Abstract
Background
Leukocytes collected from hematopoietic stem cell transplant donors are often given to the recipient to speed immune recovery or treat disease relapse. The post-thaw recovery and viability of cryopreserved donor leukocytes, stored for as long as seven years, was assessed.
Methods
Total nucleated cell (TNC) cell recovery, CD3+ cell recovery, and TNC viability were measured in 311 clinical donor leukocyte products: 168 products were unmanipulated or minimally manipulated and 143 products were extensively manipulated. An additional 45 products were selected because they were stored for a longer duration; these were tested using both standard methods and global transcriptional analysis. All products were cryopreserved in 5% DMSO plus 6% pentastarch and stored in liquid nitrogen.
Results
The mean duration of storage of the 311 products was 143 days. Their TNC recovery was 92 ± 17%, CD3+ cell recovery was 76 ± 19% and the TNC viability was 84 ± 6%. Duration of storage had no effect on TNC recovery, CD3+ cell recovery or TNC viability of the 311 products. The mean duration of storage of the long-term stored products was 5.2 years; their TNC recovery (93 ± 14%) and the TNC viability (78 ± 13%) did not differ from the 311 products, but their CD3 cell recovery was greater (86 ± 22%; p=0.0042). Gene expression profiles of the long-term stored products revealed no differences due to storage duration.
Conclusions
Donor leukocyte products cryopreserved in 5% DMSO and 6% pentastarch can be stored in liquid nitrogen for at least 7 years.
Introduction
Peripheral blood mononuclear cells (PBMCs) collected by apheresis from allogeneic hematopoietic stem cell (HSC) transplant donors are often given to the transplant recipient as post-transplant immune therapy.1, 2 Unmanipulated or minimally manipulated PBMCs are given to speed immune recovery following transplantation with T cell depleted grafts; they are also used to prevent and treat leukemia relapse following transplantation. Some of these donor leukocyte products are processed extensively. For example, in some cases, T cells are treated to polarize them to a TH2 phenotype3 or to deplete allo-reactive T cells4 in order to reduce the risk of GVHD while maintaining a graft versus tumor effect.
Minimally manipulated and extensively manipulated donor lymphocytes are often collected and processed near the time of HSC donation, cryopreserved and stored in the vapor or liquid phase of liquid nitrogen until they are needed. In most cases, the products are stored for a few days to a few months; however, some of these products are stored for several years. When lymphocytes are cryopreserved in solutions containing 10% DMSO using a controlled-rate freezer and are stored in liquid nitrogen, they can remain functional for a long period of time.5–8
For many years, DMSO at a final concentration of 10% was the standard cryoprotectant. However, the transfusion of DMSO with thawed cellular therapies may cause nausea, vomiting, headache, flushing, chest tightness, hypotension, hypertension, bradycardia or abdominal cramps.9–13 In order to reduce these toxicities, several groups have investigated the cryopreservation of cells with lower concentrations of DMSO and some have found the that post-thaw recovery of cells stored in 5% DMSO is superior than those stored in 10% DMSO.14–16 One comparison of the post-thaw cellular recoveries of CD34+, T and NK cell subsets from mobilized PBSCs stored in 2, 4, 5, and 10% DMSO found the best post-thaw recovery in cells stored in 4 or 5% DMSO.17,18 Comparison of the clinical engraftment of cryopreserved autologous peripheral blood stem cell (PBSC) components found no differences in platelet and neutrophil recovery between transplants performed with PBSCs stored in 5 and 10% DMSO.19 While the post-thaw recoveries of HSCs and leukocytes have been similar in 5% and 10% DMSO, almost all of the studies assessed cells from granulocyte colony-stimulating factor (G-CSF) mobilized PBSC components.
To further explore the effectiveness of 5% DMSO as a cryoprotectant for long-term storage of leukocytes, we evaluated a large number of clinical donor leukocyte products cryopreserved, stored and thawed in a clinical cell processing laboratory. We assessed the post-thaw recovery of total nucleated cells (TNCs), CD3+ cells and TNC viability of unmanipulated, minimally manipulated and extensively manipulated products tested as part of our laboratory’s routine post-thaw quality assurance testing. We also tested leukocyte products that were intended for clinical use but were to be discarded and had been stored a longer duration of time, up to 7.8 years. These products were assessed using standard methods as well as with global transcriptional analysis to provide a more comprehensive analysis of the thawed cells.
Materials and Methods
Study Design
We studied donor leukocyte products that had been collected and cryopreserved for use in clinical cell therapy protocols. A total of 356 leukocyte products were tested including unmanipulated, minimally manipulated, and extensively manipulated products. The minimally manipulated products were processed to remove plasma or red blood cells to prevent hemolytic reactions due to ABO incompatibility between the donor and recipient. Extensively manipulated products included PBMC concentrates that were treated with cytokines or growth factors, were stimulated with CD3/CD8 beads, were enriched for subpopulations, or were where co-cultured with other cells. The products studied were used, or were intended to be used, for clinical donor lymphocytes infusions.
