Abstract
Heightened cortisol response to stress due to hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis may stimulate appetite and food intake. In this study, we assessed cortisol responsivity to a cold pressor test (CPT) as well as appetite ratings and subsequent test meal intake (TMI) in obese women. Following an overnight fast on two counterbalanced days, 20 obese women immersed their non-dominant hand for 2 min in ice water (CPT) or warm water (WW) as a control. Plasma cortisol (ng/ml), heart rate, and blood pressure, as well as ratings of stress, pain, and appetite, were serially acquired. An ad libitum liquid meal was offered at 45 min and intake measured covertly. Fasting cortisol was higher at 15 min (mean peak cortisol) following the CPT compared to WW. Higher stress was reported at 2 and 15 min for the CPT compared to WW. Pain, an indirect marker of the acute stress, systolic and diastolic blood pressure increased following the CPT at 2 min compared to WW Hunger decreased after the CPT at 2 and 15 min, and desire to eat ratings were lower following CPT compared to WW . Subjects did not have greater test meal intake (TMI) following CPT compared to WW. There was also no significant relationship between cortisol levels following stress and TMI, indicating that cortisol did not predict subsequent intake in obese women.
Keywords: Stress, obesity, cortisol, food intake
1. Introduction
Stress has a varied effect on appetite and behavior (1). Acute and/or severe stressors (e.g., injury, harmful threats) can result in decreased food intake (2), whereas repeated exposure to life stressors (e.g., job pressures) may lead some persons to seek and consume palatable foods (3, 4). Stressors, whether acute or chronic, are a composite of physiological, psychological, and environmental factors that can activate two pathways, the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal axis (HPA). During stress, the SNS induces a “fight or flight” response, whereas HPA stimulation promotes adrenocortical secretion. In addition, psychobiological stress responses to appraisals of threat are mediated by neural substrates involved in higher order executive functioning (5, 6).
Several lines of evidence suggest that stress-induced cortisol release may be a potential mediator of disordered eating, as exaggerated cortisol responses following acute laboratory stress tests have been observed in women with anorexia nervosa (7), bulimia nervosa (8), and in obese women with (9) and without (10) binge eating disorder (BED). Persistent stress appears to hyperactivate the HPA axis, and may lead to increased food intake and central adiposity (11). Obese people tend to report stress as a precipitant of overeating in clinical practice (12), and recent studies have found an association between stress and greater binge frequency and size (13, 14).
In the present study, we investigated the role of cortisol as a key mediator of stress-induced eating in obese women. We measured endogenously secreted plasma cortisol levels prior to and following a cold pressor test (CPT), compared to a control warm water (WW) condition. Serial measures of heart rate (HR), blood pressure (BP) and pain were used as indicators of SNS activation and stress response . We expected that the CPT would increase cortisol levels compared to the control, and that stress-induced cortisol release would stimulate appetite and subsequent test meal intake (TMI).
2. Methods
2.1 Participants
The participants (Subjects; Ss) were 20 severely obese pre-menopausal non-diabetic women (age=35.9±6.2 SD; BMI=37.0±5.2). All Ss were evaluated by medical history and a physical examination that included an electrocardiogram and fasting blood samples for a chemistry panel and complete blood cell count. Women taking oral contraceptives were excluded, since prolonged use can alter HPA axis responsivity. None of the Ss had current suicidal ideation or had been hospitalized for depression or other psychiatric disorders. Ss were advised that the aim of the study was to examine the relationship between stress and hormones in healthy obese women. The study protocol and consent forms were approved by the St. Luke’s-Roosevelt Hospital Institution Review Board.
2.2 Procedure
During the initial consultation, anthropometric measurements (height, weight) were taken, and Ss were given a taste test of a complete nutritional liquid food (Boost - High Protein, Mead Johnson) to determine their preferred flavor (vanilla, chocolate, strawberry) for an ad libitum meal. Bioelectrical impedance analysis (BIA) was performed using a Tanita instrument (TBF-300A Tokyo, Japan) to estimate the percentage of body fat (15, 16).
Following an overnight fast on two non-consecutive counterbalanced days within a 7-day period, Ss underwent a cold pressor test (CPT) or warm water (WW) control starting at 10 am on a weekday. An indwelling butterfly needle catheter was inserted into the non-dominant forearm vein and remained connected to a slow drip normal-saline bag for 2.5 hours to prevent intermittent clotting. Ss rested for 30 min after the catheter was inserted to avoid a cortisol stress response secondary to venipuncture. At time periods of −30, 0, 2, 5, 15, 30, 45, 60, 90, and 120 min (using 0 min as the baseline) on each experimental day, Ss had blood draws, heart rate (HR) and blood pressure (BP) measurements, and were asked to rate verbally their stress, pain, hunger, and desire to eat on a visual analogue scale (VAS) from 0 - 100.
