Abstract
Study Objectives:
Females with obstructive sleep apnea (OSA) show different psychological and physiological symptoms from males, which may be associated with sex-related variations in neural injury occurring with the disorder. To determine whether male- or female-specific brain injury is present in OSA, we assessed influences of sex on white matter changes in the condition.
Design:
Two-group factorial.
Setting:
University medical center.
Patients or Participants:
80 subjects total, with newly diagnosed, untreated OSA groups of 10 female (age mean ± SE: 52.6 ± 2.4 years, AHI 22.5 ± 4.1 events/h) and 20 male (age 48.9 ± 1.7, AHI 25.5 ± 2.9) patients, and 20 female (age 50.3 ± 1.7) and 30 male (age 49.2 ± 1.4) healthy control subjects.
Interventions:
None.
Measurements and Results:
Brain fiber integrity was assessed with fractional anisotropy (FA), a diffusion tensor imaging-derived measure. Sleep quality, daytime sleepiness, depression, and anxiety were assessed with questionnaires. We identified regions of differing injury in male versus female OSA patients by assessing brain regions with significant interaction effects of OSA and sex on FA. Areas of sex-specific, OSA-related FA reductions appeared in females relative to males, including in the bilateral cingulum bundle adjacent to the mid hippocampus, right stria terminalis near the amygdala, prefrontal and posterior-parietal white matter, corpus callosum, and left superior cerebellar peduncle. Females with OSA showed higher daytime sleepiness, anxiety and depression levels, and reduced sleep quality.
Conclusions:
Sex differences in white matter structural integrity appeared in OSA patients, with females more affected than males. These female-specific structural changes may contribute to or derive from neuropsychological and physiological symptom differences between sexes.
Citation:
Macey PM; Kumar R; Yan-Go FL; Woo MA; Harper RM. Sex differences in white matter alterations accompanying obstructive sleep apnea. SLEEP 2012;35(12):1603-1613.
Keywords: Magnetic resonance imaging, diffusion tensor imaging, hypoxia, autonomic nervous system, cingulum, superior cerebellar peduncles, stria terminalis
INTRODUCTION
Obstructive sleep apnea (OSA), characterized by repeated obstructions of the upper airway with intermittent hypoxic exposure, is associated with multiple detrimental physiological and psychological consequences, and occurs in at least 5% of the population.1 The disorder is less common in females than males, with women showing an OSA prevalence less than half of that in men.2 Furthermore, OSA characteristics differ between sexes; women with OSA typically report different sleep-related complaints from males, and have a higher incidence of chronic pulmonary disease, vascular issues, including heart failure and possibly related tissue fluid retention, and hypothyroidism, while male OSA patients show higher levels of cardiovascular disease and arrhythmia than females.3–5 Other physiological differences appear in overnight blood pressure changes and upper airway muscle tone.6–9 Disease severity, as measured by apneic events per hour (apnea/hypopnea index [AHI]), tends to be lower in females, but women are symptomatic at lower AHI levels.5 The incidence of certain neuropsychological characteristics in OSA, including depression and anxiety symptoms, is higher in women.3,4,10–13 The mechanisms underlying these sex differences in symptoms are unclear, but many physiological and psychological consequences may result from neural dysfunction arising from the injury in the condition.
Alterations in neural structure, reflected as gray matter loss, changes in metabolite levels, and alterations in water diffusion, appear in OSA.14–27 These alterations are accompanied by functional consequences,28–32 which likely contribute to the deleterious characteristics of the sleep disorder, including psychological and physiological comorbidities. These differences could result, in part, from sex-related variation in neural alterations from hypoxia, since animal models of intermittent hypoxia show relative neuroprotection in females.33,34 Estrogen may play a protective role, as shown in animal models,35,36 and as reflected in the post-menopause rapid increase in incidence of OSA in women, which approaches that in men.37 However, the postmenopausal elevation of prevalence of OSA might develop from an array of processes other than direct neural influences, such as upper airway morphological changes accompanying cervical vertebrae alterations,38 although those anatomical changes may also be hormonally based. Direct evidence of sex differences in central nervous system injury related to OSA is lacking.
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) procedure sensitive to changes in water diffusion associated with alterations in structure. One DTI-derived measure is fractional anisotropy (FA), which is an index of axonal structural integrity.39,40 The FA measure reflects the directionality of water movement in tissue; in regions of dense, closely aligned, well-myelinated fiber tracts, water movement is relatively constrained along the direction of those tracts, but movement perpendicular to the fibers is impeded. The FA index reflects that directionality, being the ratio of diffusivity along the direction of easiest movement (termed axial direction) to the diffusivity across the plane perpendicular to the axial direction. Lower FA values reflect lower impedance in the plane perpendicular to the axial direction, which can result from less axonal white matter myelin, thus reflecting reduced axonal integrity. Obstructive sleep apnea patients show extensive structural changes as indicated by lower FA,15 and the procedure has been used elsewhere to demonstrate other sex-related white matter changes.41–44 The purpose of this study was to test the hypothesis that male-female differences in axonal injury are present in OSA, as measured with FA. Psychological symptoms were also measured to assist interpretation of white matter findings, and specifically to determine whether potential brain changes occurred independently of psychological factors. Further studies will be needed to establish interactions of injury with psychological variables, since this study was designed only to determine whether structural brain changes differ by sex.
METHODS
Subjects
We studied 10 female and 20 male OSA patients recruited from the UCLA Sleep Laboratory, and 20 female and 30 male control subjects recruited from the Los Angeles community. These subjects were a subset of those used in a previous study.15 No OSA subjects had started treatment for the sleep disorder, and all were recently diagnosed; they were referred to the study at the time of their diagnostic overnight polysomnographic sleep assessment and were typically scanned within 4 weeks. OSA patients were included if they were diagnosed as moderate or severe, based on AHI being ≥ 15 events/h.45 Calculated parameters included AHI, mean and nadir oxygen saturation, and classification as REM-dominant OSA if inspection illustrated apneas occurring exclusively (or almost so) in REM sleep. After verification of inclusion and exclusion criteria, the control subjects were interviewed for the presence of symptoms of OSA (e.g., daytime sleepiness, snoring, non-restorative sleep, bed partner report of disturbed breathing). Control subjects who reported such symptoms were scheduled for a full sleep study to verify their status. Five subjects in total were screened, 2 of whom were included in the study (AHI 2 and 3), one of whom was ultimately diagnosed with OSA, and the remaining 2 of whom were diagnosed with mild OSA (5 < AHI < 15) and excluded from the study. Male and female OSA groups were matched for severity, and all 4 groups (control male and female, OSA male and female) were age-matched. No subjects had a history of psychiatric disorders, cardiovascular disease, stroke, or other major illness, and no subjects were taking psychotropic or cardiovascular medications. Scanner limitations precluded patients with metallic implants or weight over 125 kg. All procedures were in accordance with the Declaration of Helsinki and approved by the UCLA Institutional Review Board, and subjects provided written informed consent.