Three hundred eleven of the leukocyte products were tested at the time they were thawed and infused as part of our laboratory’s routine quality assurance testing. The products were cryopreserved from October 2000 through December 2008 and were tested from March 2001 through April 2009. TNC counts, 7AAD-viability, and proportion of cells expressing CD3 were measured both pre-cryopreservation and post-thaw.
An additional 45 leukocyte products that were to be discarded because they were no longer needed for clinical care were also tested. In most cases, this was because the intended recipient of the product had died. These products were cryopreserved between February 2001 and December 2008 and were thawed between June 2008 and January 2009. All of these products were thawed and tested using our laboratory’s thawing and post-thaw testing procedures. Cell counts, viability and number of cells expressing CD3 were measured pre-cryopreservation and post-thaw. The post-thaw samples were also assessed by global transcriptome analysis.
Cryopreservation
All products were cryopreserved using a controlled rate freezer (Kryosave, Integra, Planer plc, Sunbury-on-Thames, UK) and 5% DMSO and 6% pentastarch with 4% HSA as a cryoprotectant. The products were stored in aliquots with a final volume of 4.5, 25 or 50 mL; aliquots whose final volume was 4.5 mL were stored in vials (Nunc Cryotube Vials, Roskilde, Denmark) and aliquots of 25 and 50 mL were stored in bags. The cell concentration of products frozen in vials ranged from 1 × 106 per mL to 1 × 108 per mL while that of products frozen in bags ranged from 2 × 107 to 3 × 108 cells per mL. The cryopreserved bags and vials were stored in both the vapor and liquid phases of liquid nitrogen.
From October 2000 through January 22, 2002 the products were cryopreserved in poly(ethylene co-vinyl acetate) (EVA) plastic bags (Cryocyte freezing container, PL 269 plastic, Baxter Healthcare, Deerfield, IL) and from January 23, 2002 through December 2008 in polyfluoroethylene polyfluoropropylene (FEP) bags (KryoSure, American Fluroseal Corporation, Gaithersburg MD).20
Thawing leukocyte products
The products were thawed in a water bath at 37°C. Immediately after thawing, the products that had been cryopreserved in vials were diluted to a final volume of 30 mL with Plasma-Lyte A (Baxter Healthcare) containing 10 units per mL of preservative free heparin. When the 25 mL bags were thawed, 6 mL of Plasma-Lyte A was added and 12 mL of Plasma-Lyte A was added to 50 mL aliquots cryopreserved in bags.
Post-thaw quality control testing
An aliquot of the product was diluted 5-fold in Plasma-Lyte A immediately after thawing. Cell counts were performed using an automated cell counter (Abbott CellDyn 3500) and the cells were analyzed by flow cytometry (BD Biosciences, San Jose, CA) with anti-CD3 and CD45 (BD Biosciences). Viability was assessed by flow cytometry and 7-AAD staining.
RNA preparation, RNA amplification and labeling for oligonucleotide microarray
Total RNA was extracted from a sample from each of the thawed leukocyte products using Trizol reagent (Invitrogen, Carlsbad, CA). RNA integrity was assessed using the Agilent 2100 Bioanalyser (Agilent Technologies, Waldbronn, Germany). Total RNA (3μg) from the leukocytes was amplified into anti-sense RNA (aRNA). Also, total RNA from PBMCs pooled from six normal donors was extracted and amplified into aRNA to serve as the reference. Pooled reference and test aRNA were isolated and amplified in identical conditions during the same amplification/hybridization procedure to avoid possible inter-experimental biases. Both reference and test aRNA were directly labeled using ULS aRNA Fluorescent Labeling kit (Kreatech, Amsterdam, The Netherlands) with Cy3 for reference and Cy5 for test samples.
Whole-genome human 36K oligonucleotide microarrays were printed in the Infectious Disease and Immunogenetics Section, DTM, Clinical Center, NIH (Bethesda, MD) using a commercial probe set which contains 35,035 oligonucleotide probes, representing approximately 25,100 unique genes and 39,600 transcripts excluding control oligonucleotides (Operon Human Genome Array-Ready Oligo Set version 4.0, Huntsville, AL, USA). The design is based on the Ensemble Human Database build (NCBI-35c), with a full coverage on NCBI human Refseq dataset (04/04/2005). The microarray was composed of 48 blocks; one spot was printed per probe per slide. Hybridization was carried out in a water bath at 42°C for 18 to 24 hours and the arrays were then washed and scanned on a GenePix scanner Pro 4.0 (Axon, Sunnyvale, CA) at variable photomultiplier tube to obtain optimized signal intensities with minimum (<1% spots) intensity saturation.
Statistical analysis
The resulting data files were uploaded to the mAdb database (http://nciarray.nci.nih.gov/) and further analyzed using BRBArrayTools developed by the Biometric Research Branch, National Cancer Institute (http://linus.nci.nih.gov/BRB-ArrayTools.html) and Cluster and TreeView software. Briefly, the raw data set was filtered according to a standard procedure to exclude spots with a minimum intensity that was arbitrarily set to an intensity parameter of 200 for both fluorescence channels. If the fluorescence intensity of one channel was greater than 200 and that of the other was less than 200, the fluorescence of the low intensity channel was arbitrarily set to 200. Spots with diameters < 20μm were excluded from the analysis. The filtered data were normalized using median over entire array.
