Abstract
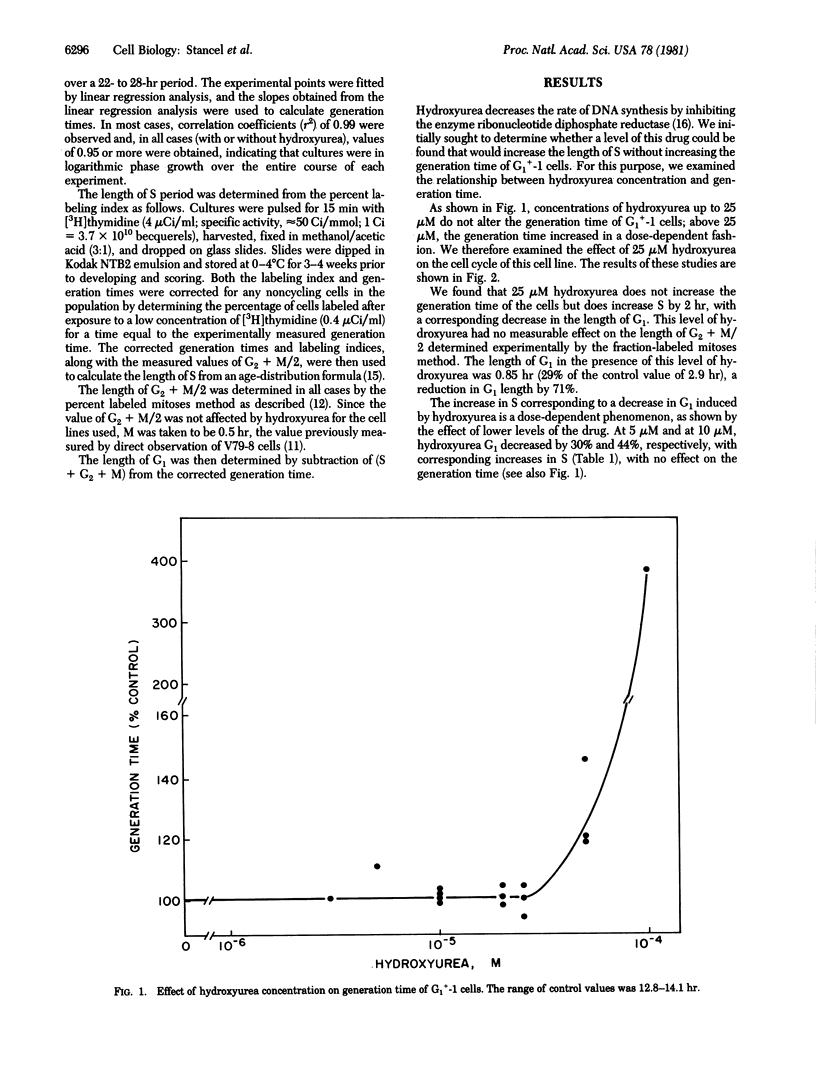
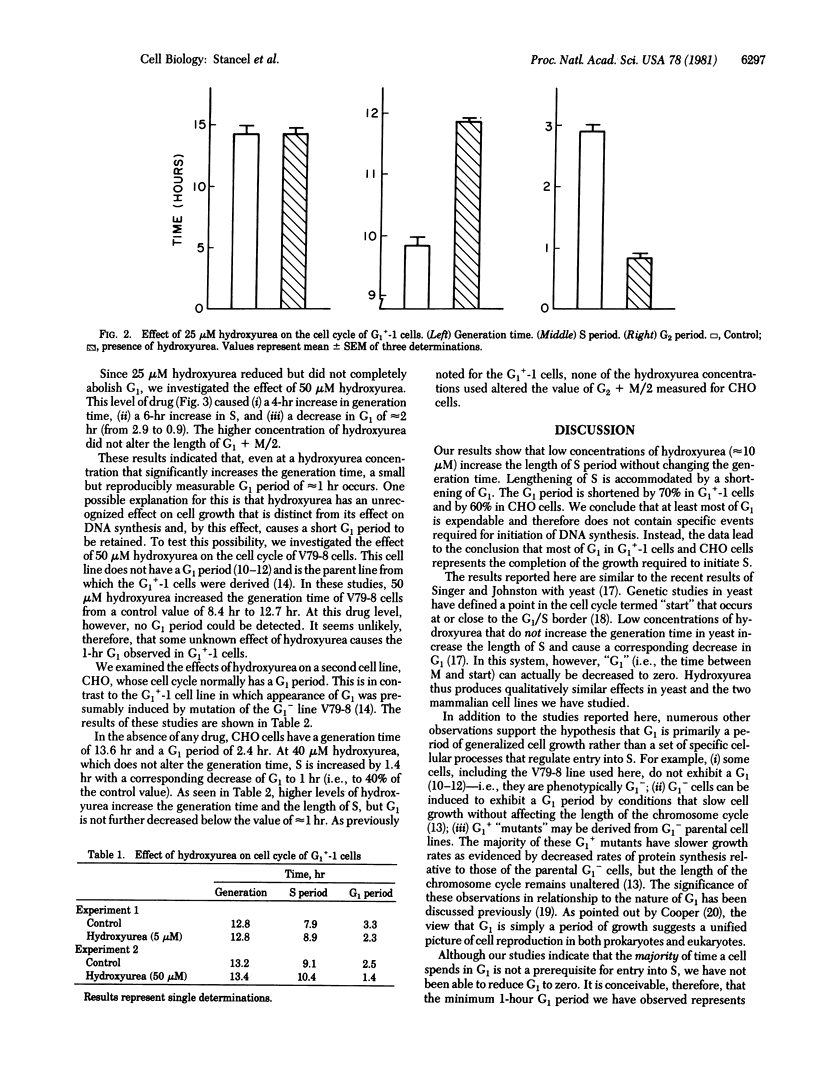
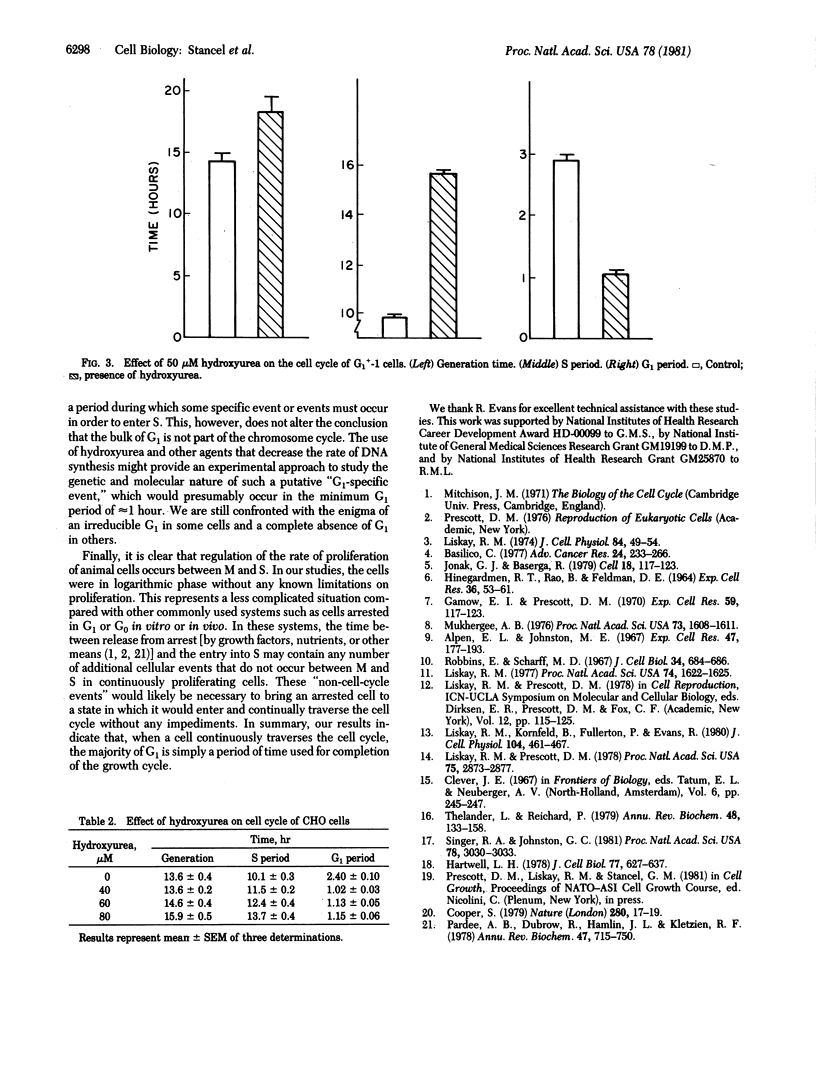
Two Chinese hamster cell lines, G1+-1 and CHO, have been grown in the presence of low concentrations of hydroxyurea to determine how a slowing DNA synthesis (i.e., a lengthening of the S period) affects the length of the G1 period. Hydroxyurea concentrations of approximately 10 microM do not alter the generation times of these cell lines but do cause increases in S with corresponding decreases in G1. In both cell lines, 10 microM hydroxyurea reduces G1 to an absolute value of 1 hr, which represents decreases of 70% (G1+-1) and 60% (CHO) from control values. Higher concentrations of hydroxyurea increase the generation times and lengths of S for both cell lines but do not reduce G1 below the minimum value of 1 hr. These observations indicate that the majority of G1 is expendable and most of G1 therefore cannot contain specific events required for the initiation of DNA synthesis. This result supports the hypothesis that G1 is a portion of the cell growth cycle but not of the chromosome cycle.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpen E. L., Johnston M. E. DNA synthetic rate and DNA content of nucleated erythroid cells. Exp Cell Res. 1967 Aug;47(1):177–192. doi: 10.1016/0014-4827(67)90221-2. [DOI] [PubMed] [Google Scholar]