Psychological Symptoms
We assessed daytime sleepiness with the Epworth Sleepiness Scale (ESS),46 and sleep quality with the Pittsburgh Sleep Quality Index (PSQI).47 We also assessed depressive symptoms with the Beck Depression Inventory-II (BDI)48,49 and anxiety symptoms with the Beck Anxiety Inventory (BAI).50 The ESS is scored between 0 and 24; a score ≥ 10 is considered above normal. The PSQI is a 19-item questionnaire assessing self-reported sleep quality and sleep times, with a score ≥ 5 indicating clinically meaningful disturbed sleep. The BAI is a 21-question instrument with a maximum score of 63, where scores ≥ 8 indicate higher than normal levels of anxiety. Similarly, the BDI is a 21-item questionnaire, with scores ≥ 10 considered mild or greater levels of depressive symptoms, although higher cutoffs are sometimes used.
MRI Scanning
We collected DTI and other anatomical scans with a 3.0 Tesla MRI scanner (Siemens Magnetom Tim-Trio, 8-channel head coil). Diffusion tensor imaging data consisted of 4 separate series using a single-shot multi-section spin-echo echo-planar pulse sequence (repetition time [TR] = 10,000 ms; echo-time [TE] = 87 ms; flip angle = 90°) in the axial plane, with a 128 × 128 matrix size, 230 × 230 mm field of view (FOV), 2.0 mm slice thickness, 75 slices and no interslice gap, and a readout bandwidth of 1346 Hz/pixel. Diffusion gradients were applied along 12 directions with b = 700 s/mm2, in addition to the b = 0 s/mm2 (b0) images. An acceleration factor of 2 was applied using the parallel imaging generalized autocalibrating partially parallel acquisition technique. To enable correction for field-related distortions, we also collected phase-difference and magnitude images (1st TE = 4.98 ms, 2nd TE = 7.44 ms; TR = 880 ms; flip angle = 90°; matrix size = 64 × 64; FOV = 192 × 192 mm; slice thickness = 3.0 mm; number of slices = 36). High-resolution 3-dimensional T1-weighted anatomical scans were collected using a magnetization-prepared rapid-acquisition-gradient-echo pulse sequence (TR = 2200 ms; TE = 2.2 ms; inversion time = 900 ms; flip angle = 9°; matrix size = 256 × 256; FOV = 230 × 230 mm; slice thickness = 1.0 mm; number of slices = 176).
Analysis: Preprocessing
The 3 later DTI series were realigned to the first series using the non-diffusion weighted (b0) images; the diffusion gradient direction vectors of the images were rotated accordingly. Corrections for eddy-current related distortions were performed using an affine coregistration.51,52 Phase distortions due to magnetic field inhomogeneities were corrected using fieldmaps calculated from the phase-difference and magnitude images, with software from the SPM5 “Fieldmap” toolbox.53 The diffusion tensor was calculated at each voxel using a linear model, and FA derived using the SPM5 “Diffusion” toolbox. The b0 images were spatially normalized to the Montreal Neurological Institute (MNI) template, and the FA maps had the same transformation applied (see Appendix A in supplemental material for details). Finally, the spatially normalized FA maps were smoothed (isotropic Gaussian filter with full-width half-maximum = 10 mm).
Analysis: Statistical Modeling
We implemented a factorial model in SPM554; this earlier version of the SPM software was used to maintain consistency with our previous findings.15 As FA is a quantitative measure, we implemented a model to assess absolute means rather than the SPM5 default of relative means (as used in functional magnetic resonance imaging analyses). We created a linear model with sex and OSA terms and an interaction variable. We also included a constant term. We created a mask for the statistical analysis to include all regions with large axonal groups including white matter, and to exclude non-brain regions and areas with very low FA, which could have high variability. The purpose was to restrict analysis to areas which likely included axonal groups, but with a conservative approach to ensure all brain areas of potential interest were included. The mask was created based on a threshold of 0.15 applied to an FA map of the mean of all subjects' normalized images; the threshold level was relatively low (0.2 or 0.3 is used on some studies of healthy people55,56) to ensure inclusion of all regions with axonal groups, and to allow for inclusion of regions with reduced FA which would occur with injury. We first assessed the effects of interest (sex and OSA status) across the whole brain using an F test at a threshold of 0.05 false discovery rate (FDR), to be consistent with previous findings.15 We performed post hoc testing in brain regions showing significant effects to determine the direction and magnitude of changes.
RESULTS
Subjects
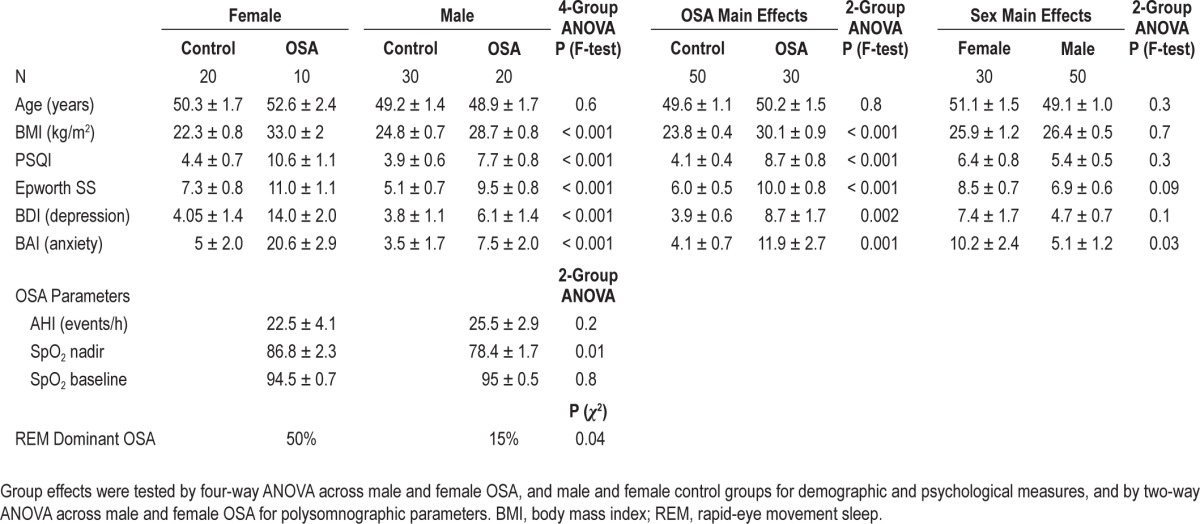
Detailed characteristics of the subjects are shown in Table 1. All subgroups were age-matched (overall age mean ± SD = 49.8 ± 7.6 years), and the disease severity was similar for male and female OSA subgroups (overall AHI = 23.8 ± 12.7 events/h). Significant group differences appeared in all symptoms (Table 1), with OSA females showing the highest levels of each symptom (BAI, BDI, ESS, and PSQI). Physiologically, the oxygen saturation nadir was lower in males, and REM-related apneas dominated in 50% of females versus 15% of males.