For each sample the TNC recovery was equal to (pre-cryopreservation TNC × product volume)/(post-thaw TNC × post thaw volume) × 100 and the CD3+ cell recovery was (pre-cryopreservation TNC × pre-cryopreservation CD3% × product volume)/(post-thaw TNC × post-thaw CD3% × post thaw volume) × 100. All data were expressed as mean ± one standard deviation. Groups were compared using t-tests or correlation coefficients (Excel, Microsoft, Redmond, Washington)
Results
Recovery of thawed leukocytes
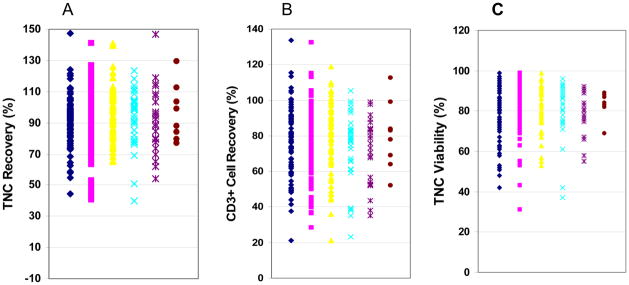
The duration of storage of the 311 clinical leukocyte products evaluated as part of the laboratory’s routine quality assurance program ranged from 1 day to 5.5 years, and the mean duration of storage was 143 days. The TNC recovery was 92 ± 17%, the CD3+ cell recovery was 76 ± 19% and the TNC viability was 84 ± 6%. The post-thaw recovery was analyzed in leukocyte products stored for various periods of time; the duration of storage had no effect on the recoveries of TNCs, the CD3+ cells, or TNC viability (Table 1 and Figure 1).
Table 1.
Recovery of total nucleated cells (TNCs) and CD3+ cells following cryopreservation, storage, and thawing of 311 leukocyte components; effects of duration of storage
| Storage Duration | Number | Post Thaw Recovery (%)
|
Post Thaw TNC Viability (%) | |
|---|---|---|---|---|
| TNC | CD3 | |||
| 1 to 30 days | 93 | 90 ± 16 | 78 ± 19 | 83 ± 13 |
| 31 to 90 days | 100 | 90 ± 18 | 76 ± 18 | 83 ± 11 |
| 91 to 180 | 61 | 97 ± 17 | 78 ± 20 | 82 ± 10 |
| 181 to 365 | 31 | 92 ± 17 | 72 ± 20 | 79 ± 15 |
| 1 to 2 years | 18 | 88 ± 19 | 70 ± 19 | 79 ± 9 |
| > 2 years | 8 | 96 ± 18 | 80 ± 19 | 82 ± 6 |
|
| ||||
| All | 311 | 92 ± 17 | 76 ± 19 | 84 ± 6 |
Figure 1.
Impact of storage duration on the post-thaw recovery of TNCs and CD3+ cells and TNC viability in clinical donor leukocyte products. Three hundred eleven clinical leukocyte products were cryopreserved in 5% DMSO and 6% pentastarch, were stored in liquid nitrogen, and were thawed. The post-thaw recovery of TNCs for products stored for various durations of time is shown in panel A, the post-thaw CD3+ cell recovery is show in panel B and the post-thaw TNC viability in panel C. Products were grouped by those stored up to 30 days (black diamonds), 31 to 90 days (red squares), 91 to 180 days (yellow triangles), 181 to 365 days (blue Xs), 1 to 2 years (purple Xs), and more than 2 years (brown circles).
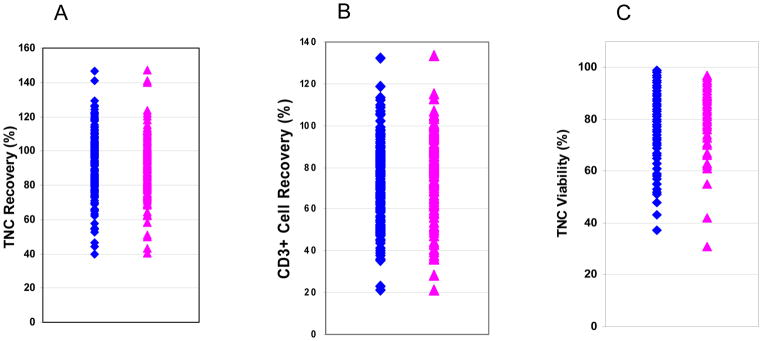
Among the 311 leukocyte products, 168 were minimally or unmanipulated lymphocyte products and 143 were extensively manipulated. The duration of storage of the 168 minimally and unmanipulated leukocytes was significantly greater than the extensively manipulated leukocytes (181 ± 288 days versus 81 ± 92 days, p = 8.13 × 10−5). The TNC recovery of the 168 minimally and unmanipulated lymphocytes was 93 ± 18%, the CD3+ cell recovery was 74 ± 19% and the TNC viability was 80 ±12%. The TNC recovery of the 143 extensively manipulated leukocyte products (90 ± 17%) did not differ from that of minimally and unmanipulated products, but the CD3+ cell recovery and post-thaw TNC viability was slightly greater for extensively manipulated leukocyte products (79 ± 19%, p = 0.015 and 85 ± 11% respectively, p < 0.001) (Figure 2). Storage duration did not affect the TNC recovery, CD3 cell recovery or TNC viability of minimally and unmanipulated leukocyte (Table 2) or extensively manipulated leukocyte products (Table 3).
