Abstract
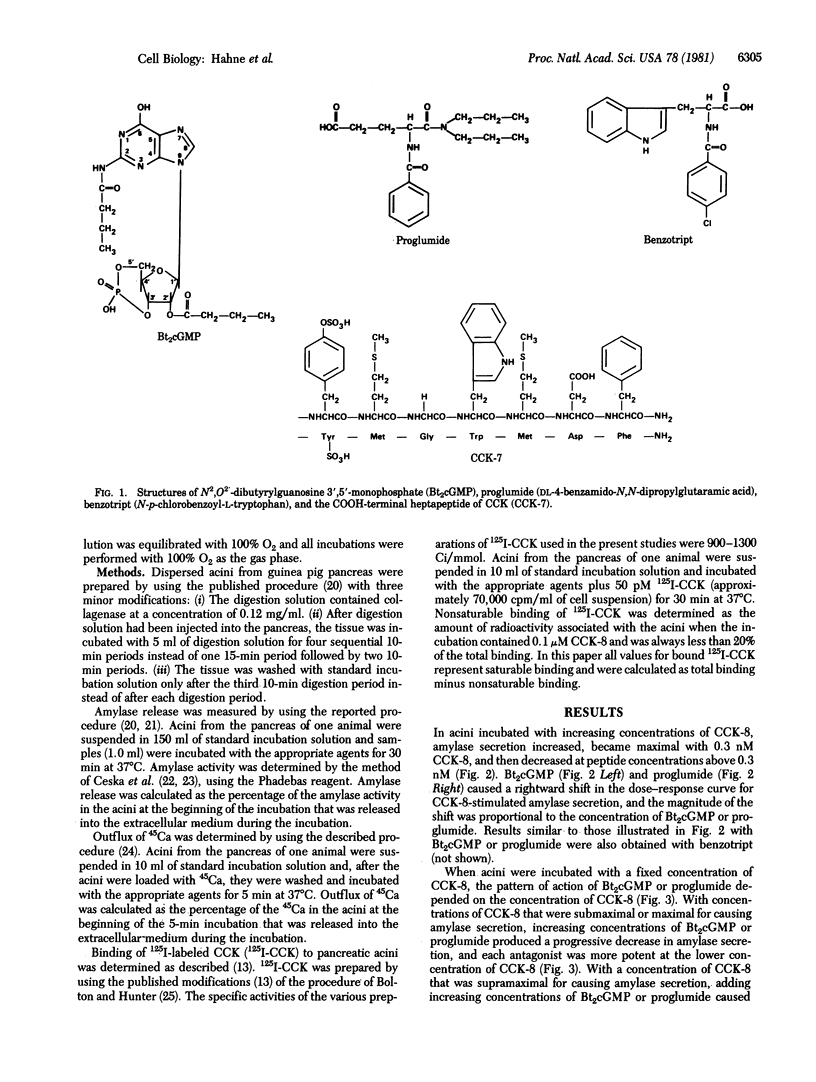
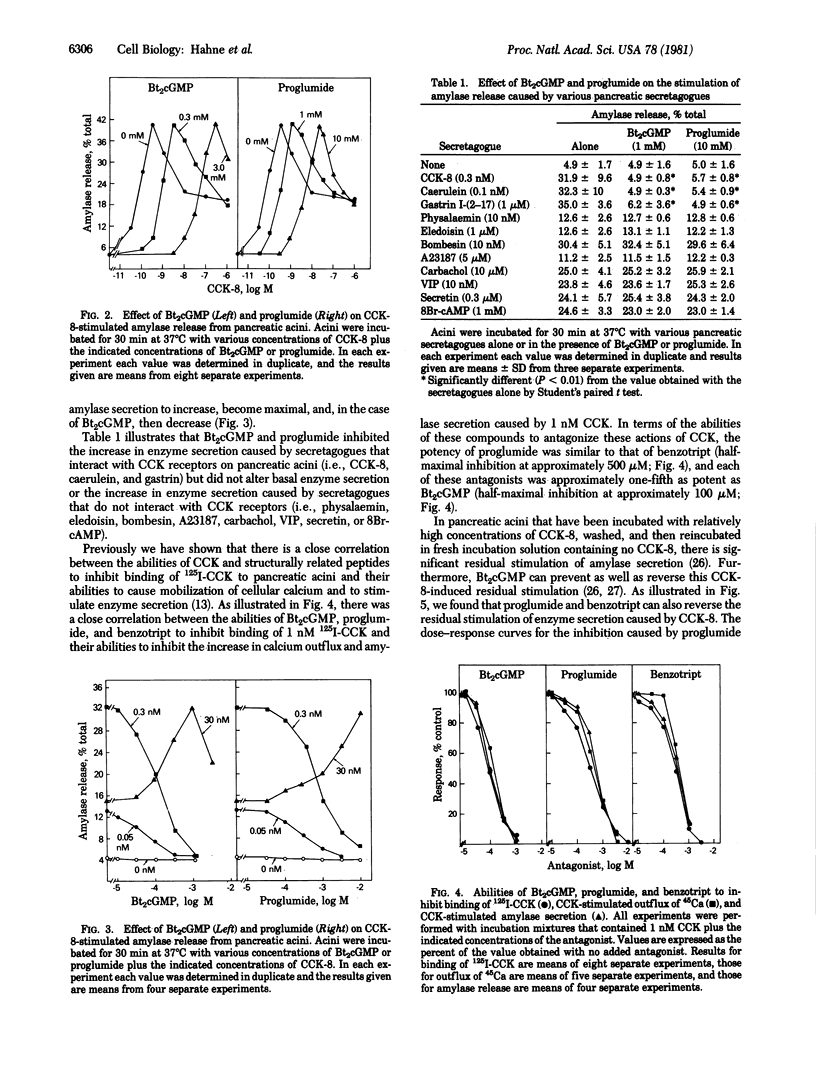
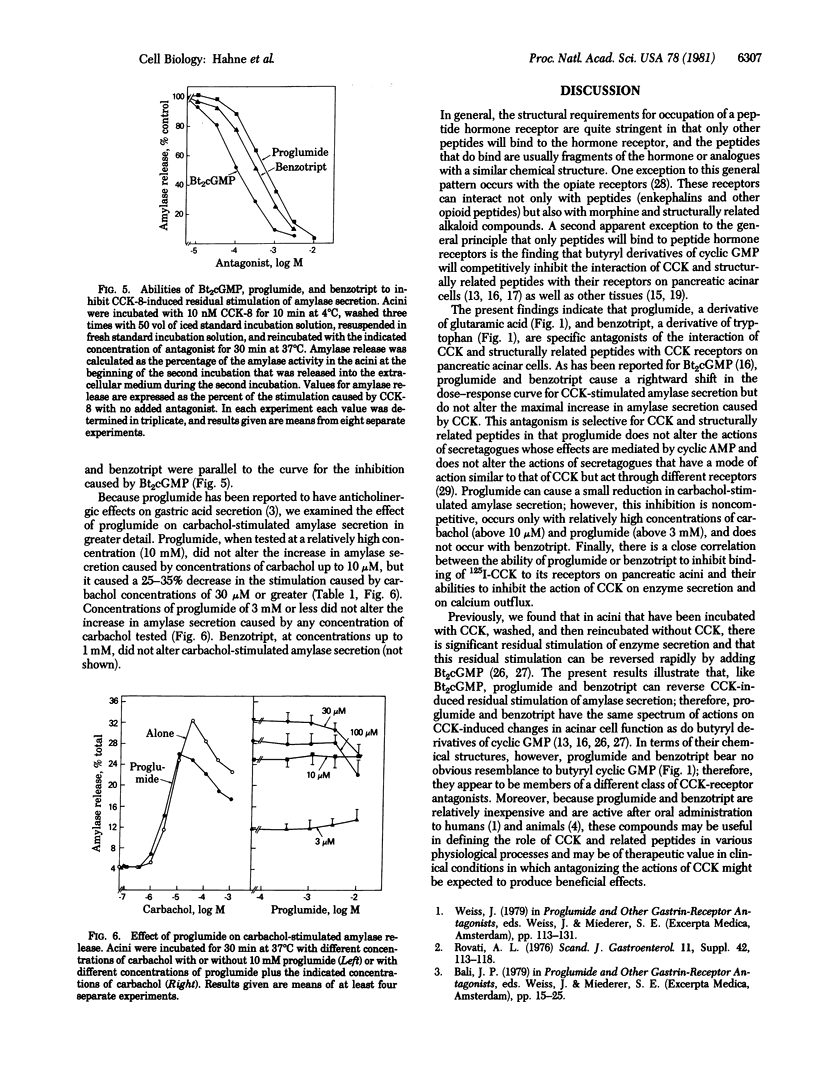
In dispersed acini from guinea pig pancreas, proglumide (DL-4-benzamido-N, N-dipropylglutaramic acid) and benzotript (N-p-chlorobenzoyl-L-tryptophan) caused a rightward shift in the dose--response curve for cholecystokinin-stimulated amylase secretion but did not alter the maximal increase in amylase secretion caused by cholecystokinin. At relatively low concentrations, proglumide did not alter the stimulation of enzyme secretion caused by secretagogues whose effects are mediated by adenosine 3'5'-monophosphate (e.g., vasoactive intestinal peptide or secretin) and did not alter the stimulation of enzyme secretion caused by secretagogues that have a mode of action similar to that of cholecystokinin but act through different receptors (e.g., bombesin, physalaemin, eledoisin, and ionophore A23187). There was a close correlation between the ability of proglumide or benzotript to inhibit binding of 125I-labeled cholecystokinin to its receptors on pancreatic acini and the abilities of these compounds to inhibit the action of cholecystokinin on enzyme secretion and on calcium outflux. These results indicate that proglumide and benzotript are members of a different class of cholecystokinin receptor antagonists.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelmoumene S., Gardner J. D. Cholecystokinin-induced desensitization of enzyme secretion in dispersed acini from guinea pig pancreas. Am J Physiol. 1980 Oct;239(4):G272–G279. doi: 10.1152/ajpgi.1980.239.4.G272. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceska M., Birath K., Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Optimal conditions. Clin Chim Acta. 1969 Dec;26(3):437–444. doi: 10.1016/0009-8981(69)90071-0. [DOI] [PubMed] [Google Scholar]
