Abstract
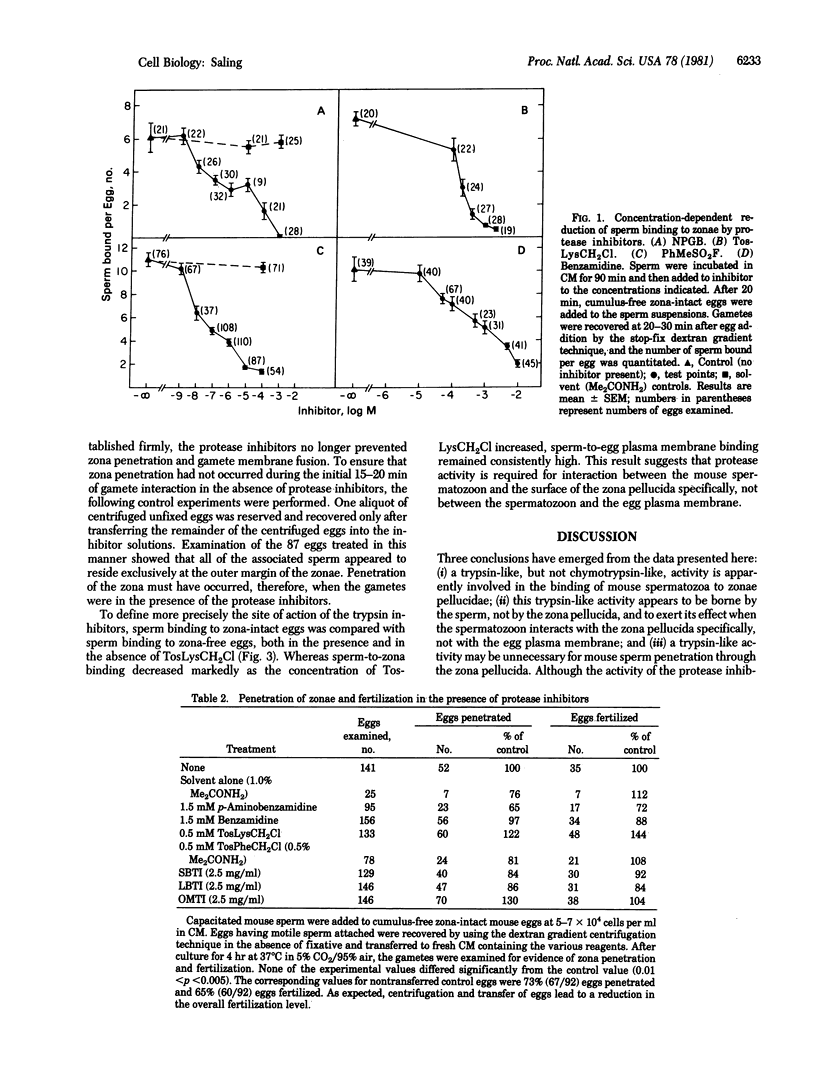
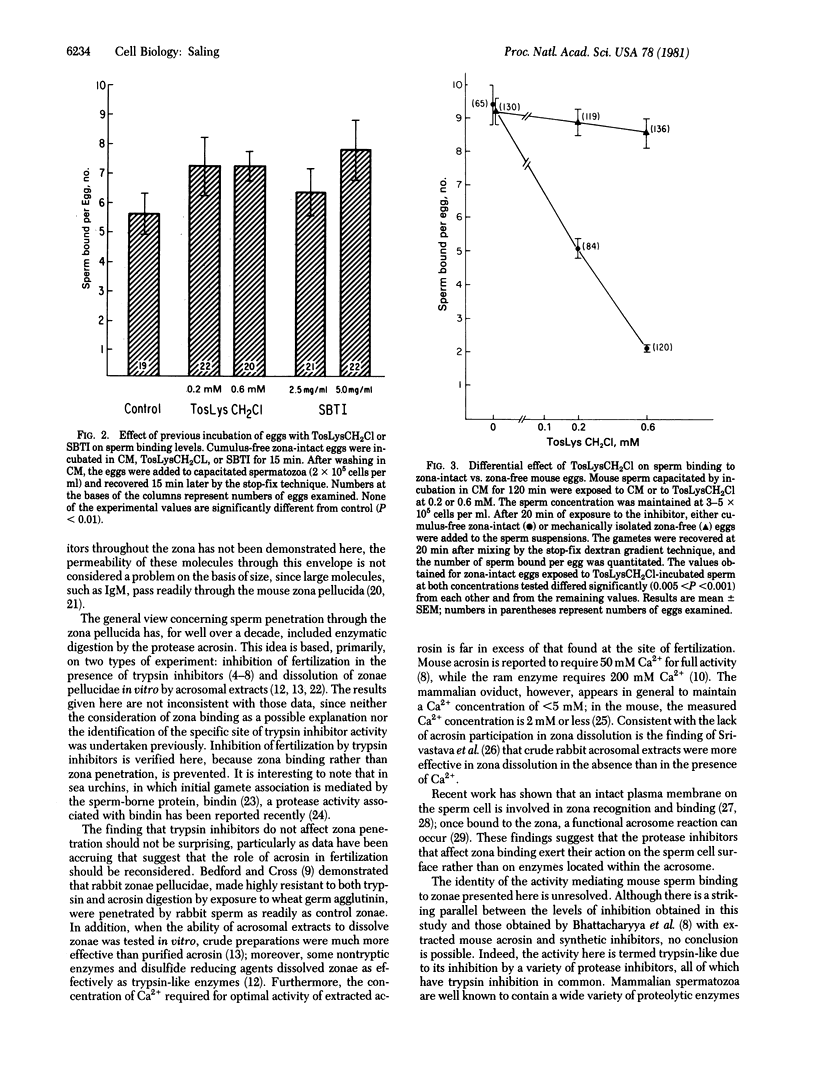
Work from a number of laboratories has shown that fertilization is blocked in the presence of protease inhibitors, although the specific site of inhibition has not been identified. The present experiments were designed to discriminate between sperm binding to zonae pellucidae as opposed to sperm penetration through zonae, so as to assess the effect of protease inhibitors on these two distinct events. Exposure of capacitated mouse spermatozoa to a variety of protease inhibitors directed against trypsin blocked sperm binding to zonae in a concentration-dependent manner. A chymotrypsin-directed inhibitor was not capable of blocking sperm binding to zonae. The trypsin inhibitors did not affect sperm penetration though zonae nor gamete membrane fusion if the sperm had established a firm association with the zona surface before addition of the inhibitors. Previous incubation of zona-intact eggs with the inhibitors did not lead to a reduction in sperm binding, indicating that the activity affected by the inhibitors is borne by spermatozoa. Interaction between spermatozoa and the zona surface appeared to be the specific locus of inhibition; sperm binding to zona-free eggs (i.e., binding to the egg plasma membrane) was unaltered by the trypsin inhibitors. These results suggest a reevaluation of the function of proteases in fertilization focusing on their role in initial sperm contact with the zona pellucida.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedford J. M., Cross N. L. Normal penetration of rabbit spermatozoa through a trypsin- and acrosin-resistant zona pellucida. J Reprod Fertil. 1978 Nov;54(2):385–392. doi: 10.1530/jrf.0.0540385. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A. K., Goodpasture J. C., Zaneveld L. J. Acrosin of mouse spermatozoa. Am J Physiol. 1979 Jul;237(1):E40–E44. doi: 10.1152/ajpendo.1979.237.1.E40. [DOI] [PubMed] [Google Scholar]
- Borland R. M., Hazra S., Biggers J. D., Lechene C. P. The elemental composition of the environments of the gametes and preimplantation embryo during the initiation of pregnancy. Biol Reprod. 1977 Mar;16(2):147–157. doi: 10.1095/biolreprod16.2.147. [DOI] [PubMed] [Google Scholar]