Figure 2.
Comparison of post-thaw analysis of minimally and extensively manipulated donor leukocyte products. Three hundred eleven donor lymphocyte products cryopreserved in 5% DMSO and 6% pentastarch and were stored in liquid or vapor phase of liquid nitrogen. The post-thaw recovery TNCs of 168 minimally (blue diamonds) 143 extensively (red triangles) manipulated products are shown in panel A. The post-thaw CD3+ cell recovery are shown in panel B and the post-thaw TNC viability in panel C.
Table 2.
Recovery of total nucleated cells (TNCs) and CD3+ cells following cryopreservation, storage, and thawing of 168 unmanipulated and minimally manipulated donor leukocyte products; effects of duration of storage
| Storage Duration | Number | Post Thaw Recovery (%)
|
Post Thaw TNC Viability (%) | |
|---|---|---|---|---|
| TNC | CD3 | |||
| 1 to 30 days | 38 | 88 ± 17 | 69 ± 19 | 76 ± 14 |
| 31 to 90 days | 53 | 93 ± 17 | 74 ± 16 | 81 ± 11 |
| 91 to 180 | 37 | 97 ± 17 | 78 ± 20 | 81 ± 11 |
| 181 to 365 | 17 | 93 ± 18 | 75 ± 21 | 81 ± 13 |
| 1 to 2 years | 15 | 87 ± 19 | 67 ± 21 | 77 ± 14 |
| > 2 years | 8 | 96 ± 18 | 80 ± 19 | 84 ± 6 |
|
| ||||
| All | 168 | 93 ± 18 | 74 ± 19 | 80 ± 12 |
Table 3.
Recovery of Total Nucleated Cells (TNCs) and CD3+ cells following cryopreservation, storage, and thawing of 143 extensively manipulated donor leukocyte products; effects of duration of storage
| Storage Duration | Number | Post Thaw Recovery (%)
|
Post Thaw TNC Viability (%) | |
|---|---|---|---|---|
| TNC | CD3 | |||
| 1 to 30 days | 55 | 91 ± 15 | 84 ± 18 | 88 ± 10 |
| 31 to 90 days | 47 | 87 ± 18 | 78 ± 21 | 86 ± 11 |
| 91 to 180 | 24 | 95 ± 18 | 78 ± 21 | 84 ± 9 |
| 181 to 365 | 13 | 90 ± 18 | 72 ± 18 | 78 ± 15 |
| 1 to 2 years | 4 | 92 ± 15 | 72 ± 14 | 75 ± 10 |
| > 2 years | 0 | ---- | ---- | ---- |
|
| ||||
| All | 143 | 90 ± 17 | 79 ± 19 | 85 ± 11 |
The post-thaw recoveries of 43 products stored in Cryocyte PL 269 plastic bags were compared to the post-thaw recoveries of 247 products stored in KryoSafe FEP bags. Although products cryopreserved in PL 269 bags had been stored longer than those stored in FEP bags (239 ± 432 days compared to 124 ± 171 days, p < 0.01, respectively for PL 269 and FEP bags), there were no differences in TNC recovery (89.9 ± 16.2% versus 91.2 ± 18.2%), CD3+ cell recovery (71.2 ± 20.1 versus 77.2 ± 19.3%) and TNC viability (83.4 ± 9.7% versus 82.9 ± 11.7%).
The concentration of cryopreserved TNCs in each product ranged from 1.87 × 107 to 3.33 × 108 cells/mL. When all 311 products were analyzed higher cell concentrations were weakly associated with decreased TNC and CD3+ cell recoveries (r = −0.17; p<0.003 and r = −0.23; p<0.001 respectively) but not post-thaw TNC viability (r = −0.097; p>0.05). There were no differences in cell concentration between minimally manipulated and unmanipulated lymphocytes compared to extensively manipulated products (7.63 × 107 ± 6.92 × 107 cells/mL versus 8.17 × 107 ± 5.30 × 107 cells/mL, p = 0.27), but products stored in PL 269 had a slightly higher concentration of cells of TNCs than those stored in FEP bags (1.00 × 108 ± 6.92 × 107 cells/mL versus 7.31 × 107 ± 6.02 × 107 cells/mL, p = 0.018)
Molecular analysis of long-term stored leukocyte products
We analyzed the leukocyte recoveries in 45 long-term stored clinical leukocyte products. The duration of storage of these products (mean = 5.22 years, range 48 days to 7.80 years) was significantly longer than the other 311 products analyzed; p < 1 × 10−6). These 45 products were products from 37 subjects. Two products were collected, processed, cryopreserved, and thawed at the same time from 3 different people, (donors 2, 3 and 5), 3 products from one person (donor 1) and 4 from one person (donor 4). When duplicate products were excluded and only the first product thawed from each subject was considered, 37 products were analyzed. The total nucleated cell recovery for these 37 products was 93 ± 14%, the CD3 cell recovery was 86 ± 22% and the post thaw TNC viability was 78 ± 13% (Table 4). There were no differences in the TNC and post-thaw TNC viability between the 37 long-term stored products and the 311 short-term stored products, but the CD3+ cell recovery was greater in the group of 37 products that had been stored longer (p = 0.0042).