Table 1.
Subject information across groups, with adjusted means and standard errors (± SE)
White Matter Structural Changes
An absolute FA threshold of 0.15 led to the exclusion of non-brain regions and outer cortical areas from the analysis, and the inclusion of all white matter, and mixed regions (Figure 1). The inclusion of some ventricular and purely gray matter regions likely reflects variations in spatial normalization, but the consequence of these additional regions would be only a very modest reduction in sensitivity resulting from a more stringent multiple comparisons threshold due to the slight increase in number of voxels in the search area.57 (See Figure S1 in supplemental material for statistical maps of the F-test of the entire model at 2 FA thresholds, 0.015 and 0.05, along with an average of the 80 subjects' anatomical scans for reference.)
Figure 1.
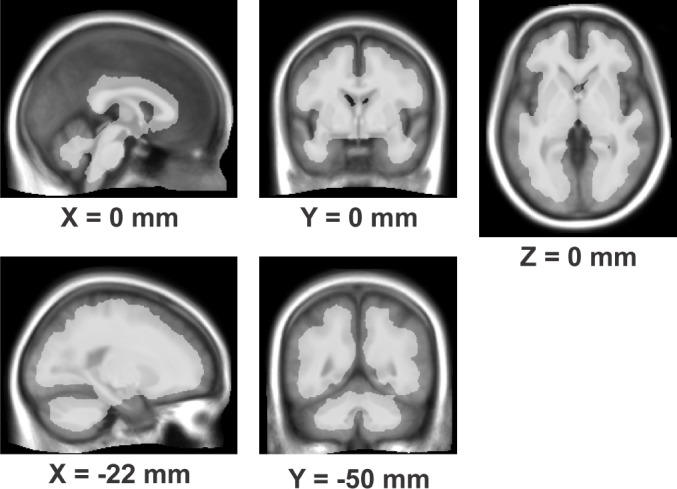
Regions (white semi-transparent overlay) meeting the criterion of FA > 0.15, overlaid onto average of 80 subjects' anatomical images normalized to MNI space. Locations of slices are in MNI coordinates.
Overall Effects
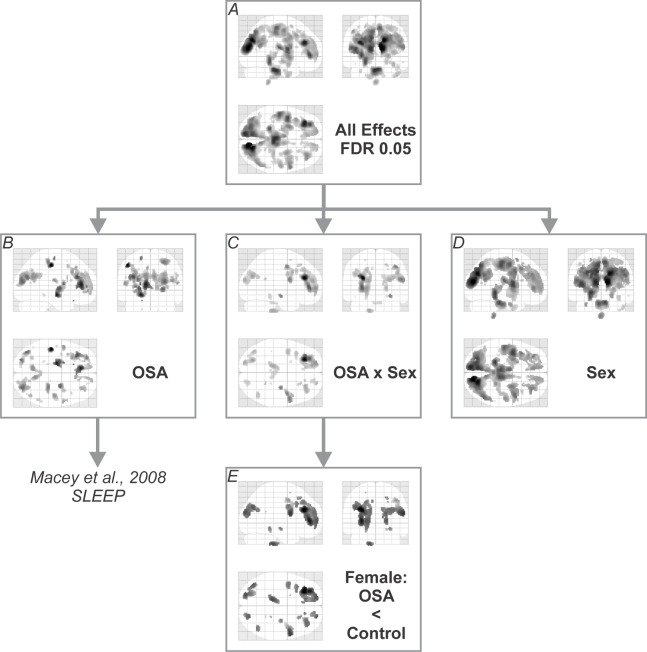
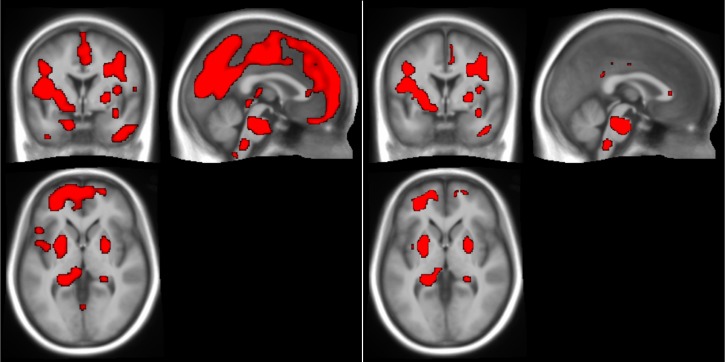
The model showed significance in the F-test of the effects of interest across a wide number of brain regions (Figure 2A), with 0.297 liters (approximately 18% of brain volume) showing effects at the 0.05 level (FDR correction). Significant differences were present across many brain regions for the previously reported15 OSA effect (Figure 2B), a sex-by-OSA interaction (Figure 2C), and a direct sex effect (Figure 2D).
Figure 2.
Glass brain view of areas showing significant effects (F test, FDR < 0.05) for A: all effects (sex, OSA, and interaction); B: OSA effects, i.e., the equivalent to our previous report;15 C: interaction of sex and OSA; D: sex effects. E: post hoc test of OSA × sex interaction illustrating regions of significantly lower FA in female OSA versus female control subjects (ANCOVA, t statistic at FDR 0.05). (In the glass brain view, all regions of significance throughout the volume are projected onto sagittal, coronal and axial views, giving a visual indication of overall extent and approximate location of significant effects.)
