanucleosides 26-29 (40 mg, 0.11 mmol) were treated with a saturated solution of ammonia in MeOH (3 mL) and stirred for 1 h at 100 °C in a sealed tube. The reaction is cooled to room temperature, MeOH evaporated and the residue purified by column chromatography (5–10% MeOH/CH2Cl2) to afford nucleosides 30-33 as white solids (72–75%).
9-[(2R,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-adenine (30)
72% yield. Rf (10% MeOH/CH2Cl2): 0.31. 1H NMR (THF-d8, 400.13 MHz): δ 1.25 (s, 9H, Boc), 2.60 (m, 1H, H-4), 3.01 (m, 1H, H-4), 3.70 (d, 1H, CH2O, JHH 10.8 Hz), 4.07 (m, 1H, H-5), 4.28 (d, 1H, CH2O, JHH 10.4 Hz), 4.67 (br s, 1H, OH), 6.40 (d, 1H, H-2, JHF 13.6 Hz), 6.47 (br s, 2H, NH2), 8.14 (s, 1H, H-2 or H-8), 8.39 (s, 1H, H-8 or H-2). 13C NMR (THF-d8, 100.61 MHz): δ 25.3 (Boc), 31.4 (t, C-4, JCF 11.3 Hz), 57.7 (C-5), 58.9 (CH2O), 69.0 (m, C-2), 79.2 (Boc), 117.7 (C-5B), 122.5 (t, C-3, JCF 252.3 Hz), 136.8 (C-8B), 148.3 (C-4B), 150.8 (C-2), 151.7 (C-6B), 161.8 (C=O). HRMS (ESI+) calcd for C15H21F2N6O3 [M+H]+ 371.1638, found 371.1642.
9-[(2S,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-adenine (31)
72% yield. Rf (10% MeOH/CH2Cl2): 0.29. mp: 105–107 °C. [α]D20 = −2 (c 0.5, MeOH). UV λmax (MeOH) 260 nm (8975 M−1 cm−1). 1H NMR (CDCl3, 400.13 MHz): δ 1.16 (rotamers, 9H, Boc), 2.58 (t, 1H, H-4, JHH 15.2 Hz), 3.15 (m, 1H, H-4), 3.75 (dd, 1H, CH2O, JHH 6.4 Hz, JHH 10.9 Hz), 4.05 (dd, 1H, CH2O, JHH 5.2 Hz, JHH 10.9 Hz), 4.63 (s, 1H, H-5), 6.07 (d, 1H, H-2, JHF 9.6 Hz), 6.13 (s, 2H, NH2), 7.81 (s, 1H, H-2 or H-8), 8.33 (s, 1H, H-8 or H-2). 13C NMR (CDCl3, 75.5 MHz): δ 27.7 (Boc), 34.3 (t, C-4, JCF 22.5 Hz), 58.5 (C-5), 63.9 (CH2O), 71.8 (m, C-2), 82.6 (Boc), 119.5 (C-5B), 124.8 (t, C-3, JCF 252.3 Hz), 139.4 (C-8B), 149.3 (C-4B), 153.3, 155.8 (C-2B + C-6B + C=O), 151.7 (C-6B), 161.8 (C=O). HRMS (ESI+) calcd for C15H21F2N6O3 [M+H]+ 371.1638, found 371.1628.
7-[(2R,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-adenine (32)
75% yield. Rf (10% MeOH/CH2Cl2): 0.29. mp: 225–227 °C. 1H NMR (THF-d8, 300.13 MHz): δ 1.29 (s, 9H, Boc), 2.68 (m, 2H, H-4), 3.63 (d, 1H, CH2O, JHH 11.1 Hz), 4.06 (m, 2H, CH2O + H-5), 5.38 (t, 1H, OH, JHH 9.6 Hz), 6.75 (d, 1H, H-2, JHF 10.5 Hz), 6.87 (s, 2H, NH2), 8.24 (s, 1H, H-2 or H-8), 8.86 (s, 1H, H-8 or H-2). 13C NMR (MeOH-d4, 75.5 MHz): δ 28.0 (Boc), 31.4 (t, C-4, JCF 22.3 Hz), 57.4 (C-5), 60.2 (CH2O), 73.7 (m, C-2), 81.9 (Boc), 111.4 (C-5B), 124.6 (t, C-3, JCF 252.9 Hz), 143.5 (C-8B), 151.6 (C-4B), 152.9 (C-2B), 153.8, 160.4 (C-6B + C=O). HRMS (ESI+) calcd for C15H21F2N6O3 [M+H]+ 371.1638, found 371.1648.