Table 4.
Recovery of Total Nucleated Cells (TNCs) and CD3+ cells following cryopreservation, storage, and thawing of 37 long-term stored leukocyte products
| Storage Duration | Number | Post Thaw Recovery (%)
|
Post Thaw TNC Viability (%) | |
|---|---|---|---|---|
| TNC | CD3 | |||
| Up to 2 years | 4 | 97 ± 7 | 84 ± 11 | 79 ± 11 |
| 2 to <4 years | 5 | 102 ± 11 | 98 ± 19 | 90 ± 11 |
| 4 to <6 years | 9 | 98 ± 9 | 96 ± 24 | 79 ± 9 |
| >6 years | 19 | 87 ± 18 | 78 ± 21 | 74 ± 14 |
|
| ||||
| All | 37 | 93 ± 14 | 86 ± 22 | 78 ± 13 |
Thirty-seven of the 45 long-term stored products were minimally or unmanipulated and 8 were extensively manipulated products. Among the 37 minimally and unmanipulated lymphocyte products, two products were collected, cryopreserved, and thawed at the same time from 3 people (donors 2, 3, and 5) and the remaining 31 products were from separate subjects. The second product from each of the 3 donors with duplicate products was excluded from the post-thaw analysis and the post-thaw recoveries were determined for the remaining 34 minimally and unmanipulated products. The TNC recovery for these 34 minimally and unmanipulated lymphocytes collected from unique subjects was 88 ± 20%, the CD3+ cell recovery 82 ± 17% and the post-thaw TNC viability was 82 ± 8% Although these 34 minimally and unmanipulated products were stored for a longer period of time than the 168 minimally and unmanipulated products that were not analyzed at a molecular level, there were no differences in the TNC and post-thaw TNC viability between the two groups, and the CD3+ cell recovery was greater in the group of 34 products that had been stored longer (p = 0.0298).
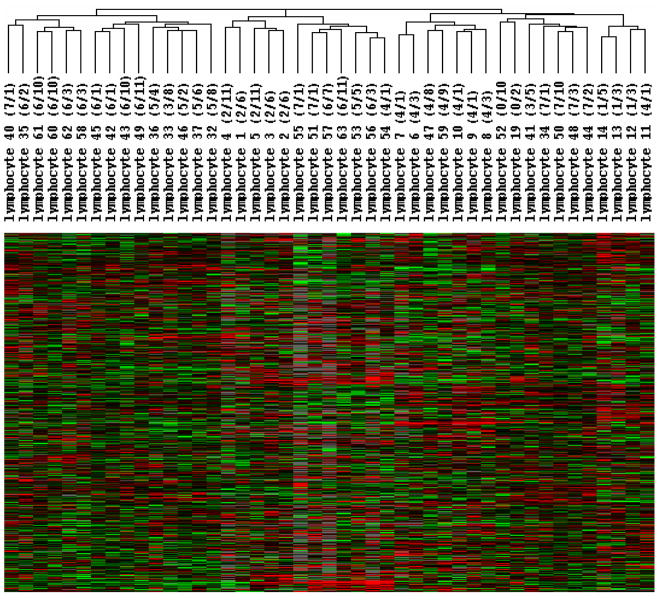
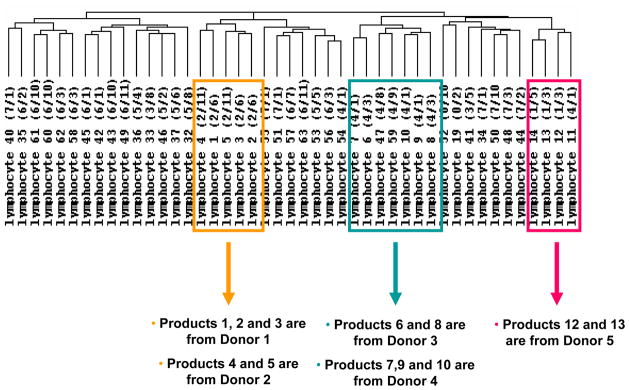
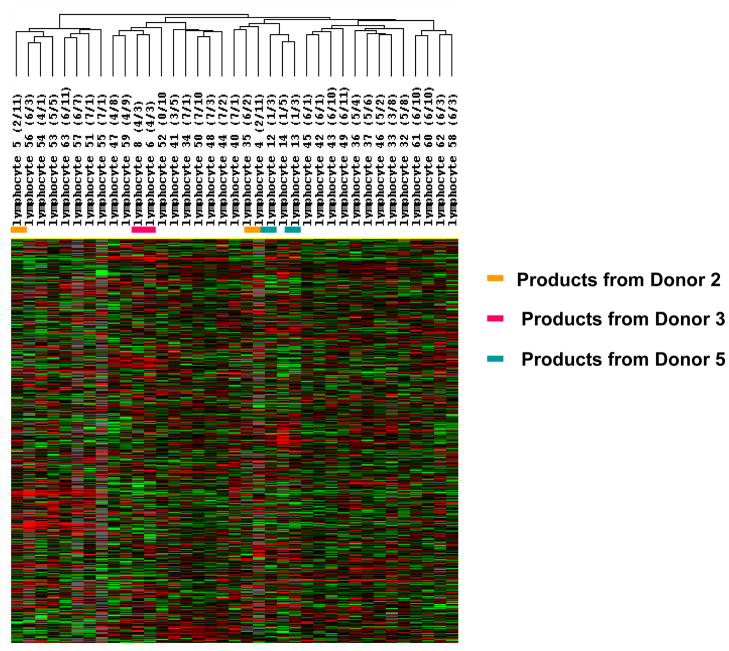
All 45 long-term stored products were analyzed by global gene expression profiling to determine any differences in leukocytes stored for a longer duration of time. Unsupervised hierarchical clustering analysis of all 45 samples revealed several clusters, but the samples did not cluster by storage duration. Instead, they clustered by donor (Figure 3). Products 1, 2 and 3, from donor 1, were all found in the same cluster (Figure 4). This same cluster also contained products 4 and 5 which were from donor 2. Products 7, 9, and 10 were from one donor (donor 4); they were all in the same cluster, but a fourth product from this same donor (product 11) was in a different cluster. Products 6 and 8 from donor 3 also clustered together as did products 12 and 13 from donor 5.