- Brown C. R., Andani Z., Hartree E. F. Studies on ram acrosin. Fluorimetric titratiion of operational molarity with 4-methylumbelliferyl p-guanidinobenzoate. Biochem J. 1975 Jul;149(1):147–154. doi: 10.1042/bj1490147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. G. Application of in vitro fertilization. Fed Proc. 1973 Oct;32(10):2069–2074. [PubMed] [Google Scholar]
- Green J. D., Summers R. G. Ultrastructural demonstration of trypsin-like protease in acrosomes of sea urchin sperm. Science. 1980 Jul 18;209(4454):398–400. doi: 10.1126/science.6992277. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. The Eleventh CIBA Medal Lecture: Spermatazoa, eggs and proteinases. Biochem Soc Trans. 1977;5(2):375–394. doi: 10.1042/bst0050375. [DOI] [PubMed] [Google Scholar]
- Hastings R. A., 2nd, Enders A. C., Schlafke S. Permeability of the zona pellucida to protein tracers. Biol Reprod. 1972 Oct;7(2):288–296. doi: 10.1093/biolreprod/7.2.288. [DOI] [PubMed] [Google Scholar]
- Inoue M., Wolf D. P. Sperm binding characteristics of the murine zona pellucida. Biol Reprod. 1975 Oct;13(3):340–346. doi: 10.1095/biolreprod13.3.340. [DOI] [PubMed] [Google Scholar]
- McRorie R. A., Williams W. L. Biochemistry of mammalian fertilization. Annu Rev Biochem. 1974;43(0):777–803. doi: 10.1146/annurev.bi.43.070174.004021. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Chang M. C. Effects of protease inhibitors on the fertilizing capacity of hamster spermatozoa. Biol Reprod. 1973 Dec;9(5):533–537. doi: 10.1093/biolreprod/9.5.533. [DOI] [PubMed] [Google Scholar]
- Phillips D. M., Shalgi R. M. Surface properties of the zona pellucida. J Exp Zool. 1980 Jul;213(1):1–8. doi: 10.1002/jez.1402130102. [DOI] [PubMed] [Google Scholar]
- Polakoski K. L., McRorie R. A. Boar acrosin. II. Classification, inhibition, and specificity studies of a proteinase from sperm acrosomes. J Biol Chem. 1973 Dec 10;248(23):8183–8188. [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- SRIVASTAVA P. N., ADAMS C. E., HARTREE E. F. ENZYMIC ACTION OF ACROSOMAL PREPARATIONS ON THE RABBIT OVUM IN VITRO. J Reprod Fertil. 1965 Aug;10:61–67. doi: 10.1530/jrf.0.0100061. [DOI] [PubMed] [Google Scholar]
- Saling P. M., Sowinski J., Storey B. T. An ultrastructural study of epididymal mouse spermatozoa binding to zonae pellucidae in vitro: sequential relationship to the acrosome reaction. J Exp Zool. 1979 Aug;209(2):229–238. doi: 10.1002/jez.1402090205. [DOI] [PubMed] [Google Scholar]