From 0 min through 2 min, Ss immersed their non-dominant hand, with fingers spread apart, into a circular container of either ice water (CPT) at 0 °C or warm water (WW) at 34 °C. To prevent a temperature rise during the CPT, a strainer bag with ice was kept in the water, monitored with a digital thermometer. At 45 min, Ss were given a test meal of a commercially available liquid formula (Boost - High Protein, 1 kcal/ ml, Mead Johnson) in a covered large cooler container which had been refrigerated. Ss were asked to drink through a straw, to prevent visual feedback, and told to “eat as much as you’d like” and to complete appetite ratings before and after the ad libitum meal. The liquid formula meal was weighed before and after consumption (unobserved by the Ss), and energy values calculated.
2.3 Measures
Test tubes for plasma cortisol (ng/ml) contained EDTA and were cold centrifuged (3000 RPM) for 15 min, then stored in cryo-microtubes at −80°C at the New York Obesity Nutrition Research (NYONRC) Laboratory.
2.4 Data Analysis
Cortisol, heart rate (HR), blood pressure (BP) including systolic and diastolic , pain, stress, and appetite ratings were assessed before and after the CPT and WW. Peak and AUC values before and after the meal for were analyzed with two-way (condition, time) repeated measures ANOVA. Correlations between plasma cortisol and ad libitum TMI were performed by Pearson’s r. All statistical analyses were conducted using SPSS (version 20; IBM SPSS, Chicago, IL) with two-tailed tests of significance (p<0.05).
2.5 Power and Sample Size
A sample size calculation was based on George et al. (17), who compared energy intake following corticotropin-releasing hormone (CRH) infusion, which led to increased cortisol levels relative to placebo in 14 lean individuals [F(1,13) = 7.79, p = .015], with effect size d = 1.55. For power = .80 with two tailed α =.05, the required n = 18. Thus the sample size n = 20 in our study had adequate power to detect a difference between conditions.
3. Results
Descriptive statistics of cortisol, HR, BP, pain, stress and appetite ratings at baseline (0 min), hand withdrawal (2 min), and mean peak cortisol (15 min) are expressed as means ± SD (Table 1). The mean time for the cortisol to peak was at 15 min whereas the mean time for the peak HR, BP, stress and pain ratings were at 2 min at hand withdrawal.
Table 1.
Cortisol Levels and Stress-related and Appetite Ratings at baseline (0 min), hand withdrawal (2 min), and at mean time for peak cortisol (15 min) in all Obese Women.
| Baseline (0 min) |
Hand Withdrawal (2 min) |
Time at Mean Peak Cortisol (15 min) |
||||
|---|---|---|---|---|---|---|
| CPT | WW | CPT | WW | CPT | WW | |
| Cortisol (ng/ml) |
5.1±1.5 | 5.1±1.8 | 5.1±1.6 | 4.8±1.9†† | 6.0±2.3† | 4.5±1.7** ††† |
| Heart Rate beats/min |
68.8±8.0 | 66.5±8.4 | 67.7±9.3 | 67±7.8 | 65.5±9.1 | 67±8.1 |
| Systolic BP (mmHg) |
122±14.1 | 121.4±15.0 | 129.2±15.7†† | 117.2±14.2** | 121.7±13.1 | 120±13.7 |
| Diastolic BP (mmHg) |
73.1±10.2 | 72.7±11.1 | 75.6±10.8† | 71.3±12.4* | 71.4±16.5 | 70.8±11.4 |
| Stress (0-100) |
6.0±13.1 | 5.5±10.0 | 21±30.8† | 5.5±11** | 7±14.9 | 5.0±11.5 |
| Pain (0-100) |
1.0±4.5 | 2.0±4.1 | 59.3±33.4*** ††† |
1.5±3.7 | 2.0±4.1 | 1.25±3.2 |
| Hunger (0-100) |
48.3±34.4 | 60.5±25.5 | 34±36† | 53.3±32* | 45.8±34.7 | 64.3±27.2* |
| Desire to eat (0-100) |
51.3±33.4 | 53.8±27.5 | 34.8±35.6†† | 56.0±25.9** | 46.0±33.0 | 66.2±25.5*† |
Means (± SD) for cortisol concentrations, heart rate, BP (blood pressure), stress, pain, and appetite ratings in obese women (n = 20) are shown.