Interaction of Sex and OSA
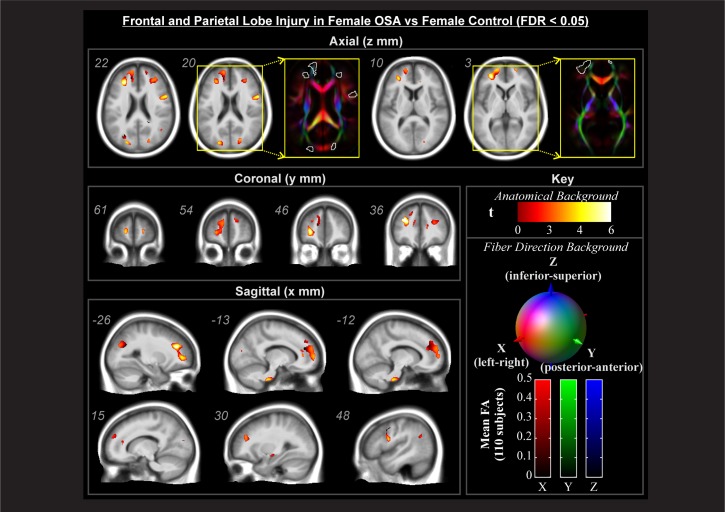
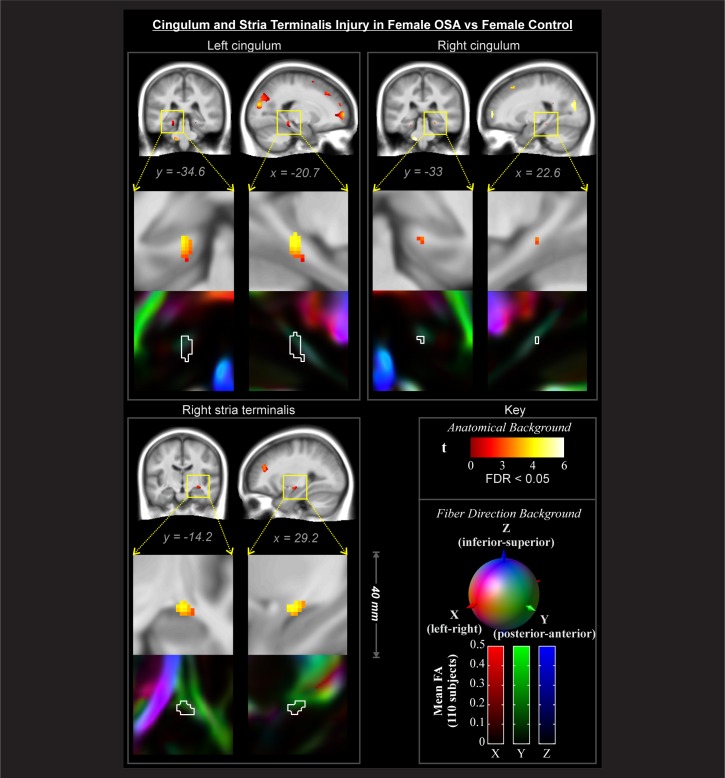
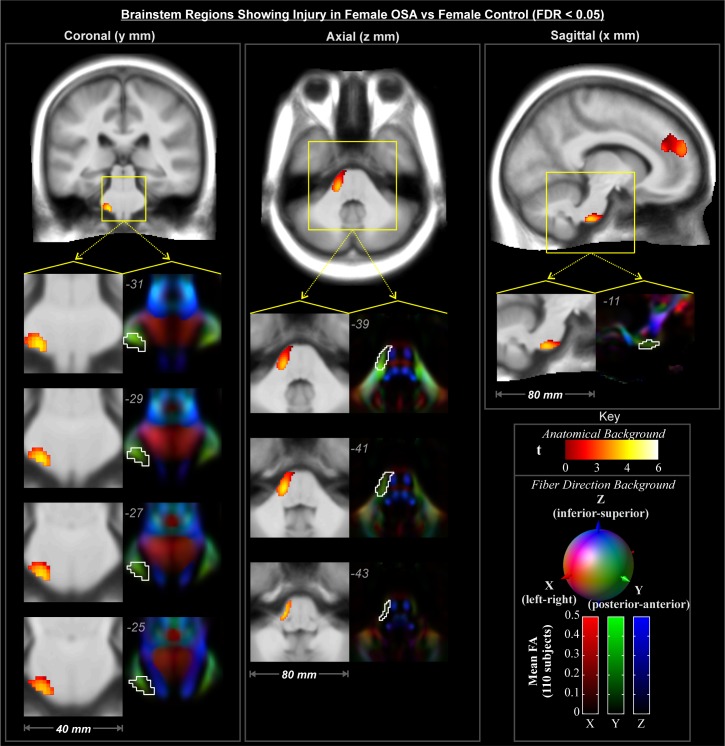
Regions showing an interaction effect (Figure 2C) were tested across conditional means with post hoc tests to characterize differences of interest. In these areas, male OSA patients did not show effects relative to male controls at this statistical threshold. In contrast, female OSA patients showed lower FA than healthy female subjects in many white matter structures (Figures 3–5). These areas included fibers projecting to prefrontal regions (Figure 3, coronal and sagittal views), the bilateral forceps minor (Figure 3, axial z = 3, 20, and 22) with greater impact on the left (Figure 3, axial z = 3, 10, 20), and projections from the anterior corona radiata (Figure 3, axial z = 20, 22) with greater differences on the left (Figure 3, axial z = 3, 10). Transverse projections to the lateral frontal cortex on the right, in the region of the postcentral gyrus, showed lower FA (Figure 3, axial z = 20 and 22). Bilaterally, the posterior end of the forceps major extending into the parietal cortex was affected (Figure 3, axial z = 20 and 22). A bilateral region of the inferior aspect of the cingulum bundle, in the region of the mid-to-posterior hippocampus, was affected (Figure 4), with greater extent of change on the left. The right stria terminalis near its terminus in the amygdala was also affected (Figure 4). The brainstem showed an area of significantly reduced FA in the left superior cerebellar peduncle (Figure 5).
Figure 3.
Forebrain regions showing significant interaction effects (Figure 2E) with post-hoc tests of significant female-OSA compromised structure (lower FA) relative to female-control (FDR < 0.05), color-coded according to significance level; numbers indicate locations in MNI coordinates (mm). Backgrounds are the average of 80 subjects' spatially normalized T1 anatomical volumes (grayscale), and DTI-derived tensor 1st eigenvector direction images (color).
Figure 4.
Bilateral cingulum and right stria terminalis regions showing significant interaction effects (Figure 2E) with post hoc tests of significant female-OSA compromised structure (lower FA) relative to female-control (FDR < 0.05), color-coded according to significance level; numbers indicate locations in MNI coordinates (mm). Backgrounds are the average of 80 subjects' spatially normalized T1 anatomical volumes (grayscale), and DTI-derived tensor 1st eigenvector direction images (color).
Figure 5.
Brainstem regions showing significant interaction effects (Figure 2E) with post-hoc tests of significant female-OSA compromised structures (lower FA) relative to female-control (FDR < 0.05), color-coded according to significance level; numbers indicate locations in MNI coordinates (mm). Backgrounds are the average of 80 subjects' spatially normalized T1 anatomical volumes (grayscale), and DTI-derived tensor 1st eigenvector direction images (color).
DISCUSSION
Changes in white matter in OSA differ by sex, with injury appearing specifically in female OSA subjects. The white matter injury here is inconsistent with female neuroprotection from hypoxia injury in animal models,33,34 and to stroke in humans,58,59 but the changes may help explain symptom differences between the sexes. Alternatively, the symptom differences in anxiety and depression in particular may contribute to the neural injury, perhaps in spite of female neuroprotection. Affected axonal bundles included interconnecting fibers to limbic structures, such as the bilateral cingulum and right stria terminalis, and axons to bilateral frontal and parietal cortices and the left superior cerebellar peduncle. Although the origin of the injury cannot be established, the compromised white matter may mediate aspects of the increased psychological symptoms in OSA females over males, particularly anxiety and depression. Limbic and cerebellar areas are also involved in autonomic regulation; thus, the sex differences may play a role in the variations in cardiovascular symptoms between males and females. The findings add white matter injury to the range of characteristics that differ between the males and females with OSA, and reinforce the importance of considering sex as a factor in studies of the central nervous system in the sleep disorder.