- Basilico C. Temperature-sensitive mutations in animal cells. Adv Cancer Res. 1977;24:223–266. doi: 10.1016/s0065-230x(08)61016-7. [DOI] [PubMed] [Google Scholar]
- Cooper S. A unifying model for the G1 period in prokaryotes and eukaryotes. Nature. 1979 Jul 5;280(5717):17–19. doi: 10.1038/280017a0. [DOI] [PubMed] [Google Scholar]
- Gamow E. I., Prescott D. M. The cell life cycle during early embryogenesis of the mouse. Exp Cell Res. 1970 Jan;59(1):117–123. doi: 10.1016/0014-4827(70)90630-0. [DOI] [PubMed] [Google Scholar]
- HINEGARDNER R. T., RAO B., FELDMAN D. E. THE DNA SYNTHETIC PERIOD DURING EARLY DEVELOPMENT OF THE SEA URCHIN EGG. Exp Cell Res. 1964 Oct;36:53–61. doi: 10.1016/0014-4827(64)90159-4. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Cell division from a genetic perspective. J Cell Biol. 1978 Jun;77(3):627–637. doi: 10.1083/jcb.77.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak G. J., Baserga R. Cytoplasmic regulation of two G1-specific temperature-sensitive functions. Cell. 1979 Sep;18(1):117–123. doi: 10.1016/0092-8674(79)90360-x. [DOI] [PubMed] [Google Scholar]
- Liskay R. M. A mammalian somatic "cell cycle" mutant defective in G1. J Cell Physiol. 1974 Aug;84(1):49–55. doi: 10.1002/jcp.1040840106. [DOI] [PubMed] [Google Scholar]
- Liskay R. M. Absence of a measurable G2 phase in two Chinese hamster cell lines. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1622–1625. doi: 10.1073/pnas.74.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Kornfeld B., Fullerton P., Evans R. Protein synthesis and the presence of absence of a measurable G1 in cultured Chinese hamster cells. J Cell Physiol. 1980 Sep;104(3):461–467. doi: 10.1002/jcp.1041040318. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Prescott D. M. Genetic analysis of the G1 period: isolation of mutants (or variants) with a G1 perior from a Chinese hamster cell line lacking G1. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2873–2877. doi: 10.1073/pnas.75.6.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A. B. Cell cycle analysis and X-chromosome inactivation in the developing mouse. Proc Natl Acad Sci U S A. 1976 May;73(5):1608–1611. doi: 10.1073/pnas.73.5.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Robbins E., Scharff M. D. The absence of a detectable G1 phase in a cultured strain of Chinese hamster lung cell. J Cell Biol. 1967 Aug;34(2):684–686. doi: 10.1083/jcb.34.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. A., Johnston G. C. Nature of the G1 phase of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 May;78(5):3030–3033. doi: 10.1073/pnas.78.5.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]