- Saling P. M., Storey B. T. Mouse gamete interactions during fertilization in vitro. Chlortetracycline as a fluorescent probe for the mouse sperm acrosome reaction. J Cell Biol. 1979 Dec;83(3):544–555. doi: 10.1083/jcb.83.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling P. M., Storey B. T., Wolf D. P. Calcium-dependent binding of mouse epididymal spermatozoa to the zona pellucida. Dev Biol. 1978 Aug;65(2):515–525. doi: 10.1016/0012-1606(78)90046-5. [DOI] [PubMed] [Google Scholar]
- Sellens M. H., Jenkinson E. J. Permeability of the mouse zona pellucida to immunoglobulin. J Reprod Fertil. 1975 Jan;42(1):153–157. doi: 10.1530/jrf.0.0420153. [DOI] [PubMed] [Google Scholar]
- Srivastava P. N., Akruk S. R., Williams W. L. Dissolution of rabbit zona by sperm acrosomal extract: effect of calcium (1). J Exp Zool. 1979 Mar;207(3):521–529. doi: 10.1002/jez.1402070321. [DOI] [PubMed] [Google Scholar]
- Stambaugh R., Brackett B. G., Mastroianni L. Inhibition of in vitro fertilization of rabbit ova by trypsin inhibitors. Biol Reprod. 1969 Sep;1(3):223–227. doi: 10.1095/biolreprod1.3.223. [DOI] [PubMed] [Google Scholar]
- Stambaugh R., Buckley J. Identification and subcellular localization of the enzymes effecting penetration of the zona pellucida by rabbit spermatozoa. J Reprod Fertil. 1969 Aug;19(3):423–432. doi: 10.1530/jrf.0.0190423. [DOI] [PubMed] [Google Scholar]
- Toyoda Y., Chang M. C. Fertilization of rat eggs in vitro by epididymal spermatozoa and the development of eggs following transfer. J Reprod Fertil. 1974 Jan;36(1):9–22. doi: 10.1530/jrf.0.0360009. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Moy G. W. Isolation of bindin: the protein responsible for adhesion of sperm to sea urchin eggs. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2456–2460. doi: 10.1073/pnas.74.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. P., Inoue M. Sperm concentration dependency in the fertilization and zonae sperm binding properties of mouse eggs inseminated in vitro. J Exp Zool. 1976 Apr;196(1):27–37. doi: 10.1002/jez.1401960104. [DOI] [PubMed] [Google Scholar]
- Zaneveld L. J., Robertson R. T., Kessler M., Williams W. L. Inhibition of fertilization in vivo by pancreatic and seminal plasma trypsin inhibitors. J Reprod Fertil. 1971 Jun;25(3):387–392. doi: 10.1530/jrf.0.0250387. [DOI] [PubMed] [Google Scholar]


