Abstract
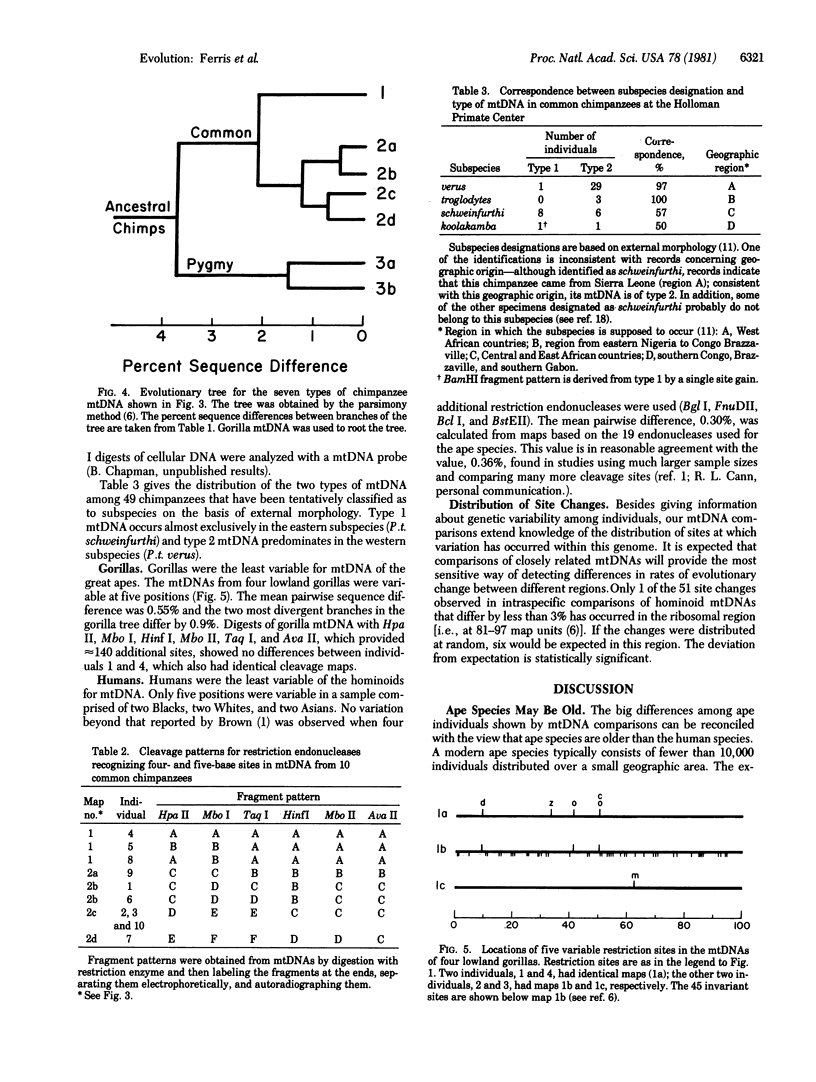
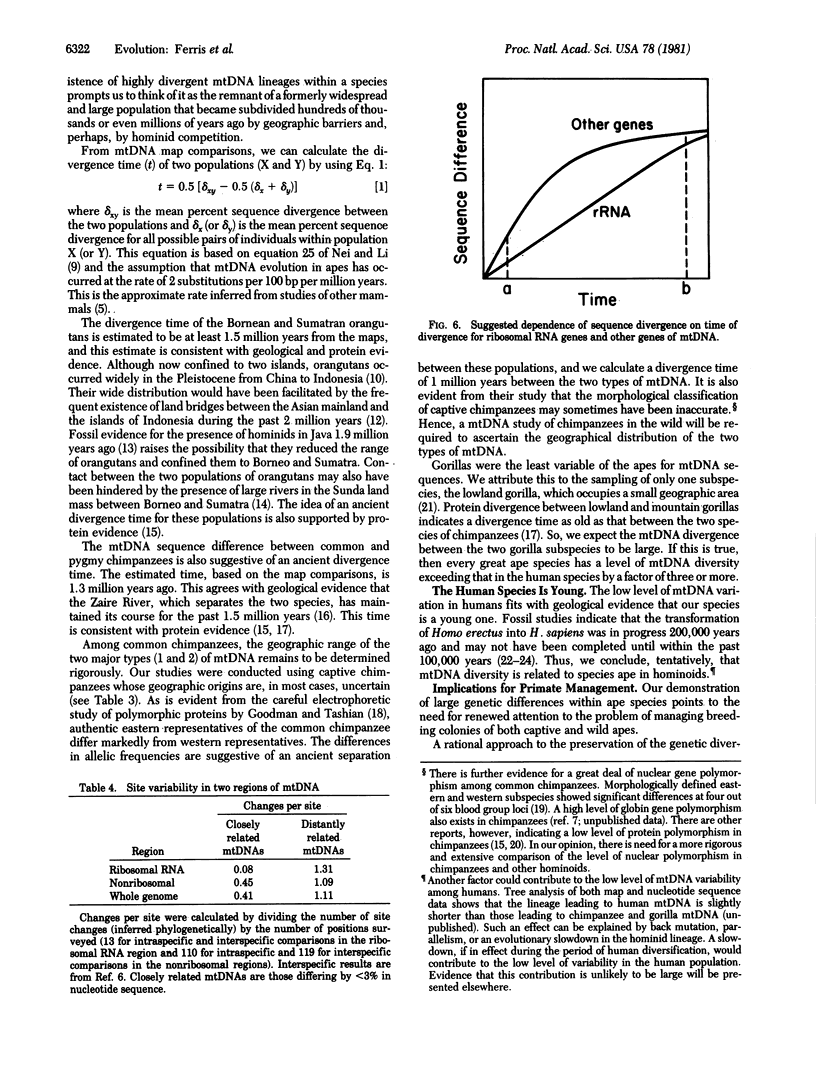
Ape species are 2-10 times more variable than the human species with respect to the nucleotide sequence of mtDNA, even though ape populations have been smaller than the human population for at least 10,000 years. This finding was made by comparing purified mtDNAs from 27 individuals with the aid of 25 restriction endonucleases; for an additional 59 individuals, comparisons were made with fewer enzymes by using the blot hybridization method. The amount of intraspecific sequence divergence was greatest between orangutans of Borneo and Sumatra. Among common chimpanzees, a large component of the variation is due to two highly distinct forms of mtDNA that may reflect a major geographic subdivision. The least amount of sequence variation occurred among lowland gorillas, which exhibit only twice as much sequence variation as humans. The large intraspecific differences among apes, together with the geological and protein evidence, leads us to propose that each ape species is the remnant of an ancient and widespread population that became subdivided geographically and reduced in size and range, perhaps by hominid competition. The low variation among human mtDNAs is consistent with geological evidence that the human species is young. The distribution of site changes within the mitochondrial genome was also examined. Comparison of closely related mtDNAs shows that the ribosomal RNA genes have diverged more slowly than the rest of the genome.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avise J. C., Giblin-Davidson C., Laerm J., Patton J. C., Lansman R. A. Mitochondrial DNA clones and matriarchal phylogeny within and among geographic populations of the pocket gopher, Geomys pinetis. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6694–6698. doi: 10.1073/pnas.76.12.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise J. C., Lansman R. A., Shade R. O. The use of restriction endonucleases to measure mitochondrial DNA sequence relatedness in natural populations. I. Population structure and evolution in the genus Peromyscus. Genetics. 