- Ceska M., Brown B., Birath K. Ranges of alpha-amylase activities in human serum and urine and correlations with some other alpha-amylase methods. Clin Chim Acta. 1969 Dec;26(3):445–453. doi: 10.1016/0009-8981(69)90072-2. [DOI] [PubMed] [Google Scholar]
- Collins S. M., Abdelmoumene S., Jensen R. T., Gardner J. D. Reversal of cholecystokinin-induced persistent stimulation of pancreatic enzyme secretion by dibutyryl cyclic GMP. Am J Physiol. 1981 Jun;240(6):G466–G471. doi: 10.1152/ajpgi.1981.240.6.G466. [DOI] [PubMed] [Google Scholar]
- Danhof I. A. Attività della xylamide, CR 242, nella secrezione gastrica del cane dopo stimoli ipersecretivi. Minerva Med. 1967 Oct 27;58(86):3670–3677. [PubMed] [Google Scholar]
- Desvigne C., Vagne M., Faivre M., Desvigne A. Action du proglumide sur la sécrétion acide et la motricité gastriques stimulées par la pentagastrine chez le chat éveillé. Gastroenterol Clin Biol. 1977;1(5):447–454. [PubMed] [Google Scholar]
- Gardner J. D., Costenbader C. L., Uhlemann E. R. Effect of extracellular calcium on amylase release from dispersed pancreatic acini. Am J Physiol. 1979 Jun;236(6):E754–E762. doi: 10.1152/ajpendo.1979.236.6.E754. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Jackson M. J. Regulation of amylase release from dispersed pancreatic acinar cells. J Physiol. 1977 Sep;270(2):439–454. doi: 10.1113/jphysiol.1977.sp011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Jensen R. T. Receptor for secretagogues on pancreatic acinar cells. Am J Physiol. 1980 Feb;238(2):G63–G66. doi: 10.1152/ajpgi.1980.238.2.G63. [DOI] [PubMed] [Google Scholar]