Potential Mechanisms of Neural Alterations
Hypoxic and ischemic injury associated with obstructive events likely plays a major role in the changes found in brain structures in OSA. Animal models of intermittent hypoxia show neural injury, with sex-related variations.33,34,60 Resting blood flow is higher in females,61,62 which presumably is protective against ischemic conditions, such as may occur with large blood pressure swings that occur with obstructive apneic events.63 However, whether this vascular advantage is present in female OSA subjects is unknown. Estrogen also confers neuroprotection in females, at least in animal models35,36; thus, lower levels of this hormone in post-menopausal females might place those subjects at greater risk relative to their younger counterparts. Other factors may contribute to the structural brain changes, including hypertension and chronic stress and immune system activation. The demonstration of very high levels of type 2 diabetes in OSA patients raises the possibility that additive effects of metabolic disturbances and OSA may contribute to neural injury, at least in general OSA populations,64 as diabetes alone is associated with structural brain changes,65,66 but the patterns of neural injury in diabetes across sexes have not been examined. Obesity is another factor associated with neural changes,67,68 presumably from a combination of metabolic and inflammatory factors contributing to the pathophysiology that injures the brain, although many other factors are likely involved.69 The presence of psychological symptoms of depression and anxiety in females is likely associated with increased sympathetic and chronic inflammatory activation,70–72 but the extent of neural injury resulting from these comorbid conditions is also unknown.
Relationship of Neural Changes with Physiological Symptoms
The left superior cerebellar peduncle was affected in female OSA subjects. Those fibers normally communicate between cerebellar nuclei, such as the fastigial autonomic nucleus, to more rostral brain areas, including cardiovascular regulatory areas.73–77 Autonomic functions are altered in combined male-female OSA populations,78,79 with elevated sympathetic activity a particular concern, potentially leading to the development of hypertension80 and cardiac arrhythmias.81,82 The findings here may relate to the sex-specific variations in cardiovascular symptoms.
Male-female differences in upper airway muscle tone and chemical ventilatory drive have been described in sleep disordered breathing populations,83–89 and healthy females show reduced upper airway collapsibility over males, an effect associated with higher tongue genioglossal activity.90,91 Resolution of brainstem areas was too low to determine if other, smaller structures were specifically affected in females, such as serotonergic projections from the raphe which project to the hypoglossal nucleus. Those raphe projections show functional sex-related differences in rats,92,93 which may also be present in humans.
Age differences were not significant, although the female OSA group was slightly older than the control and male OSA groups (from 2.3 to 3.7 years older). Since age was a covariate in the model, simple aging effects were likely factored out in the findings. The more complex age-related influence of menopausal status could not be addressed, but the sample included pre-, peri-, and post-menopausal females, so the levels of the potentially neuroprotective35,36 estrogen hormones will likely have been highly variable in this sample.94 Furthermore, any beneficial effects would have been present for the duration of the disorder, which would likely be many years prior to their recent diagnosis.95
The sleep parameter variations between the sexes do not explain the neural changes. A polysomnography parameter that differed between OSA males and females was the SpO2 nadir, but the males were substantially lower than females, suggesting that, if anything, the women patients experienced a more modest level of OSA. Although the oxygen saturation nadir is a crude indicator of hypoxic exposure, the finding suggests that female OSA patients did not experience hypoxia to the extent males did, and therefore, hypoxic exposure is unlikely to be a major factor in the neural differences. An additional parameter showing differences was the greater incidence of apneas in REM sleep in females, but the relevance of REM-only versus all sleep state events is unknown.
Relationship of Neural Changes with Psychological Symptoms
Depression is more common in women with OSA than men,4,13,96,97 a finding replicated in the present study. The locations of the structural changes are consistent with this higher prevalence in women, including injury in the cingulum bundle adjacent to the hippocampus, and the stria terminalis near its terminus in the amygdala. We earlier showed that the anterior cingulate, hippocampus, and amygdala all showed more injury in depressed OSA subjects than OSA subjects without depression (not partitioned by sex),98 and the cingulum bundle carries fibers between all three structures.99,100 Communication between the cerebellum and limbic regions could additionally be affected by the injury to the superior cerebellar peduncle.101,102 Fibers to prefrontal regions adjacent to the genu of the anterior cingulate cortex, also injured in depressed OSA subjects, were affected in OSA females, and could be associated with impaired mood regulation. The cingulate areas showing female OSA injury are similar to those areas affected in depression independent of OSA.103–105 Other psychological symptoms, including anxiety and daytime fatigue, tend to be higher in female OSA patients.4,88 Earlier, we found that anxiety (not partitioned by sex) was associated with enhanced injury in OSA patients in particular regions which included the cingulum bundle as well as targets of the bundle, including the hippocampus and amygdala.106 The findings here, especially in the cingulum bundle and stria terminalis, the latter carrying fibers from the caudate to the amygdala, two areas having major roles in both depression and anxiety,107–110 suggest that females may have contributed to a major portion of the earlier description of anxiety and depression-related findings of structural brain changes in OSA. However, whether depression and anxiety are triggered by OSA-induced brain changes or whether such symptoms interact with OSA patterns to induce injury is unclear.
Considerations
The measure used here, FA, is an indirect measure of brain injury that reflects structure, specifically the parallel organization of brain tissue.39 The index is high in large axonal tracks, but low in gray matter (and close to zero in cerebrospinal fluid). Reductions in FA appear in the presence of fiber injury, including demyelination, shrinkage, and cell death.111 Other pathologies that lead to reduced FA include fluid accumulation from micro-vascular disease or larger lesions. Additional measures, such as specialized MRI procedures which can partition myelin from axonal and neuronal injury (e.g., magnetization transfer imaging,112 axial and radial diffusivity,113 axial and radial kurtosis114) may provide further understanding of the structural changes, and will be useful in determining whether the injury developed from hypoxia and other accompaniments of OSA, or preexisted the condition.
The groups in the present sample showed significant differences in BMI. One pattern that could potentially have contributed to the white matter findings is the greater difference in BMI between OSA and controls in females as opposed to males (i.e., 10.7 versus 3.9 kg/m2). Obesity-related contributions to neural changes cannot be isolated in the present study, and other known factors, especially chronic inflammatory action which is increased with obesity, as well as elevated blood pressure, will need to be considered to distinguish the source of neural pathology in obese OSA patients.68,115–117
Although the male and female OSA groups were matched for AHI, the OSA characteristics differed between sexes, consistent with other studies (e.g., Resta et al.118). The sleep characteristics, including sleep onset time, awakenings, and time in different sleep stages likely differed between sexes, based on the PSQI and depressive symptom scores (the BDI includes disturbed sleep symptoms). Oxygen desaturation was less severe in females, and REM-dominant OSA was present in significantly more female that male OSA patients. The impact on neural injury of these characteristics is unclear, but what is apparent is that the exposure to OSA phenomena differed between male and female subjects, and these sleep physiology differences could be related to the brain structural differences reported here.