7-[(2S,5S)-N-tert-Butyloxycarbonyl-3,3-difluoro-5-(hydroxymethyl)pyrrolidin-2-yl]-adenine (33)
73% yield. Rf (10% MeOH/CH2Cl2): 0.27. mp: 206–208 °C. [α]D20 = +29 (c 0.5, MeOH). UV λmax (MeOH) 275 nm (4644 M−1 cm−1). 1H NMR (MeOH-d4, 300.13 MHz) δ 1.56 (rotamers, 9H, Boc), 2.80 (m, 2H, H-4), 3.61 (t, 1H, CH2O, JHH 9.9 Hz), 4.03 (d, 1H, CH2O, JHH 9.0 Hz), 4.52 (s, 1H, H-5), 6.68 (d, 1H, H-2, JHF 9.3 Hz), 8.33 (s, 1H, H-2 or H-8), 8.54 (s, 1H, H-8 or H-2). 13C NMR (MeOH-d4, 75.5 MHz): δ 26.6 (Boc), 33.9 (m, C-4), 56.9 (C-5), 60.3 (CH2O), 72.1 (m, C-2), 82.1 (Boc), 111.4 (C-5B), 124.6 (t, C-3, JCF 256.7 Hz), 142.6 (C-8B), 151.8, 152.1 (C-4B or C=O or C-6B), 152.6 (C-2B), 158.9 (C-4B or C=O or C-6B). HRMS (ESI+) calcd for C15H21F2N6O3 [M+H]+ 371.1638, found 371.1628.
Supplementary Material
Acknowledgments
Financial support of this work by the Spanish Ministerio de Educación y Ciencia (MEC) (Project MEC-CTQ-2007-61126) and PCTI (2006–2009)/FEDER (2007–2013) Asturias (Project EQUIP09-07) are gratefully acknowledged. S.M.-M. thanks MEC for a predoctoral fellowship. We also acknowledge the National Institutes of Health (NIH) for financial support of this work through Grant Number R01 GM081484. We thank the National Science Foundation for instrumentation grants CHE9709183 and CHE0741968. We also thank Dr. Y. Su (UCSD Mass Spectrometry) for mass analysis. This work was also supported in part by NIH grant 2P30-AI-050409 and the Department of Veterans Affairs.
Footnotes
Electronic supplementary material available: 1H, 13C NMR spectral data. The level of purity is indicated by the inclusion of copies of 1H and 13C NMR spectra. In addition, some 2D NMR experiments are shown, which were used to assign the peaks.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Parker WB. Chem Rev. 2009;109:2880–2893. doi: 10.1021/cr900028p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Morris PE, Kamath VP. Modified Nucleosides in Biochemistry, Biotechnology and Medicine. Wiley-VCH; Weinheim (Germany): 2008. [Google Scholar]; b) De Clercq E. J Clin Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]; c) Chu CK, Baker DC, editors. Nucleosides and Nucleotides as Antitumor and Antiviral Agents. Plenum Press; New York: 1993. [Google Scholar]
- 3.Ferrero M, Gotor V. Chem Rev. 2000;100:4319–4347. doi: 10.1021/cr000446y. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama M. Synthesis. 2000:1637–1655. [Google Scholar]
- 5.a) Boto A, Hernández D, Hernández R. Eur J Org Chem. 2010:3847–3857. [Google Scholar]; b) Boto A, Hernández D, Hernández R. Tetrahedron Lett. 2008;49:455–458. [Google Scholar]; c) Costenaro ER, Fontoura LAM, Oliveira DF, Correia CRD. Tetrahedron Lett. 2001;42:1599–1602. [Google Scholar]; d) Yokoyama M, Momotake A. Synthesis. 1999:1541–1554. [Google Scholar]
- 6.a) Jeong LS, Tosh DK, Choi WJ, Lee SK, Kang YJ, Choi S, Lee JH, Lee H, Lee HW, Kim HO. J Med Chem. 2009;52:5303–5306. doi: 10.1021/jm900852b. [DOI] [PubMed] [Google Scholar]; b) Jeong LS, Tosh DK, Kim HO, Wang T, Hou X, Yun HS, Kwon Y, Lee SK, Choi J, Zhao LX. Org Lett. 2008;10:209–212. doi: 10.1021/ol7025558. [DOI] [PubMed] [Google Scholar]
