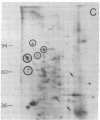
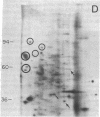
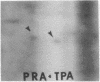
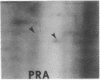
Abstract
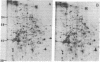
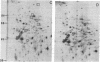
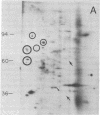
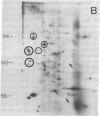
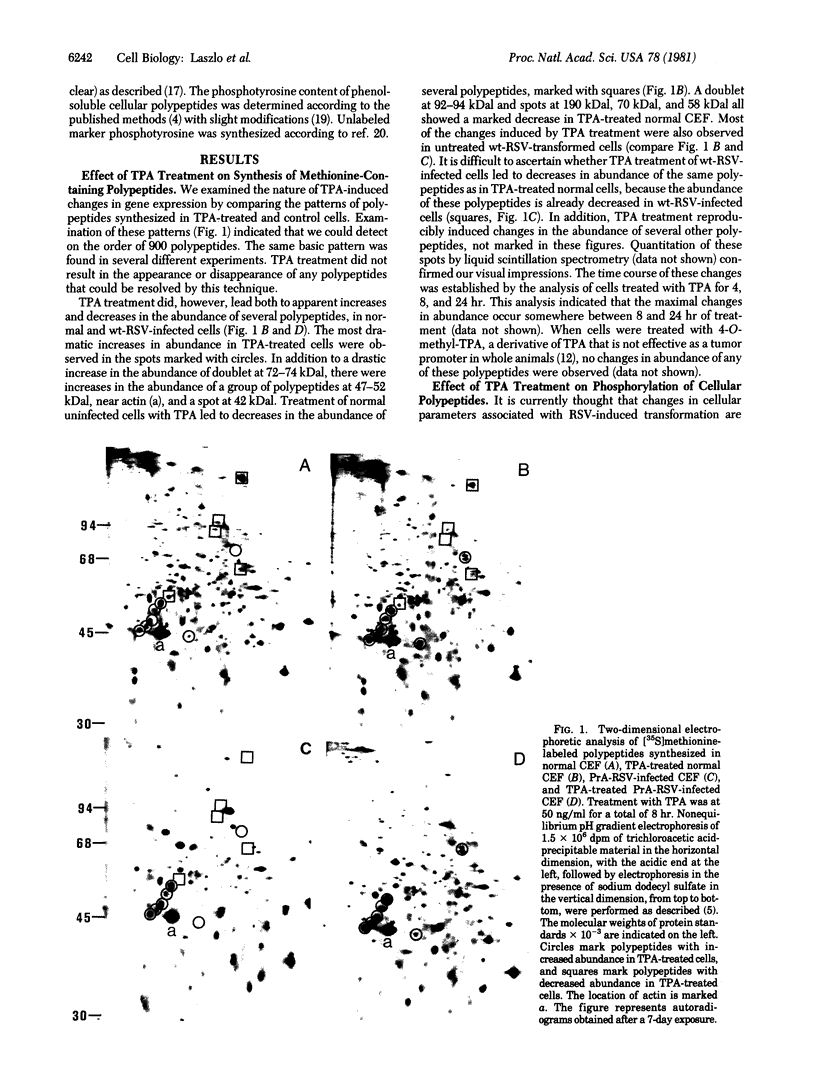
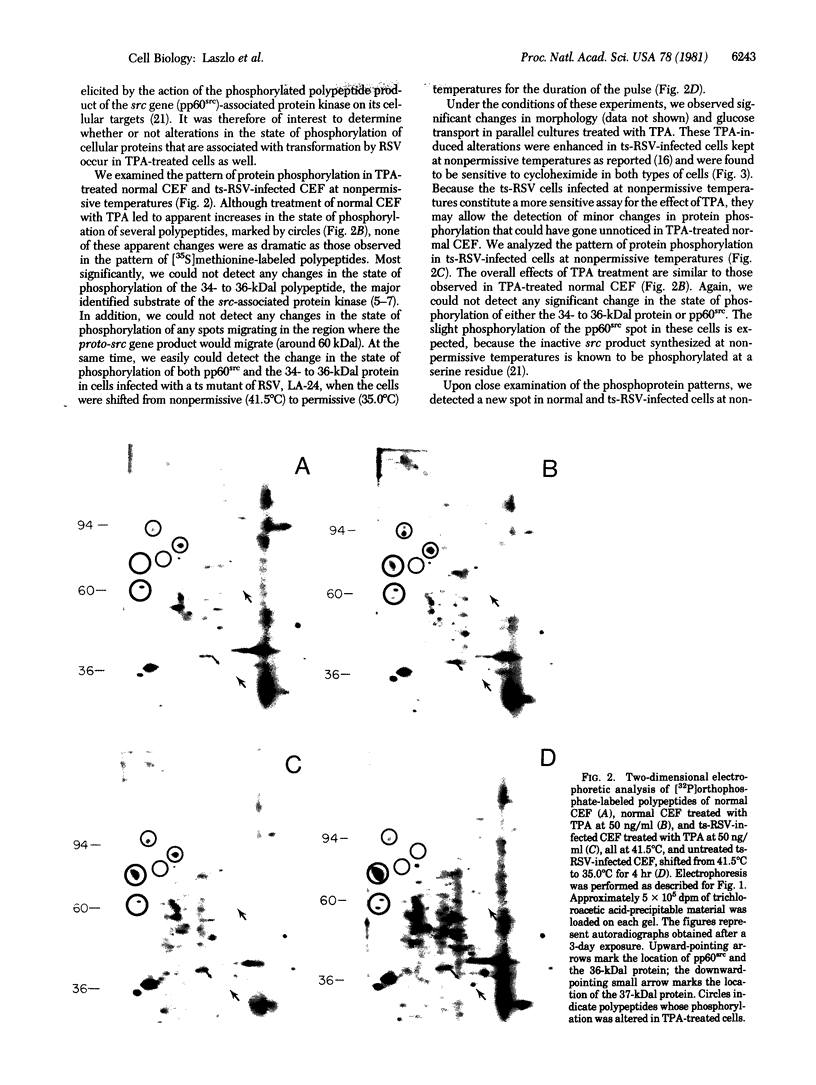
We have investigated the effect of 12-O-tetradecanoylphorbol 13-acetate (TPA) on the synthesis and modification of polypeptides in normal avian cells and cells infected by wild-type and temperature-sensitive Rous sarcoma virus (RSV). Using two-dimensional gel electrophoresis, we have detected alterations in both the abundance of cellular polypeptides and in their phosphorylation that seem unique to TPA treatment. However, the state of phosphorylation of the major putative substrate for the action of the src gene-associated protein kinase, the 34- to 36-kilodalton protein, was not altered. Moreover, examination of the phosphorylated amino acid content of total cellular phosphoproteins revealed that the response to TPA was not associated with detectable increases in their phosphotyrosine content. These results make it unlikely that TPA acts by the activation of the phosphorylating activity of the cellular proto-src gene or by the activation of other cellular phosphotyrosine-specific kinases. We have shown previously that temperature-sensitive RSV-infected cells at nonpermissive temperature demonstrate an increased sensitivity to TPA treatment [Bissell, M. J., Hatie, C. & Calvin, M. (1979) Proc. Natl. Acad. Sci. USA 76, 348-352]. Our present results indicate that this is not due to reactivation of the phosphorylating activity of the defective src gene product or to its leakiness, and they lend support to the notion of multistep viral carcinogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Farson D., Tung A. S. Cell shape and hexose transport in normal and virus-transformed cells in culture. J Supramol Struct. 1977;6(1):1–12. doi: 10.1002/jss.400060102. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hatié C., Calvin M. Is the product of the src gene a promoter? Proc Natl Acad Sci U S A. 1979 Jan;76(1):348–352. doi: 10.1073/pnas.76.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Purchio A. F., Brugge J. S., Erikson R. L. