Abstract
Silicon (Si) is a beneficial element for plant growth. In barley (Hordeum vulgare), Si uptake by the roots is mainly mediated by a Si channel, Low Silicon1 (HvLsi1), and an efflux transporter, HvLsi2. However, transporters involved in the distribution of Si in the shoots have not been identified. Here, we report the functional characterization of a homolog of HvLsi1, HvLsi6. HvLsi6 showed permeability for Si and localized to the plasma membrane. At the vegetative growth stage, HvLsi6 was expressed in both the roots and shoots. The expression level was unaffected by Si supply. In the roots, HvLsi6 was localized in epidermis and cortex cells of the tips, while in the leaf blades and sheaths, HvLsi6 was only localized at parenchyma cells of vascular bundles. At the reproductive growth stage, high expression of HvLsi6 was also found in the nodes. HvLsi6 in node I was polarly located at the transfer cells surrounding the enlarged vascular bundles toward the numerous xylem vessels. These results suggest that HvLsi6 is involved in Si uptake in the root tips, xylem unloading of Si in leaf blade and sheath, and intervascular transfer of Si in the nodes. Furthermore, HvLsi2 was found to be localized at the parenchyma cell layer adjacent to the transfer cells with opposite polarity of HvLsi6, suggesting that the coupling of HvLsi6 and HvLsi2 is involved in the intervascular transfer of Si at the nodes. Si translocated via the enlarged vascular bundles is unloaded to the transfer cells by HvLsi6, followed by HvLsi2 to reload Si to the diffuse vascular bundles, which are connected to the upper part of the plant, especially the panicles, the ultimate Si sink.
Silicon (Si) is a beneficial element for plant growth. It enhances the resistance of plants to various biotic and abiotic stresses (Epstein, 1999; Ma and Takahashi, 2002; Ma and Yamaji, 2006). For example, Si reduces the epidemics of both leaf and panicle blast in rice (Oryza sativa; Datnoff and Rodrigues, 2005) and decreases the incidence of powdery mildew in cucumber (Cucumis sativus), barley (Hordeum vulgare), and wheat (Triticum aestivum; Fauteux et al., 2005). Si also suppresses insect pests such as stem borer (Chilo suppressalis), brown planthopper (Nilaparvata lugens), and rice green leafhopper (Nephotettix cincticeps; Savant et al., 1997). Resistance to the damage by wild rabbit in wheat is also enhanced by an increased amount of Si in leaf tissue (Cotterill et al., 2007). Si is also able to alleviate lodging, drought, and low- and high-temperature stresses (Ma, 2004). The beneficial effects of Si under phosphate deficiency, phosphate excess, and manganese and salt toxicity stresses have been observed in many plants (Ma and Takahashi, 2002). Usually, the more Si that accumulates in the shoots, the greater its effect in enhancing the plant’s response. This is because most effects of Si are expressed through the formation of silica gel, which is deposited on leaves, stems, and other organs of plants (Ma and Yamaji, 2006). Therefore, for the plant to benefit from Si, a high accumulation is required. However, Si accumulation greatly varies with plant species, and this difference has been attributed to the ability of plants to take up Si.
Transporters responsible for Si uptake by roots have been identified in several plant species, including barley, maize (Zea mays), pumpkin (Cucurbita moschata), rice, wheat (Ma et al., 2011), and most recently in horsetail (Equisetum arvense; EaNIP3s [for Nod26-like major intrinsic protein3]; Grégoire et al., 2012). Two different types of transporter, Si-permeable channel and efflux transporter, are involved in the Si-uptake process. Low Silicon1 (Lsi1) belongs to a NIP subfamily of aquaporin-like proteins and functions as a Si-permeable channel. Lsi1 in rice is localized in the distal side of root exodermis and endodermis (Ma et al., 2006), but Lsi1 in barley, maize, and pumpkin is localized in the epidermis and cortex (Chiba et al., 2009; Mitani et al., 2009b, 2011). On the other hand, Lsi2 functions as an efflux Si transporter and belongs to a putative anion transporter family without any similarity to Lsi1. Lsi2 in rice is also localized at the root exodermis and endodermis as Lsi1, but it is polarly localized at the proximal side (Ma et al., 2007). By contrast, Lsi2 in barley and maize is localized only to the endodermis of roots. Furthermore, these transporters do not show polar localization in barley and maize (Mitani et al., 2009a). Therefore, Si uptake mediated by Lsi1 and Lsi2 shows different pathways between rice and other plant species (Ma et al., 2011).