- 7.a) Clarke ML, Damaraju VL, Zhang J, Mowles D, Tackaberry T, Lang T, Smith KM, Young JD, Tomkinson B, Cass CE. Mol Pharmacol. 2006;70:303–310. doi: 10.1124/mol.105.021543. [DOI] [PubMed] [Google Scholar]; b) Parker WB, Shaddix SC, Rose LM, Waud WR, Shewach DS, Tiwari KN, Secrist JA., III Biochem Pharmacol. 2000;60:1925–1932. doi: 10.1016/s0006-2952(00)00520-7. [DOI] [PubMed] [Google Scholar]
- 8.Schinazi RF, Chu CK, Peck A, McMillan A, Mathis R, Cannon D, Jeon LS, Beach JW, Choi WB, Yeola S, Liotta DC. Antimicrob Agents Chemother. 1992;36:672–676. doi: 10.1128/aac.36.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiacchio U, Borrello L, Crispino L, Rescifina A, Merino P, Macchi B, Balestrieri A, Mastino A, Piperno A, Romeo G. J Med Chem. 2009;52:4054–4057. doi: 10.1021/jm900197j. [DOI] [PubMed] [Google Scholar]
- 10.Romeo G, Chiacchio U, Corsaro A, Merino P. Chem Rev. 2010;110:3337–3370. doi: 10.1021/cr800464r. [DOI] [PubMed] [Google Scholar]
- 11.For recent reviews on fluorinated nucleosides see: Qiu XL, Xu XH, Qing FL. Tetrahedron. 2010;66:789–843.Liu P, Sharon A, Chu CK. J Fluor Chem. 2008;129:743–766. doi: 10.1016/j.jfluchem.2008.06.007.
- 12.a) Hagmann WK. J Med Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]; b) Muller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; b) Bohm HJ, Banner D, Kuhn B, Muller K, Obst-Sander U, Stahl M, Bendels S, Kansy M. Chem Bio Chem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- 13.a) Mitsuya H, Broder S. Nature. 1987;325:773–778. doi: 10.1038/325773a0. [DOI] [PubMed] [Google Scholar]; b) Mitsuya H, Broder S. Proc Natl Acad Sci. 1986;83:1911–1915. doi: 10.1073/pnas.83.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Martin JA, Bushnell DJ, Duncan IB, Dunsdon SJ, Hall MJ, Machin PJ, Merrett JH, Parkes KEB, Roberts NA, Thomas GJ, Galpin SA, Kinchington D. J Med Chem. 1990;33:2137–2145. doi: 10.1021/jm00170a015. [DOI] [PubMed] [Google Scholar]; b) Sterzycki RZ, Ismail G, Brankovan V, Martin JC, Mansur MM. J Med Chem. 1990;33:2150–2157. doi: 10.1021/jm00170a017. [DOI] [PubMed] [Google Scholar]
- 15.Balzarini J, Baba M, Pauwels R, Herdewijn P, De Clercq E. Biochem Pharmacol. 1988;37:2847–2856. doi: 10.1016/0006-2952(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 16.Ferradina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, Del Medico P, Scaltriti L, Katsaros D, Priolo D, Scambia G. J Clin Oncol. 2008;26:890–896. doi: 10.1200/JCO.2007.13.6606. [DOI] [PubMed] [Google Scholar]
- 17.Vulfovich M, Rocha-Lima C. Expert Rev Anticancer Ther. 2008;8:993–1002. doi: 10.1586/14737140.8.6.993. [DOI] [PubMed] [Google Scholar]
- 18.Silvestris N, Cinieri S, La Torre I, Pezzela G, Numico G, Orlando L, Lorusso V. Breast. 2008;17:220–226. doi: 10.1016/j.breast.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 19.a) Bonate PL, Arthaud L, Stephenson K, Secrist JA, Weitman S. Nat Rev Drug Discov. 2006;5:855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]; b) Faderl S, Gandhi V, Keating MJ, Jeha S, Plunket W, Kantarjian HM. Cancer. 2005;103:1985–1995. doi: 10.1002/cncr.21005. [DOI] [PubMed] [Google Scholar]