1979 May;92(1):279–295. doi: 10.1093/genetics/92.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. Small is beautiful--portrait of a mitochondrial genome. Nature. 1981 Apr 9;290(5806):443–444. doi: 10.1038/290443a0. [DOI] [PubMed] [Google Scholar]
- Brown G. G., Simpson M. V. Intra- and interspecific variation of the mitochondrial genome in Rattus norvegicus and Rattus rattus: restriction enzyme analysis of variant mitochondrial DNA molecules and their evolutionary relationships. Genetics. 1981 Jan;97(1):125–143. doi: 10.1093/genetics/97.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J. E., Boaz N. T., Stringer C. B., Rak Y. Tempo and mode in hominid evolution. Nature. 1981 Jul 9;292(5819):113–122. doi: 10.1038/292113a0. [DOI] [PubMed] [Google Scholar]
- Ferris S. D., Wilson A. C., Brown W. M. Evolutionary tree for apes and humans based on cleavage maps of mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2432–2436. doi: 10.1073/pnas.78.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M., Tashian R. E. A geographic variation in the serum transferrin and red cell phosphoglucomutase polymorphisms of chimpanzees. Hum Biol. 1969 May;41(2):237–249. [PubMed] [Google Scholar]
- Kennedy G. E. The emergence of modern man. Nature. 1980 Mar 6;284(5751):11–12. doi: 10.1038/284011a0. [DOI] [PubMed] [Google Scholar]
- King M. C., Wilson A. C. Evolution at two levels in humans and chimpanzees. Science. 1975 Apr 11;188(4184):107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich V. M. Rates, sample sizes, and the neutrality hypothesis for electrophoresis in evolutionary studies. Nature. 1977 Jan 6;265(5589):24–28. doi: 10.1038/265024a0. [DOI] [PubMed] [Google Scholar]
- Zimmer E. A., Martin S. L., Beverley S. M., Kan Y. W., Wilson A. C. Rapid duplication and loss of genes coding for the alpha chains of hemoglobin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2158–2162. doi: 10.1073/pnas.77.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]