Following uptake by the roots through Lsi1 and Lsi2, Si is translocated to the aboveground part and distributed in different tissues. Lsi6, a homolog of Lsi1, is involved in xylem unloading of Si in rice (Yamaji et al., 2008). Lsi6 is localized on the adaxial side of the xylem parenchyma cells in the leaf sheaths and leaf blades. Knockout of Lsi6 resulted in altered distribution of Si in the leaf cells. Furthermore, at the reproductive growth stage of rice, Lsi6 is also highly expressed at the nodes (Yamaji and Ma, 2009). At node I below the panicle, Lsi6 is mainly localized at the xylem transfer cells with polarity facing toward the xylem vessel (Yamaji and Ma, 2009). Knockout of Lsi6 decreased Si accumulation in the panicle but increased Si accumulation in the flag leaf. These findings indicate that Lsi6 is also required for the intervascular transfer of Si in rice, transferring Si from the enlarged vascular bundles coming from the roots to the diffuse vascular bundles connected to the panicle.
Barley is a Si-accumulating species, although the accumulation extent is lower than that of rice. Transporters responsible for Si uptake in barley roots have been identified (Chiba et al., 2009; Mitani et al., 2009a); however, transporters for Si distribution in aboveground plant tissues are unknown. In this study, we functionally characterized a rice Lsi6 homolog gene in barley, HvLsi6, in terms of transport activity and expression pattern, as well as cellular and subcellular localizations. We found that HvLsi6 is probably involved in Si uptake in the root tip, xylem unloading in the leaf, and intervascular transfer of Si at the nodes in barley. We further found that HvLsi2 was also expressed in the nodes and involved in the intervascular transfer by coupling with HvLsi6.
RESULTS
Phylogenetic Analysis of HvLsi6
A BLAST search with the rice Lsi1 cDNA sequence against the barley full-length cDNA library detected the cDNA of HvLsi6. The open reading frame (ORF) of HvLsi6 is 900 bp long, encoding a peptide with 300 amino acids (Supplemental Fig. S1A). HvLsi6 exhibited 88.2% identity with rice Lsi6 at the amino acid level (Supplemental Fig. S1B). Similar to other Si-permeable channels, HvLsi6 is characterized by two Asn-Pro-Ala motifs and a distinct aromatic/Arg selectivity filter, Gly, Ser, Gly, and Arg (Supplemental Fig. S1A).
Si Transport Activity of HvLsi6 in Xenopus laevis Oocytes
To examine the transport activity of HvLsi6 for silicic acid, HvLsi6 was expressed in X. laevis oocytes and the uptake of silicic acid labeled with 68Ge was determined (Mitani et al., 2008). Compared with the control injected with water, oocytes injected with HvLsi6 complementary RNA (cRNA) showed a significantly higher uptake activity for Si (Fig. 1). This result indicates that, like OsLsi6, HvLsi6 is also permeable to silicic acid.
Figure 1.