- 20.a) Zhu W, Chong Y, Choo H, Mathews J, Schinazi RF, Chu CK. J Med Chem. 2004;47:1631–1640. doi: 10.1021/jm0303148. [DOI] [PubMed] [Google Scholar]; b) Choo Y, Chong Y, Choi Y, Mathews J, Schinazi RF, Chu CK. J Med Chem. 2003;46:389–398. doi: 10.1021/jm020376i. [DOI] [PubMed] [Google Scholar]; c) Choi Y, Choo Y, Chong Y, Lee S, Olgen S, Schinazi RF, Chu CK. Org Lett. 2002;4:305–307. doi: 10.1021/ol0171665. [DOI] [PubMed] [Google Scholar]; d) Stuyver LJ, Lostia S, Adams M, Mathew JS, Pai BS, Grier J, Tharnish PM, Choi Y, Chong Y, Choo H, Chu CK, Otto MJ, Schinazi RF. Antimicrob Agents Chemother. 2002;46:3854–3860. doi: 10.1128/AAC.46.12.3854-3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Qiu XL, Qing FL. Bioorg Med Chem. 2005;13:277–283. doi: 10.1016/j.bmc.2004.09.040. [DOI] [PubMed] [Google Scholar]; b) Qiu XL, Qing FL. J Org Chem. 2005;70:3826–3837. doi: 10.1021/jo050057+. [DOI] [PubMed] [Google Scholar]; c) Qiu XL, Qing FL. Synthesis. 2004:334–340. [Google Scholar]
- 22.a) Martínez-Montero S, Fernández S, Sanghvi YS, Chattopadhyaya J, Ganesan M, Ramesh NG, Gotor V, Ferrero M. J Org Chem. 2012;77:4671–4678. doi: 10.1021/jo3004452. [DOI] [PubMed] [Google Scholar]; b) Martínez-Montero S, Fernández S, Sanghvi YS, Gotor V, Ferrero M. Org Biomol Chem. 2011;9:5960–5966. doi: 10.1039/c1ob05739a. [DOI] [PubMed] [Google Scholar]; c) Rodríguez-Perez T, Fernández S, Sanghvi YS, Detorio D, Schinazi RF, Gotor V, Ferrero M. Bioconjugate Chem. 2010;21:2239–2249. doi: 10.1021/bc1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Martínez-Montero S, Fernández S, Sanghvi YS, Gotor V, Ferrero M. J Org Chem. 2010;75:6605–6613. doi: 10.1021/jo101368z. [DOI] [PubMed] [Google Scholar]; e) Rodríguez-Perez T, Fernández S, Martínez-Montero S, González-García T, Sanghvi YS, Gotor V, Ferrero M. Eur J Org Chem. 2010:1736–1744. [Google Scholar]; f) Martínez-Montero S, Fernández S, Sanghvi YS, Wen K, Gotor V, Ferrero M. Eur J Org Chem. 2009:3265–3271. [Google Scholar]; g) Díaz-Rodríguez A, Sanghvi YS, Fernández S, Schinazi RF, Theodorakis E, Ferrero M, Gotor V. Org Biomol Chem. 2009;7:1415–1423. doi: 10.1039/b818707j. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) García J, Díaz-Rodríguez A, Fernández S, Sanghvi YS, Ferrero M, Gotor V. J Org Chem. 2006;71:9765–9771. doi: 10.1021/jo062033o. [DOI] [PubMed] [Google Scholar]; i) Díaz-Rodríguez A, Fernández S, Sanghvi YS, Ferrero M, Gotor V. Org Proc Res Dev. 2006;10:581–587. [Google Scholar]
- 23.Saijo S, Wada M, Himizu JI, Ishida A. Chem Pharm Bull. 1980;28:1449–1458. [Google Scholar]
- 24.a) Coward JK, Konas DW. J Org Chem. 2001;66:8831–8842. doi: 10.1021/jo0106804. [DOI] [PubMed] [Google Scholar]; b) Coward JK, Konas DW. Org Lett. 1999;1:2105–2107. doi: 10.1021/ol991150l. [DOI] [PubMed] [Google Scholar]
- 25.Vorbrüggen H, Ruh-Pohlwnz C. Handbook of nucleoside synthesis. Wiley Interscience; 2001. [Google Scholar]
- 26.a) Reist EJ, Fisher LV, Goodman L. J Org Chem. 1967;32:2541–2544. doi: 10.1021/jo01283a038. [DOI] [PubMed] [Google Scholar]; b) Reist EJ, Gueffroy DE, Blackford RW, Goodman L. J Org Chem. 1966;31:4025–4030. [Google Scholar]
- 27.Schinazi RF, Sommadossi JP, Saalman V, Cannon DL, Xie MW, Hart GC, Smith GA, Hahan EF. Antimicrob Agents Chemother. 1990;34:1061–1067. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuyver LJ, Whitaker T, McBrayer TR, Hernández-Santiago BI, Lostia S, Tharnish PM, Ramesh M, Chu CK, Jordan R, Shi J, Rachakonda S, Watanabe KA, Otto MJ, Schinazi RF. Antimicrob Agents Chemother. 2003;47:244–254. doi: 10.1128/AAC.47.1.244-254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.