p<0.05
p<0.01
p<0.001 change from each respective baseline condition.
p<0.05
p<0.01
p<0.001 difference between CPT and WW control at each time point
Cortisol
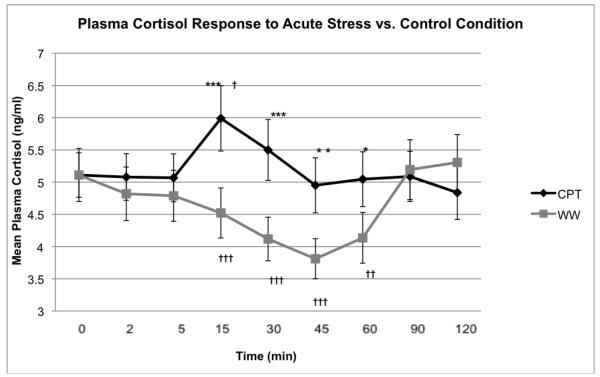
Changes in plasma cortisol levels (ng/ml) from baseline (0 min) to hand withdrawal (2 min) after CPT did not differ from the WW control (1.2±0.2 ng/ml SEM vs.1.4±0.2, p=0.30). However, cortisol changes from baseline (0 min) to mean time for peak cortisol (15 min) following the CPT were greater compared to the WW control (0.9±0.4 ng/ml; vs. −0.6±0.1, p=0.003) (Figure 1).
Figure 1.
Cortisol Prior to and Following a Cold Pressor Test (CPT) and Warm Water (WW) Control
Cortisol was serially measured for 120 mins to adequately assess change in levels due to the stressor compared to change related to diurnal variation. The stressor was administered between 0 and 2 min and the test meal was given at 45 min. a) Mean plasma cortisol (± SEM) levels significantly increased following the CPT, between 0 and 15 min whereas cortisol following WW steadily declined from 5 min in all obese women. The decline in cortisol measures before the meal and the rise after food intake in the warm water control are likely secondary to cortisol’s diurnal variation of secretion.
† p<0.05, ††p<0.01, †††p<0.001 - change from each respective baseline condition.
*p<0.05, ** p<0.01, ***p<0.001- difference between CPT and WW control at each time point.
Sympathetic Activity
From baseline to time of hand withdrawal, the change in systolic blood pressure SBP (F=15.4, p=0.001) and diastolic blood pressure DBP (F=5.4, p=0.03), were greater for CPT, although heart rate was not significantly greater (F=0.08, p=0.78). From baseline (0 min) to the time of hand withdrawal (0 – 2 min) changes in stress (F=6.4, p=0.02) and pain (F=59.4, p<0.00001) ratings, were significantly higher after the CPT compared to the WW control. Similarly, from baseline to time of hand withdrawal, the change in systolic blood pressure SBP (F=15.4, p=0.001) and diastolic blood pressure DBP (F=5.4, p=0.03), were greater for CPT, although heart rate was not significantly greater (F=0.08, p=0.78). There were no significant changes from baseline to 15 min (mean peak cortisol) in stress, pain, SBP, DBP, and HR between conditions (Table 1).
Pain
From baseline (0 min) to the time of hand withdrawal (0 – 2 min) changes in pain (F=59.4, p<0.00001) ratings, were significantly higher after the CPT compared to the WW control. There was no significant change from baseline to 15 min (mean peak cortisol) in pain between conditions (F=0.55, p=0.65). From baseline to 15 min (at mean peak cortisol) change in VAS pain ratings correlated with change in VAS stress ratings (r=0.52, p=0.19) but did not correlate with change in cortisol levels (r=-0.43, p=0.06).
Appetite Ratings
Ss reported decreasing hunger (F=3.8, p=0.03) scores from baseline to hand withdrawal at 2 min and to mean peak cortisol at 15 min, but these changes in hunger did not differ between conditions (F=0.58, p=0.57). Desire to eat ratings (at 2 and 15 min) were significantly lower following CPT compared to control (F=3.9, p=0.03).