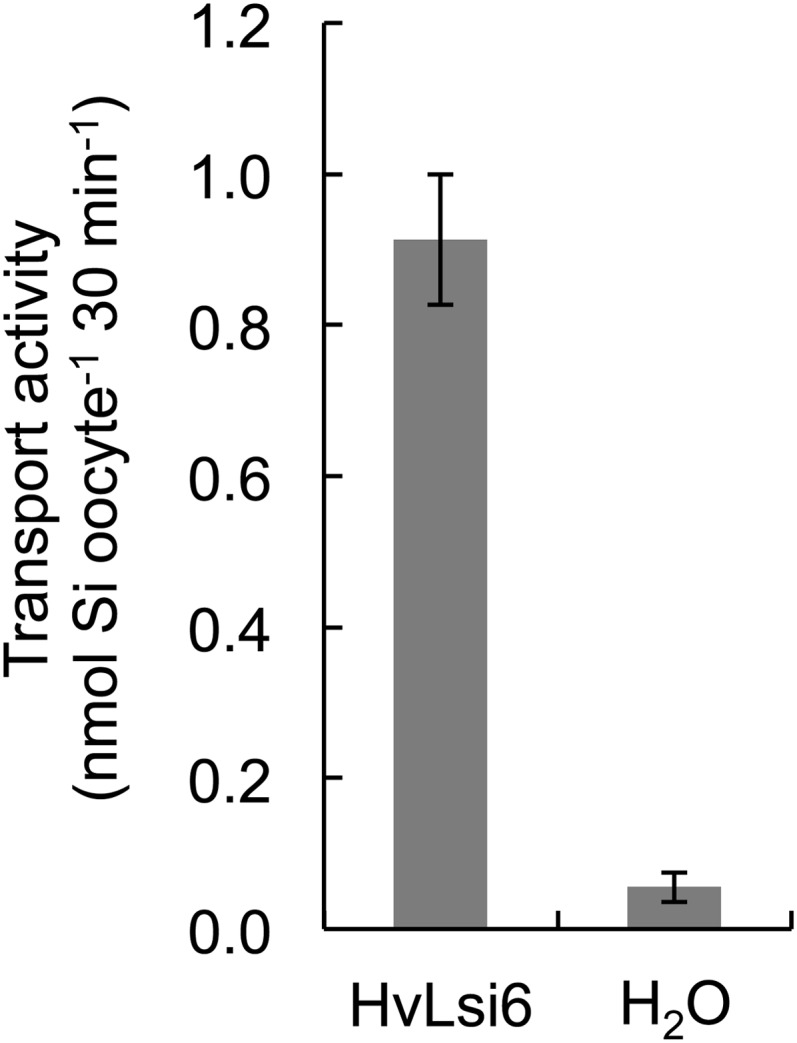
Transport activity for Si in X. laevis oocytes. cRNA of HvLsi6 or water as a negative control was injected into X. laevis oocytes. The oocytes were exposed to 1 mm silicic acid labeled with 68Ge. After 30 min of incubation, radioactivity in the oocytes was determined. Data are means ± sd of three biological replicates.
Expression Pattern of HvLsi6 at Different Growth Stages
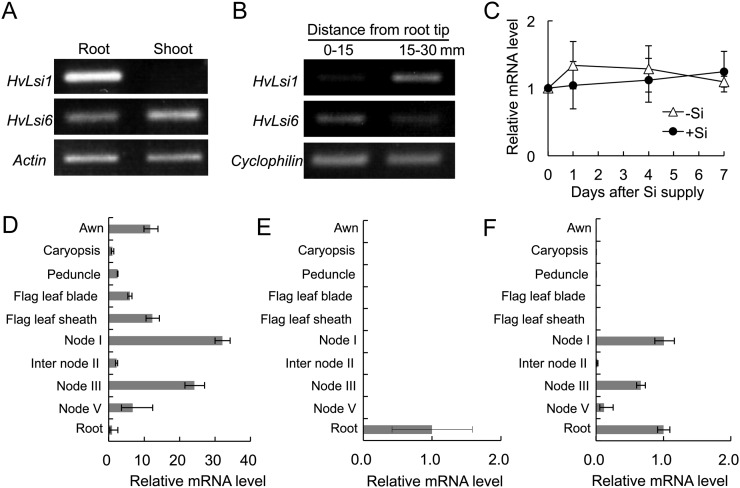
The expression pattern of HvLsi6 was investigated in different organs at different growth stages using semiquantitative and quantitative reverse transcription (RT)-PCR. At the vegetative growth stage, in contrast to HvLsi1, which was only expressed in the roots, HvLsi6 was expressed in both the roots and shoots (Fig. 2A). Spatial expression analysis showed that HvLsi6 was expressed in both the root tips (0–15 mm) and the mature region (15–30 mm; Fig. 2B), with a higher expression level in the root tips. By contrast, a higher expression of HvLsi1 was observed in the mature region of the roots (Fig. 2B; Chiba et al., 2009). Furthermore, the expression of HvLsi6 in the roots was not affected by supply of Si up to 7 d (Fig. 2C).