Figure 3.
Global gene expression profiling of 45 leukocyte products that were stored up to 7 years. The 45 minimally and extensively manipulated cryopreserved and long-term stored leukocyte products were thawed and analyzed by gene expression profiling using an oligonucleotide microarrays with more than 36,000 probes. After filtering out genes expressed in less than 80% of samples, 11,420 genes remained which were analyzed by unsupervised hierarchical cluster analysis of Eisen.
Figure 4.
Dentogram of global gene expression analysis of 45 cryopreserved and long-term stored leukocyte products showing the relationships of products collected from the same donors. The 45 minimally and extensively manipulated cryopreserved leukocyte products were analyzed by gene expression profiling and unsupervised hierarchical clustering analysis. Multiple products collected from the same donor are indicated.
The gene expression profiles of the 37 minimally and unmanipulated lymphocyte products were also analyzed by unsupervised hierarchical clustering and samples again clustered by product donor rather than by duration of product storage (Figure 5). Products 4 and 5 from donor 2 again clustered together as did products 6 and 8 from donor 3. Products 12 and 13 from donor 5, however, were in different clusters.
Figure 5.
Global gene expression profiling 37 minimally and unmanipulated leukocyte products that were stored up to 7 years. The 37 minimally and unmanipulated cryopreserved leukocyte products were thawed and analyzed by gene expression profiling using oligonucleotide microarrays with 36,000 probes. After filtering out genes expressed in less than 80% of samples, 11,848 genes remained which were analyzed by unsupervised hierarchical cluster analysis of Eisen. Duplicate products collected, processed, cryopreserved, and thawed at the same time from the same donor are indicated by dashes. Duplicate products from donor 2 are indicated by orange dashes, from donor 3 by red dashes, and from donor 5 by blue dashes.
Discussion
To determine if leukocyte and leukocyte derived cellular therapies can be stored long-term in 5% DMSO, we investigated the effects of storage duration on clinical leukocyte products stored under clinical conditions. The products were cryopreserved in 5% DMSO plus 6% pentastarch with 4% HSA and were stored, in either the liquid or vapor phase of liquid nitrogen. Two groups of products were studied; one which was tested as part of routine post-thaw quality control testing another of products that were no longer needed clinically and were stored for a much longer period of time.
The products studied included unmanipulated, minimally manipulated and extensively manipulated leukocytes. The minimally and unmanipulated products were considered as one group and were compared to extensively manipulated products. Of the 311 products tested as part of the laboratory’s routine post-thaw testing program, duration of storage did not affect TNC, TNC viability, or CD3+ cell recovery for products within each of these two groups. When the extensively and minimally and unmanipulated products were compared, there was no difference in TNC recovery; however, TNC viability and CD3+ cell recovery was greater in extensively manipulated products. The reason for these differences is not clear, but we suspect it is due to some inherent properties of extensively manipulated cells rather than due to a shorter average duration of storage of the extensively manipulated cells. The cytokine, antibody or other treatments performed on the extensively manipulated products may have changed the CD3+ cells in a way that resulted in improved post-thaw recovery.
Our process involved cryopreserving cells in fixed volumes of 4.5, 25 or 50mL. Different doses of cells were cryopreserved by varying the cell concentration. Cryopreserving cells at higher concentrations was found to be weakly associated with reduced post-thaw TNC and CD3+ cell recoveries. Since there was no difference in the concentration of cryopreserved minimally and unmanipulated cells and extensively manipulated products, cell concentration differences did not account for the increased CD3+ cell recoveries of extensively manipulated cells.
The leukocyte products that were tested during post-thaw quality control were stored for a relatively short duration of time; most for less than 90 days and almost all for less than two years. Analysis of the products stored for a longer period of time found that the TNC and TNC viability was similar to the products stored short-term; however, the CD3+ cell recovery was superior in the products stored long-term.