CONCLUSIONS
Male and female OSA patients show marked differences in disease characteristics and comorbidities between sexes; those differences are accompanied by substantial differences in extent and location of white matter injury. Females with OSA show several defined frontal, limbic, and cerebellar axonal structures with reduced integrity, which likely relate to the symptom differences between the sexes, especially variations in psychological symptoms of depression and anxiety, but potentially also physiological differences in cardiovascular characteristics. Whether the psychological symptoms contribute to the injury as opposed to the injury triggering the onset of such symptoms is unknown, and evidence exists for both possibilities. Within the OSA population, the findings suggest females are more affected than males with respect to white matter alterations, which has implications for the importance of early detection and treatment of the disorder in females.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Ms. Rebecca K. Harper for support with recruitment and data collection, and Drs. Jennifer A. Ogren and Heidi L. Richardson for editorial assistance. Work for this study was performed at the University of California at Los Angeles. Financial support was provided by the National Institutes of Health, HL-60296 and NR-011230.
ABBREVIATIONS
- AHI
apnea/hypopnea index
- BAI
Beck Anxiety Inventory
- BDI
Beck Depression Inventory-II
- BMI
body mass index
- CSF
cerebrospinal fluid
- DTI
diffusion tensor imaging
- ESS
Epworth Sleepiness Scale
- FA
fractional anisotropy
- FDR
false discovery rate
- FOV
field of view
- MNI
Montreal Neurological Institute
- MRI
magnetic resonance imaging
- OSA
obstructive sleep apnea
- PSQI
Pittsburgh Sleep Quality Index
- REM
rapid eye movement
- SpO2
oxygen saturation
- SE
standard error
- TE
echo time
- TR
repetition time
SUPPLEMENTAL MATERIAL
Appendix A:
Process of spatial normalization of DTI images to MNI space
Using standard spatial normalization techniques results in errors with DTI data due to noise and distortions inherent in those EPI-based images. These distortions are distinct from the diffusion-weighted eddy-current effect warping that is corrected with affine coregistration. We developed a process to perform the b0-to-MNI spatial normalization to account for these noise and distortions, based on matching the DTI image to the T1 anatomical scan, and applying the T1 normalization parameters to the DTI data. The adapted normalization process consists of the following steps:
The T1 anatomical image was segmented into gray and white matter and cerebrospinal fluid (CSF) using the “unified segmentation” procedure in SPM119; the output of this step is three probability maps of the likelihood of brain regions being each particular compartment type. This procedure also produces a transformation from the T1 space to the MNI template.
The b0 image was coregistered to the T1 scan using a warping transformation. This process involved applying the SPM unified segmentation/spatial normalization procedure to the b0 image while using the subjects' gray and white matter and CSF probability maps as the “tissue probability maps” (TPM) in the procedure. The TPM define the template space to which the unified segmentation attempts to warp the target image; thus, this step spatially “normalizes” the b0 image to the T1 while allowing for some warping (i.e., undistortion). The output of this step is a transformation from DTI to T1 space.
The DTI images (FA maps) are transformed and resliced into T1 space.
The T1-space FA maps are transformed and resliced into MNI space, based on the T1 spatial normalization parameters.
We visually checked this process on over 100 adult and 50 pediatric subjects using the “Check Reg” SPM tool, which allows comparison of normalized DTI, T1, and MNI template images. We found the spatial normalization to be consistently improved over standard approaches, with the improvements most notable in the brainstem and prefrontal cortex regions.
Figure S1.
Two FA masks based on alternative thresholds (left: threshold = 0.15, as used in manuscript; right: threshold = 0.05), with the mask in red on a background of the mean anatomical image. These are the coronal (top left), sagittal (top right), and axial (bottom left) views centered on the MNI origin. These were created by overlaying the thresholded FA maps on the mean anatomical background in mricron software.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 3.Smith R, Ronald J, Delaive K, Walld R, Manfreda J, Kryger MH. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest. 2002;121:164–72. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- 4.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–14. [PubMed] [Google Scholar]
- 5.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1996;156:2445–51. [PubMed] [Google Scholar]
- 6.Jordan AS, McEvoy RD. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7:377–89. doi: 10.1053/smrv.2002.0260. [DOI] [PubMed] [Google Scholar]
- 7.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99:2020–7. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohsenin V. Effects of gender on upper airway collapsibility and severity of obstructive sleep apnea. Sleep Med. 2003;4:523–9. doi: 10.1016/s1389-9457(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 9.Lavie-Nevo K, Pillar G. Evening-morning differences in blood pressure in sleep apnea syndrome: effect of gender. Am J Hypertens. 2006;19:1064–9. doi: 10.1016/j.amjhyper.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg-Dotan S, Reuveni H, Simon-Tuval T, Oksenberg A, Tarasiuk A. Gender differences in morbidity and health care utilization among adult obstructive sleep apnea patients. Sleep. 2007;30:1173–80. doi: 10.1093/sleep/30.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98:984–9. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Pillar G, Lavie P. Psychiatric symptoms in sleep apnea syndrome: effects of gender and respiratory disturbance index. Chest. 1998;114:697–703. doi: 10.1378/chest.114.3.697. [DOI] [PubMed] [Google Scholar]
- 13.Sampaio R, Pereira MG, Winck JC. Psychological morbidity, illness representations, and quality of life in female and male patients with obstructive sleep apnea syndrome. Psychol Health Med. 2012;17:136–49. doi: 10.1080/13548506.2011.579986. [DOI] [PubMed] [Google Scholar]
- 14.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 15.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 16.Morrell MJ, Jackson ML, Twigg GL, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65:908–14. doi: 10.1136/thx.2009.126730. [DOI] [PubMed] [Google Scholar]
- 17.Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–4. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 18.Joo EY, Tae WS, Lee MJ, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33:235–41. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 20.Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18:36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 21.O'Donoghue FJ, Wellard RM, Rochford PD, et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep. doi: 10.5665/sleep.1582. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonon C, Vetrugno R, Lodi R, et al. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep. 2007;30:305–11. doi: 10.1093/sleep/30.3.305. [DOI] [PubMed] [Google Scholar]