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. doi: 10.1073/pnas.76.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Purchio A. F., Erikson E., Collett M. S., Brugge J. S. Molecular events in cells transformed by Rous Sarcoma virus. J Cell Biol. 1980 Nov;87(2 Pt 1):319–325. doi: 10.1083/jcb.87.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R., Delclos K. B., Blumberg P. M. Phorbol ester action is independent of viral and cellular src kinase levels. Science. 1980 Apr 11;208(4440):191–193. doi: 10.1126/science.6244621. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. F., Krzyzek R. A., Brugge J. S., Erikson R. L., Schollmeyer J., Faras A. J. Morphological revertants of an avian sarcoma virus-transformed mammalian cell line exhibit tumorigenicity and contain pp60src. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3904–3908. doi: 10.1073/pnas.76.8.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- MITCHELL H. K., LUNAN K. D. TYROSINE-O-PHOSPHATE IN DROSOPHILA. Arch Biochem Biophys. 1964 Jul 20;106:219–222. doi: 10.1016/0003-9861(64)90179-1. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri R., Simsiman R. C., Boutwell R. K. Stimulation of the phosphorylation of mouse epidermal histones by tumor-promoting agents. Cancer Res. 1973 Jan;33(1):134–139. [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M., Pollack R. Tumor promoters induce changes in the chick embryo fibroblast cytoskeleton. Cell. 1979 Oct;18(2):361–368. doi: 10.1016/0092-8674(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Temperature-sensitive transformation by Rous sarcoma virus and temperature-sensitive protein kinase activity. J Virol. 1980 Jan;33(1):220–229. doi: 10.1128/jvi.33.1.220-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R. Polyoma virus middle t antigen: a tumor progression factor. J Virol. 1980 Aug;35(2):479–487. doi: 10.1128/jvi.35.2.479-487.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M., O'Neal S., Racker E. Phosphorylation of the beta subunit of Na+K+-ATPase in Ehrlich ascites tumor by a membrane-bound protein kinase. J Biol Chem. 1980 Sep 25;255(18):8370–8373. [PubMed] [Google Scholar]
- Wyke J. A. Complementation of transforming functions by temperature-sensitive mutants of avian sarcoma virus. Virology. 1973 Jul;54(1):28–36. doi: 10.1016/0042-6822(73)90111-6. [DOI] [PubMed] [Google Scholar]