Few recent studies have investigated the recovery and viability of stored cryopreserved leukocytes, but some studies of cryopreserved G-CSF mobilized PBSC concentrates have assessed the viability and recovery of leukocytes in these products; the results of these studies were similar to the results of our studies. One study of 20 PBSC concentrates cryopreserved in 5% DMSO, stored 5 years in liquid nitrogen, found that the post-thaw median WBC viability was 69% and ranged from 45% to 79%. The median CD3+ cell viability was 75% and ranged from 35% to 87%.18 Another study of PBSC concentrates cryopreserved in 5% DMSO from 8 subjects with sickle cell trait and 8 control subjects found that the median post-thaw TNC recovery was 82.9% and mean ±1SD was 81.4 ± 4.8% for control subjects and was 73.2 and 77.9 ± 19.7% for patients with sickle cell trait. The CD3+ cell recovery was 41.2% (41.8 ± 17.6%) for control subjects and 41.2% (44.3 ± 15.9%) for those with sickle cell trait.21
Global gene expression profiling can be effectively used to distinguish slight changes in cells. We have used gene expression to distinguish differences between embryonic stem cells and embryoid bodies,22 different types of leukocytes,23 different types of peripheral blood CD34+ cells24 and differences in PBMC products stored in liquid phase at 4°C for 48 hours.25 To more thoroughly investigate the effects of storage duration on cryopreserved leukocyte products, we studied these products by global transcriptome analysis and did not find any storage duration dependent changes. The fact that gene expression profiling clustered the products collected and processed at the same time and from the same subject, suggests that gene expression analysis maybe a useful tool for assessing cryopreserved cells, however, further studies are needed.
In 2002, our lab changed from the use of PL 269 plastic to FEP bags for the cryopreservation and storage of cellular and gene therapies.20 We compared the post-thaw recovery of cells stored in the two types of bags and found no difference in the post-thaw recoveries and viabilities. However, the products were stored for a longer duration and at a slightly higher concentration in PL 269 bags.
In conclusion, these results show that clinical products cryopreserved in 5% DMSO and 6% pentastarch with 4% HSA can be stored in clinical liquid nitrogen freezers for up to 7 years. In fact, since no changes in leukocytes were noted among cryopreserved products stored various durations of time, even longer durations of storage are reasonable. They also show that leukocytes can be stored in FEP bags.
Footnotes
The authors have no conflicts of interest to disclose. The views expressed in this paper are those of the authors and are not to be construed as the official position of the United States Department of Health and Human Services.
References
- 1.Tomblyn M, Lazarus HM. Donor lymphocyte infusions: the long and winding road: how should it be traveled? Bone Marrow Transplant. 2008 Nov;42(9):569–79. doi: 10.1038/bmt.2008.259. [DOI] [PubMed] [Google Scholar]
- 2.Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2008 Mar;41(5):483–93. doi: 10.1038/sj.bmt.1705898. [DOI] [PubMed] [Google Scholar]
- 3.Fowler DH, Odom J, Steinberg SM, Chow CK, Foley J, Kogan Y, Hou J, Gea-Banacloche J, Sportes C, Pavletic S, et al. Phase I clinical trial of costimulated, IL-4 polarized donor CD4+ T cells as augmentation of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006 Nov;12(11):1150–60. doi: 10.1016/j.bbmt.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SR, Mielke S, Savani BN, Montero A, Wisch L, Childs R, Hensel N, Schindler J, Ghetie V, Leitman SF, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005 Aug 1;106(3):1123–9. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Areman EM, Simonis TB, Carter CS, Read EJ, Klein HG. Bulk cryopreservation of lymphocytes in glycerol. Transfusion. 1988 Mar;28(2):151–6. doi: 10.1046/j.1537-2995.1988.28288179020.x. [DOI] [PubMed] [Google Scholar]
- 6.Glassman AB, Bennett CE. Cryopreservation of human lymphocytes: a brief review and evaluation of an automated liquid nitrogen freezer. Transfusion. 1979 Mar;19(2):178–81. doi: 10.1046/j.1537-2995.1979.19279160289.x. [DOI] [PubMed] [Google Scholar]
- 7.Valeri CR, Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion. 1996 Apr;36(4):303–8. doi: 10.1046/j.1537-2995.1996.36496226141.x. [DOI] [PubMed] [Google Scholar]
- 8.Heo YJ, Son CH, Chung JS, Park YS, Son JH. The cryopreservation of high concentrated PBMC for dendritic cell (DC)-based cancer immunotherapy. Cryobiology. 2009 Apr;58(2):203–9. doi: 10.1016/j.cryobiol.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Zambelli A, Poggi G, Da PG, Pedrazzoli P, Cuomo A, Miotti D, Perotti C, Preti P, Robustelli della CG. Clinical toxicity of cryopreserved circulating progenitor cells infusion. Anticancer Res. 1998 Nov;18(6B):4705–8. [PubMed] [Google Scholar]