- Hutchison J. B., Dockray G. J. Inhibition of the action of cholecystokinin octapeptide on the guinea pig ileum myenteric plexus by dibutyryl cyclic guanosine monophosphate. Brain Res. 1980 Dec 8;202(2):501–505. doi: 10.1016/0006-8993(80)90164-x. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani P., Piccinin G. L., Adorni M. C. Effetto della xylamide su alcune azioni della caeruleina. Arch Int Pharmacodyn Ther. 1971 Feb;189(2):319–327. [PubMed] [Google Scholar]
- Peikin S. R., Costenbader C. L., Gardner J. D. Actions of derivatives of cyclic nucleotides on dispersed acini from guinea pig pancreas. Discovery of a competitive antagonist of the action of cholecystokinin. J Biol Chem. 1979 Jun 25;254(12):5321–5327. [PubMed] [Google Scholar]
- Peikin S. R., Rottman A. J., Batzri S., Gardner J. D. Kinetics of amylase release by dispersed acini prepared from guinea pig pancreas. Am J Physiol. 1978 Dec;235(6):E743–E749. doi: 10.1152/ajpendo.1978.235.6.E743. [DOI] [PubMed] [Google Scholar]
- Philpott H. G., Petersen O. H. Separate activation sites for cholecystokinin and bombesin on pancreatic acini: an electrophysiological study employing a competitive antagonist for the action of CCK. Pflugers Arch. 1979 Nov;382(3):263–267. doi: 10.1007/BF00583711. [DOI] [PubMed] [Google Scholar]