Ad Libitum Test Meal Intake
Ss did not consume more test meal following the CPT (548.5 g±59.5) than WW (553.6±72.1), p=0.92. Cortisol levels at 45 min (5.0±0.4 vs.: 3.8±0.3, p=0.01) and area under the curve (AUC) cortisol from baseline to 45 min (245.2±19. vs. 195.1±15.9, p=0.001) were significantly higher in the CPT (vs. WW) condition. However, neither cortisol at 45 min (r=-0.18, p=0.46) nor AUC cortisol from baseline to 45 min (r=-0.12, p=0.63) was correlated with ad libitum TMI. Food intake was also not correlated with peak mean cortisol at 15 min (r=0.02, p=0.93), peak mean stress (r=0.02, p=0.95), pain (r=0.25, p=0.28), SBP (r=0.03, p=0.90), and DBP (r=-0.05, p=0.83) at 2 min.
4. Discussion
The objective of the study was to assess whether stress-induced cortisol was associated with overconsumption. We investigated cortisol responsivity to acute stress in women, since eating disorders and obesity are more common in females than in males (18, 19). Our results showed that cortisol rose quickly in response to the stressor, with a mean peak at 15 min. However, cortisol levels before the meal despite being higher than in the non-stress condition, did not predict intake of the ad libitum test meal given at 45 min. Food intake was tested at 45 min instead of immediately following the stressor (2 min) to ensure that peak cortisol levels were achieved prior to the meal as previous studies have demonstrated cortisol peak levels ranging from 0 – 60 min following a stressor (17, 20). The decline in cortisol levels before the meal in the control condition likely reflect cortisol’s diurnal variation of secretion which declines following a peak after awakening (21-23), and the rise in cortisol after food intake has been shown previously (24, 25).
In contrast, Epel et al. (20) showed that stress-induced cortisol reactivity was related to greater ad libitum caloric intake after exposure to a novel psychological laboratory stressor. Similarly, George et al. (17) showed that CRH-stimulated cortisol release led to greater food intake compared to placebo with the peak cortisol response correlating with total caloric intake. Variations in study results may be due to differences in stressors, (a longer 45 min psychosocial stressor (20) and a CRH infusion (17) versus an acute physiological stressor), and differences in test meal (an array of high energy dense snack foods in Epel’s and George’s study, versus a palatable liquid meal). In addition, reduced food intake following the CPT is consistent with an acute stressor activating the sympathetic nervous system to evoke a “flight or fight” response, resulting in appetite suppression (26). Although, these SNS effects were transient as can be seen in the pattern of BP, HR following the stressor , cognitive factors, including memory of the stressor’s effects may have prolonged the anorexic effects of SNS activation, overriding the effects of the HPA axis stimulation. Also in our study, the elevated cortisol levels at 15 min after the stressor had declined at 45 min just prior to the ad libitum meal, and although still significantly higher than the control, were no longer significantly higher than baseline and may not have been able to raise food intake (Figure 1). In contrast, cortisol levels prior to food intake were higher than baseline concentrations in Epel’s and George’s studies.
We used a physiological stressor, instead of a psychosocial stressor (e.g. Trier Social Stress Test-TSST ) because our goal was to reliably elicit a stress-induced cortisol response. Indeed, the CPT induced a significant stress (p=0.02) and pain response (p<0.0001) at the time of hand withdrawal. Although pain may differ from stress in influencing appetite and food intake (27), no significant relationship was observed between mean peak pain ratings at hand withdrawal (2 min) and TMI. There was also no significant correlation in baseline to 15 min changes between pain ratings and cortisol levels, suggesting that pain did not influence stress related cortisol levels.
Limitations of the study include a relatively small homogenous sample without normal weight controls. Also norepinephrine and epinephrine were not measured to better assess SNS activation in addition to HR and BP.. However, measurements of catecholamines can be problematic due to hormone stability (half lives = 1 min) (28). Lastly, laboratory studies are usually limited to testing acute stressors, and chronic stressors may have greater effects on food intake as chronic psychosocial stressors have been linked to obesity (29) and cardiometabolic disease (30). In addition, overeating is less likely to be observed in laboratory settings where food intake is being monitored, albeit covertly. In summary, in this controlled, condition counterbalanced study, elevated cortisol levels in response to an acute laboratory stressor did not lead to increased appetite ratings or test meal intake in obese women.
Highlights.
 We assessed whether stress-induced cortisol was associated with overeating in obese Ss.
We assessed whether stress-induced cortisol was associated with overeating in obese Ss. Cortisol rose in response to an acute stressor compared to the non-stress condition arm water.
Cortisol rose in response to an acute stressor compared to the non-stress condition arm water. Stress-induced cortisol not predict intake of the ad libitum test meal.
Stress-induced cortisol not predict intake of the ad libitum test meal. The sympathetic nervous system may have overridden the expected cortisol effect.