- 10.Horacek JM, Jebavy L, Jakl M, Zak P, Mericka P, Maly J. Cardiovascular changes associated with infusion of hematopoietic cell grafts in oncohematological patients -- impact of cryopreservation with dimethylsulfoxide. Exp Oncol. 2009 Jun;31(2):121–2. [PubMed] [Google Scholar]
- 11.Donmez A, Tombuloglu M, Gungor A, Soyer N, Saydam G, Cagirgan S. Clinical side effects during peripheral blood progenitor cell infusion. Transfus Apher Sci. 2007 Feb;36(1):95–101. doi: 10.1016/j.transci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Syme R, Bewick M, Stewart D, Porter K, Chadderton T, Gluck S. The role of depletion of dimethyl sulfoxide before autografting: on hematologic recovery, side effects, and toxicity. Biol Blood Marrow Transplant. 2004 Feb;10(2):135–41. doi: 10.1016/j.bbmt.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Kessinger A, Schmit-Pokorny K, Smith D, Armitage J. Cryopreservation and infusion of autologous peripheral blood stem cells. Bone Marrow Transplant. 1990 Jan;5( Suppl 1):25–7. [PubMed] [Google Scholar]
- 14.Galmes A, Besalduch J, Bargay J, Matamoros N, Duran MA, Morey M, Alvarez F, Mascaro M. Cryopreservation of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide at −80 degrees C without rate-controlled freezing. Transfusion. 1996 Sep;36(9):794–7. doi: 10.1046/j.1537-2995.1996.36996420755.x. [DOI] [PubMed] [Google Scholar]
- 15.Galmes A, Besalduch J, Bargay J, Novo A, Morey M, Guerra JM, Duran MA. Long-term storage at −80 degrees C of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide as the sole cryoprotectant. Transfusion. 1999 Jan;39(1):70–3. doi: 10.1046/j.1537-2995.1999.39199116897.x. [DOI] [PubMed] [Google Scholar]
- 16.Abrahamsen JF, Rusten L, Bakken AM, Bruserud O. Better preservation of early hematopoietic progenitor cells when human peripheral blood progenitor cells are cryopreserved with 5 percent dimethylsulfoxide instead of 10 percent dimethylsulfoxide. Transfusion. 2004 May;44(5):785–9. doi: 10.1111/j.1537-2995.2004.03336.x. [DOI] [PubMed] [Google Scholar]
- 17.Liseth K, Abrahamsen JF, Bjorsvik S, Grottebo K, Bruserud O. The viability of cryopreserved PBPC depends on the DMSO concentration and the concentration of nucleated cells in the graft. Cytotherapy. 2005;7(4):328–33. doi: 10.1080/14653240500238251. [DOI] [PubMed] [Google Scholar]
- 18.Liseth K, Ersvaer E, Abrahamsen JF, Nesthus I, Ryningen A, Bruserud O. Long-term cryopreservation of autologous stem cell grafts: a clinical and experimental study of hematopoietic and immunocompetent cells. Transfusion. 2009 Apr 20; doi: 10.1111/j.1537-2995.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- 19.Akkok CA, Liseth K, Nesthus I, Lokeland T, Tefre K, Bruserud O, Abrahamsen JF. Autologous peripheral blood progenitor cells cryopreserved with 5 and 10 percent dimethyl sulfoxide alone give comparable hematopoietic reconstitution after transplantation. Transfusion. 2008 May;48(5):877–83. doi: 10.1111/j.1537-2995.2008.01648.x. [DOI] [PubMed] [Google Scholar]
- 20.Khuu HM, Cowley H, vid-Ocampo V, Carter CS, Kasten-Sportes C, Wayne AS, Solomon SR, Bishop MR, Childs RM, Read EJ. Catastrophic failures of freezing bags for cellular therapy products: description, cause, and consequences. Cytotherapy. 2002;4(6):539–49. doi: 10.1080/146532402761624700. [DOI] [PubMed] [Google Scholar]
- 21.Kang EM, Areman EM, David-Ocampo V, Fitzhugh C, Link ME, Read EJ, Leitman SF, Rodgers GP, Tisdale JF. Mobilization, collection, and processing of peripheral blood stem cells in individuals with sickle cell trait. Blood. 2002 Feb 1;99(3):850–5. doi: 10.1182/blood.v99.3.850. [DOI] [PubMed] [Google Scholar]
- 22.Ren J, Jin P, Wang E, Marincola FM, Stroncek DF. MicroRNA and gene expression patterns in the differentiation of human embryonic stem cells. J Transl Med. 2009;7:20. doi: 10.1186/1479-5876-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin P, Wang E, Ren J, Childs R, Shin JW, Khuu H, Marincola FM, Stroncek DF. Differentiation of two types of mobilized peripheral blood stem cells by microRNA and cDNA expression analysis. J Transl Med. 2008 Jul 22;6(1):39. doi: 10.1186/1479-5876-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, Stroncek DF. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009 Sep 17;114(12):2530–41. doi: 10.1182/blood-2009-04-214403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin JW, Jin P, Fan Y, Slezak S, vid-Ocampo V, Khuu HM, Read EJ, Wang E, Marincola FM, Stroncek DF. Evaluation of gene expression profiles of immature dendritic cells prepared from peripheral blood mononuclear cells. Transfusion. 2008 Apr;48(4):647–57. doi: 10.1111/j.1537-2995.2007.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]