- 23.Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. NeuroImage. 2011;54:787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamba M, Inoue Y, Higami S, Suto Y, Ogawa T, Chen W. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry. 2001;71:334–9. doi: 10.1136/jnnp.71.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamba M, Suto Y, Ohta Y, Inoue Y, Matsuda E. Cerebral metabolism in sleep apnea evaluation by magnetic resonance spectroscopy. Am J Respir Crit Care Med. 1997;156:296–8. doi: 10.1164/ajrccm.156.1.9611063. [DOI] [PubMed] [Google Scholar]
- 26.Alchanatis M, Deligiorgis N, Zias N, et al. Frontal brain lobe impairment in obstructive sleep apnoea: a proton MR spectroscopy study. Eur Respir J. 2004;24:980–6. doi: 10.1183/09031936.04.00127603. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett DJ, Rae C, Thompson CH, et al. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Med. 2004;5:593–6. doi: 10.1016/j.sleep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Henderson LA, Woo MA, Macey PM, et al. Neural responses during Valsalva maneuvers in obstructive sleep apnea syndrome. J Appl Physiol. 2003;94:1063–74. doi: 10.1152/japplphysiol.00702.2002. [DOI] [PubMed] [Google Scholar]
- 29.Harper RM, Macey PM, Henderson LA, et al. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol. 2003;94:1583–95. doi: 10.1152/japplphysiol.00881.2002. [DOI] [PubMed] [Google Scholar]
- 30.Ayalon L, Ancoli-Israel S, Klemfuss Z, Shalauta MD, Drummond SP. Increased brain activation during verbal learning in obstructive sleep apnea. NeuroImage. 2006;31:1817–25. doi: 10.1016/j.neuroimage.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 31.Macey KE, Macey PM, Woo MA, et al. Inspiratory loading elicits aberrant fMRI signal changes in obstructive sleep apnea. Respir Physiol Neurobiol. 2006;151:44–60. doi: 10.1016/j.resp.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Macey PM, Macey KE, Henderson LA, et al. Functional magnetic resonance imaging responses to expiratory loading in obstructive sleep apnea. Respir Physiol Neurobiol. 2003;138:275–90. doi: 10.1016/j.resp.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Nijboer CH, Groenendaal F, Kavelaars A, Hagberg HH, van Bel F, Heijnen CJ. Gender-specific neuroprotection by 2-iminobiotin after hypoxia-ischemia in the neonatal rat via a nitric oxide independent pathway. J Cereb Blood Flow Metab. 2007;27:282–92. doi: 10.1038/sj.jcbfm.9600342. [DOI] [PubMed] [Google Scholar]
- 34.Sanfilippo-Cohn B, Lai S, Zhan G, et al. Sex differences in susceptibility to oxidative injury and sleepiness from intermittent hypoxia. Sleep. 2006;29:152–9. doi: 10.1093/sleep/29.2.152. [DOI] [PubMed] [Google Scholar]
- 35.Raval AP, Bramlett H, Perez-Pinzon MA. Estrogen preconditioning protects the hippocampal CA1 against ischemia. Neuroscience. 2006;141:1721–30. doi: 10.1016/j.neuroscience.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Gerstner B, Sifringer M, Dzietko M, et al. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann Neurol. 2007;61:562–73. doi: 10.1002/ana.21118. [DOI] [PubMed] [Google Scholar]
- 37.Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 2000;23:165–70. [PubMed] [Google Scholar]
- 38.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–95. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 39.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman JA, Ahrens ET, Laidlaw DH, Zhang S, Allman JM. Anatomical analysis of an aye-aye brain (Daubentonia madagascariensis, primates: Prosimii) combining histology, structural magnetic resonance imaging, and diffusion-tensor imaging. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1026–37. doi: 10.1002/ar.a.20264. [DOI] [PubMed] [Google Scholar]
- 41.Westerhausen R, Kompus K, Dramsdahl M, et al. A critical re-examination of sexual dimorphism in the corpus callosum microstructure. NeuroImage. 2011;56:874–80. doi: 10.1016/j.neuroimage.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Menzler K, Belke M, Wehrmann E, et al. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. NeuroImage. 2011;54:2557–62. doi: 10.1016/j.neuroimage.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 43.Jahanshad N, Lee AD, Barysheva M, et al. Genetic influences on brain asymmetry: a DTI study of 374 twins and siblings. NeuroImage. 2010;52:455–69. doi: 10.1016/j.neuroimage.2010.04.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar R, Macey PM, Woo MA, Harper RM. Selectively diminished corpus callosum fibers in congenital central hypoventilation syndrome. Neuroscience. 2011;178:261–9. doi: 10.1016/j.neuroscience.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 46.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 47.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 48.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 49.Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, Texas: The Psychological Corporation; 1996. [Google Scholar]
- 50.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 51.Haselgrove JC, Moore JR. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med. 1996;36:960–4. doi: 10.1002/mrm.1910360620. [DOI] [PubMed] [Google Scholar]
- 52.Ashburner J, Friston K. Multimodal image coregistration and partitioning--a unified framework. NeuroImage. 1997;6:209–17. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 53.Hutton C, Bork A, Josephs O, Deichmann R, Ashburner J, Turner R. Image distortion correction in fMRI: A quantitative evaluation. NeuroImage. 2002;16:217–40. doi: 10.1006/nimg.2001.1054. [DOI] [PubMed] [Google Scholar]
- 54.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 55.Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 56.Wen W, Zhu W, He Y, et al. Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J Neurosci. 2011;31:1204–12. doi: 10.1523/JNEUROSCI.4085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 58.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–35. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 59.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–9. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 60.Mage DT, Donner M. Female resistance to hypoxia: does it explain the sex difference in mortality rates? J Womens Health (Larchmt) 2006;15:786–94. doi: 10.1089/jwh.2006.15.786. [DOI] [PubMed] [Google Scholar]
- 61.Mathew RJ, Wilson WH, Tant SR. Determinants of resting regional cerebral blood flow in normal subjects. Biol Psychiatry. 1986;21:907–14. doi: 10.1016/0006-3223(86)90264-7. [DOI] [PubMed] [Google Scholar]
- 62.Gur RC, Gur RE, Obrist WD, et al. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science (New York, N.Y) 1982;217:659–61. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 63.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med. 1976;85:714–9. doi: 10.7326/0003-4819-85-6-714. [DOI] [PubMed] [Google Scholar]
- 64.Harper RM, Macey PM, Kumar R, Woo MA. Neural injury in diabetic versus non-diabetic obstructive sleep apnea patients: a pilot study. Sleep. 2009;32:A341–A. [Google Scholar]
- 65.Chen Z, Li L, Sun J, Ma L. Mapping the brain in type II diabetes: Voxel-based morphometry using DARTEL. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 66.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. NeuroImage. 2006;31:1419–25. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 67.Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond) 2011 doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim CK, McGorray SP, Bartholomew BA, et al. Depressive symptoms and heart rate variability in postmenopausal women. Arch Intern Med. 2005;165:1239–44. doi: 10.1001/archinte.165.11.1239. [DOI] [PubMed] [Google Scholar]
- 71.Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–6. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- 72.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Kalil K. Projections of the cerebellar and dorsal column nuclei upon the thalamus of the rhesus monkey. J Comp Neurol. 1981;195:25–50. doi: 10.1002/cne.901950105. [DOI] [PubMed] [Google Scholar]