The sympathetic nervous system may have overridden the expected cortisol effect.
Acknowledgments
Supported in part by the National Institute of Health: DK068392 (A.G.) DK07046 (M.E.G./A.G.), DK026687 (Hormone Metabolite Core Lab) and UL1RR024156 Columbia University CTSA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure information: All authors have nothing to disclose.
References
- 1.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62:853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Popper R, Smits G, Meiselman HL, Hirsch E. Eating in combat: a survey of U.S. Marines. Mil Med. 1989;154:619–623. [PubMed] [Google Scholar]
- 3.McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosom Med. 1990;52:97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ng DM, Jeffery RW. Relationships between perceived stress and health behaviors in a sample of working adults. Health Psychol. 2003;22:638–642. doi: 10.1037/0278-6133.22.6.638. [DOI] [PubMed] [Google Scholar]
- 5.Aschbacher K, Kemeny ME. New directions in linking the dynamics of affective and stress-arousal systems. Brain Behav Immun. 2011;25:230–231. doi: 10.1016/j.bbi.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abell T, Malagelada J, Lucas A. Gastric electromechanical and neurohormonal function in anorexia nervosa. Gastroenterology. 1987;93:8. doi: 10.1016/0016-5085(87)90557-9. al e. [DOI] [PubMed] [Google Scholar]
- 8.Koo-Loeb JH, Pedersen C, Girdler SS. Blunted cardiovascular and catecholamine stress reactivity in women with bulimia nervosa. Psychiatry Res. 1998;80:13–27. doi: 10.1016/s0165-1781(98)00057-2. [DOI] [PubMed] [Google Scholar]
- 9.Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- 10.Vicennati V, Pasqui F, Cavazza C, Pagotto U, Pasquali R. Stress-related development of obesity and cortisol in women. Obesity (Silver Spring) 2009;17:1678–1683. doi: 10.1038/oby.2009.76. [DOI] [PubMed] [Google Scholar]
- 11.Kyrou I, Tsigos C. Stress mechanisms and metabolic complications. Horm Metab Res. 2007;39:430–438. doi: 10.1055/s-2007-981462. [DOI] [PubMed] [Google Scholar]
- 12.Gluck ME. Stress response and binge eating disorder. Appetite. 2006;46:26–30. doi: 10.1016/j.appet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Schulz S, Laessle RG. Stress-induced laboratory eating behavior in obese women with binge eating disorder. Appetite. 2012;58:457–461. doi: 10.1016/j.appet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Sims R, Gordon S, Garcia W, Clark E, Monye D, Callender C, Campbell A. Perceived stress and eating behaviors in a community-based sample of African Americans. Eat Behav. 2008;9:137–142. doi: 10.1016/j.eatbeh.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosy-Westphal A, Later W, Hitze B, Sato T, Kossel E, Gluer CC, Heller M, Muller MJ. Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X-ray absorptiometry. Obes Facts. 2008;1:319–324. doi: 10.1159/000176061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson R, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin Nutr. 2007;26:771–777. doi: 10.1016/j.clnu.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 17.George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology. 2010;35:607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 19.Association AP. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Fourth Edition American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 20.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 21.Hucklebridge F, Hussain T, Evans P, Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Desir D, van Cauter E, Golstein J, Fang VS, Leclercq R, Refetoff S, Copinschi G. Circadian and ultradian variations of ACTH and cortisol secretion. Horm Res. 1980;13:302–316. doi: 10.1159/000179297. [DOI] [PubMed] [Google Scholar]
- 23.Kurina LM, Weiss LA, Graves SW, Parry R, Williams GH, Abney M, Ober C. Sex differences in the genetic basis of morning serum cortisol levels: genome-wide screen identifies two novel loci specific to women. J Clin Endocrinol Metab. 2005;90:4747–4752. doi: 10.1210/jc.2005-0384. [DOI] [PubMed] [Google Scholar]
- 24.Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med. 1999;61:214–224. doi: 10.1097/00006842-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetitie in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrimology. 2001;26:13. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 26.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 27.Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001;98:9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babisch W. Stress hormones in the research on cardiovascular effects of noise. Noise Health. 2003;5:1–11. [PubMed] [Google Scholar]
- 29.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Heraclides A, Chandola T, Witte DR, Brunner EJ. Psychosocial stress at work doubles the risk of type 2 diabetes in middle-aged women: evidence from the Whitehall II study. Diabetes Care. 2009;32:2230–2235. doi: 10.2337/dc09-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]