Figure 2.
Expression patterns of HvLsi6 in different organs at different growth stages. A, Expression of HvLsi6 in different organs. B, Spatial expression of HvLsi6 in the roots. C, Effect of Si on the expression of HvLsi6 in the roots. D to F, Expression of HvLsi6 (D), HvLsi1 (E), and HvLsi2 (F) in different organs at flowering stage. Expression was determined by quantitative RT-PCR. Expression relative to root is shown. Data in C to F are means ± sd of three biological replicates. Actin or Cyclophilin was used as an internal standard.
At the reproductive growth stage, HvLsi6 was expressed in the awn, peduncle, and nodes in addition to the roots, leaf sheath, and blades (Fig. 2D). Interestingly, the highest expression was found in node I. The expression of HvLsi1 and HvLsi2 was also examined at the reproductive growth stage. HvLsi1 was almost only expressed in the root (Fig. 2E), while HvLsi2 was also detected in the nodes in addition to the roots (Fig. 2F).
Intracellular and Cellular Localizations of HvLsi6
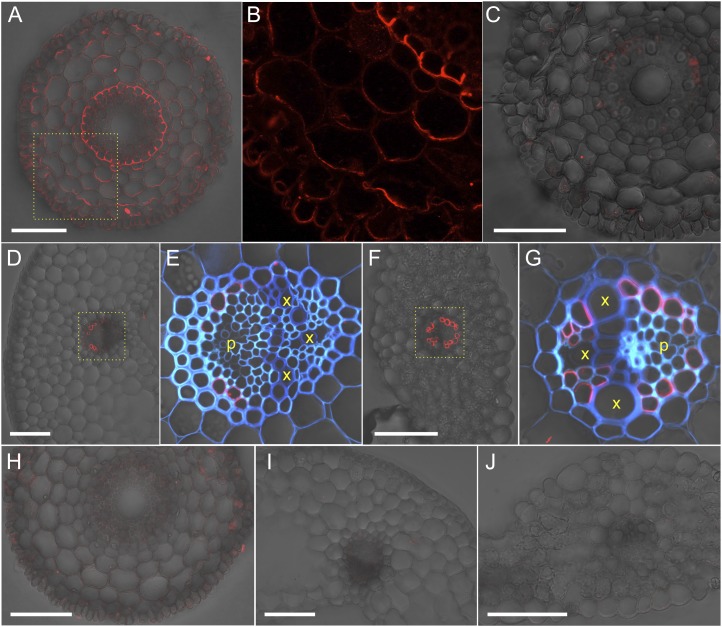
To investigate tissue and cellular localization, immunostaining using an antibody against HvLsi6 was performed in different plant organs. In the root tip, Lsi6 was localized in cell layers between the epidermis and endodermis (Fig. 3A). Higher intensity was observed at the distal side of each cell (Fig. 3B). In the mature root region, the signal was too weak to be detected (Fig. 3C), consistent with the expression level (Fig. 2B).
Figure 3.