- Poitras P., Iacino D., Walsh J. H. Dibutyryl cGMP: inhibitor of the effect of cholecystokinin and gastrin on the guinea pig gallbladder in vitro. Biochem Biophys Res Commun. 1980 Sep 16;96(1):476–482. doi: 10.1016/0006-291x(80)91239-5. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Deschodt-Lanckman M., Woussen-Colle M. C., De Neef P., Camus J. C., Christophe J. Butyryl derivatives of cyclic GMP interfere with the biological and the immunological properties of the pancreozymin-gastrin family of peptides. Mol Pharmacol. 1980 Mar;17(2):268–274. [PubMed] [Google Scholar]
- Rovati A. L., Casula P. L., Da Re G. Farmacologia e tossicologia sperimentale di un nuovo prodotto non anticolinergico ad attività antisecretiva e gastroprotettiva (CR 242-xylamide-Milid) Minerva Med. 1967 Oct 27;58(86):3656–3670. [PubMed] [Google Scholar]
- Rovati A. L. Inhibition of gastric secretion by anti-gastrinic and H2-blocking agents. Scand J Gastroenterol Suppl. 1976;42:113–118. [PubMed] [Google Scholar]
- Sampietro R. Effetti inibitori della Xylamide sulla secrezione gastrica acida massimale indotta nell'uomo. Minerva Med. 1969 Mar 21;60(23):1032–1037. [PubMed] [Google Scholar]
- Snyder S. H. Opiate receptors in the brain. N Engl J Med. 1977 Feb 3;296(5):266–271. doi: 10.1056/NEJM197702032960511. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Speir G. R., Johnson L. R. Mucosal gastrin receptor. I. Assay standardization and fulfillment of receptor criteria. Am J Physiol. 1979 Sep;237(3):E284–E294. doi: 10.1152/ajpendo.1979.237.3.E284. [DOI] [PubMed] [Google Scholar]
- Vatier J., Lepeltier M., Kuan T. S. Modèle d'étude d'un antisécrétoire gastrique: application au proglumide. Gastroenterol Clin Biol. 1977 Aug-Sep;1(8-9):643–651. [PubMed] [Google Scholar]