- 74.Zhang F, Iadecola C. Fastigial stimulation increases ischemic blood flow and reduces brain damage after focal ischemia. J Cereb Blood Flow Metab. 1993;13:1013–9. doi: 10.1038/jcbfm.1993.127. [DOI] [PubMed] [Google Scholar]
- 75.Gao L, Fei S, Qiao W, Zhang J, Xing H, Du D. Protective effect of chemical stimulation of cerebellar fastigial nucleus on stress gastric mucosal injury in rats. Life Sci. 2011;88:871–8. doi: 10.1016/j.lfs.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Miller RA, Strominger NL. An experimental study of the efferent connections of the superior cerebellar peduncle in the rhesus monkey. Brain Res. 1977;133:237–50. doi: 10.1016/0006-8993(77)90761-2. [DOI] [PubMed] [Google Scholar]
- 77.Giuditta M, Ruggiero DA, Del Bo A. Anatomical basis for the fastigial pressor response. Blood Press. 2003;12:175–80. doi: 10.1080/08037050310010912. [DOI] [PubMed] [Google Scholar]
- 78.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–8. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 79.Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–5. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- 80.Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. 1988;6:S529–31. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- 81.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shepard JW, Jr., Garrison MW, Grither DA, Dolan GF. Relationship of ventricular ectopy to oxyhemoglobin desaturation in patients with obstructive sleep apnea. Chest. 1985;88:335–40. doi: 10.1378/chest.88.3.335. [DOI] [PubMed] [Google Scholar]
- 83.Sin DD, Jones RL, Man GC. Hypercapnic ventilatory response in patients with and without obstructive sleep apnea: do age, gender, obesity, and daytime PaCO(2) matter? Chest. 2000;117:454–9. doi: 10.1378/chest.117.2.454. [DOI] [PubMed] [Google Scholar]
- 84.Jordan AS, McEvoy RD, Edwards JK, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol. 2004;558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hedemark LL, Kronenberg RS. Ventilatory and heart rate responses to hypoxia and hypercapnia during sleep in adults. J Appl Physiol. 1982;53:307–12. doi: 10.1152/jappl.1982.53.2.307. [DOI] [PubMed] [Google Scholar]
- 86.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Hypoxic ventilatory response during sleep in normal premenopausal women. Am Rev Respir Dis. 1982;126:530–3. doi: 10.1164/arrd.1982.126.3.530. [DOI] [PubMed] [Google Scholar]
- 87.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 2: mechanisms. Sleep. 2002;25:499–506. [PubMed] [Google Scholar]
- 88.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: Clinical features. Sleep. 2002;25:412–9. [PubMed] [Google Scholar]
- 89.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. J Appl Physiol. 1983;54:874–9. doi: 10.1152/jappl.1983.54.4.874. [DOI] [PubMed] [Google Scholar]
- 90.Popovic RM, White DP. Influence of gender on waking genioglossal electromyogram and upper airway resistance. Am J Respir Crit Care Med. 1995;152:725–31. doi: 10.1164/ajrccm.152.2.7633734. [DOI] [PubMed] [Google Scholar]
- 91.Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol. 2008;164:213–21. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barker JR, Thomas CF, Behan M. Serotonergic projections from the caudal raphe nuclei to the hypoglossal nucleus in male and female rats. Respir Physiol Neurobiol. 2009;165:175–84. doi: 10.1016/j.resp.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136:249–63. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 94.Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–30. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 95.Rahaghi F, Basner RC. Delayed diagnosis of obstructive sleep apnea: don't ask, don't tell. Sleep Breath. 1999;3:119–24. doi: 10.1007/s11325-999-0119-z. [DOI] [PubMed] [Google Scholar]
- 96.Wahner-Roedler DL, Olson EJ, Narayanan S, et al. Gender-specific differences in a patient population with obstructive sleep apnea-hypopnea syndrome. Gend Med. 2007;4:329–38. doi: 10.1016/s1550-8579(07)80062-3. [DOI] [PubMed] [Google Scholar]
- 97.Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep. 2007;30:312–9. doi: 10.1093/sleep/30.3.312. [DOI] [PubMed] [Google Scholar]
- 98.Cross RL, Kumar R, Macey PM, et al. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31:1103–9. [PMC free article] [PubMed] [Google Scholar]
- 99.Nimchinsky EA, Vogt BA, Morrison JH, Hof PR. Spindle neurons of the human anterior cingulate cortex. J Comp Neurol. 1995;355:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- 100.Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci. 2012;19:289–98. doi: 10.1016/j.jocn.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 101.Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res. 1976;2:133–46. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- 102.Rubio C, Custodio V, Juarez F, Paz C. Stimulation of the superior cerebellar peduncle during the development of amygdaloid kindling in rats. Brain Res. 2004;1010:151–5. doi: 10.1016/j.brainres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 103.Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–88. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 104.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann NY Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 105.Taki Y, Kinomura S, Awata S, et al. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. J Affect Disord. 2005;88:313–20. doi: 10.1016/j.jad.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 106.Kumar R, Macey PM, Cross RL, Woo MA, Yan-Go FL, Harper RM. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety. 2009;26:480–91. doi: 10.1002/da.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schienle A, Ebner F, Schafer A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261:303–7. doi: 10.1007/s00406-010-0147-5. [DOI] [PubMed] [Google Scholar]
- 108.Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–32. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 109.Ferrari MC, Busatto GF, McGuire PK, Crippa JA. Structural magnetic resonance imaging in anxiety disorders: an update of research findings. Rev Bras Psiquiatr. 2008;30:251–64. doi: 10.1590/s1516-44462008000300013. [DOI] [PubMed] [Google Scholar]
- 110.Correia S, Hubbard E, Hassenstab J, et al. Basal ganglia MR relaxometry in obsessive-compulsive disorder: T2 depends upon age of symptom onset. Brain Imaging Beh. 2010;4:35–45. doi: 10.1007/s11682-009-9083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007;205:116–31. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koenig SH. Cholesterol of myelin is the determinant of gray-white contrast in MRI of brain. Magn Reson Med. 1991;20:285–91. doi: 10.1002/mrm.1910200210. [DOI] [PubMed] [Google Scholar]
- 113.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 114.Poot DH, den Dekker AJ, Achten E, Verhoye M, Sijbers J. Optimal experimental design for diffusion kurtosis imaging. IEEE Trans Med Imaging. 2010;29:819–29. doi: 10.1109/TMI.2009.2037915. [DOI] [PubMed] [Google Scholar]
- 115.Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–62. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol. 2007;254:713–21. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- 118.Resta O, Carpanano GE, Lacedonia D, et al. Gender difference in sleep profile of severely obese patients with obstructive sleep apnea (OSA) Respir Med. 2005;99:91–6. doi: 10.1016/j.rmed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 119.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]