Localization of HvLsi6 in roots and leaves. Immunostaining with anti-HvLsi6 antibody (A–G) or preimmune serum (H–J) is shown in the root tip (10 mm from the tip; A, B, and H), root mature region (C), leaf sheath (D, E, and I) and leaf blade (F, G, and J) of barley ‘Haruna-Nijo’. B, E, and G show magnified images of the yellow dotted box regions in A, D, and F, respectively, without transmitted light (B) or with cell wall autofluorescence (blue color in E and G). p, Phloem region; x, xylem vessel. Bars = 100 µm.
In the shoots, the signal was observed in both the leaf blades and sheaths. In either tissue, HvLsi6 was only localized at parenchyma cells of vascular bundles (Fig. 3, D–G). Staining with preimmune serum did not show any signal, indicating the specificity of this antibody in both root and shoot (Fig. 3, H–J).
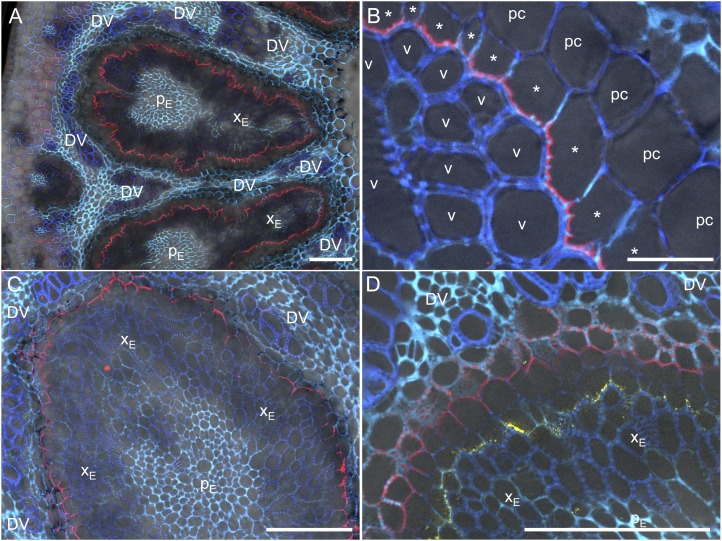
In node I, HvLsi6 was localized at specialized parenchyma cells surrounding the enlarged vascular bundles named xylem transfer cells (Fig. 4, A and B). Furthermore, HvLsi6 showed polar localization, facing toward the numerous xylem vessels in the xylem parenchyma cells of the bundles (Fig. 4B). Since HvLsi2 was also expressed in node I (Fig. 2F), we also investigated the localization of HvLsi2 with immunostaining using an antibody against HvLsi2 (Mitani et al., 2009a). HvLsi2 was localized at the outer parenchyma cell layer adjacent to the xylem transfer cells (Fig. 4, C and D). Furthermore, HvLsi2 showed opposite polarity from HvLsi6 (Fig. 4, C and D).
Figure 4.
Localization of HvLsi6 and HvLsi2 in node I. Immunostaining is shown in node I at the flowering stage with anti-HvLsi6 antibody (red color in A and B), anti-HvLsi2 antibody (red color in C), or double staining with anti-HvLsi6 (yellow) and anti-HvLsi2 (red) antibodies (D). Blue and cyan colors are UV autofluorescence from the cell wall. DV, Diffuse vascular bundle; pc, outer parenchyma cell layer next to the xylem transfer cell; v, xylem vessel; XE and PE, xylem and phloem regions of enlarged vascular bundles, respectively. Asterisks indicate xylem transfer cells. Bars = 100 µm (A, C, and D) and 20 µm (B).
The subcellular localization of HvLsi6 was investigated by expressing a GFP-HvLsi6 fusion in onion (Allium cepa) epidermal cells. GFP fluorescence from fused GFP-HvLsi6 was observed on the outer layer of a cell, whereas the signal from GFP alone was observed both in the cytoplasm and nucleus (Supplemental Fig. S2).
Spatial Si Uptake
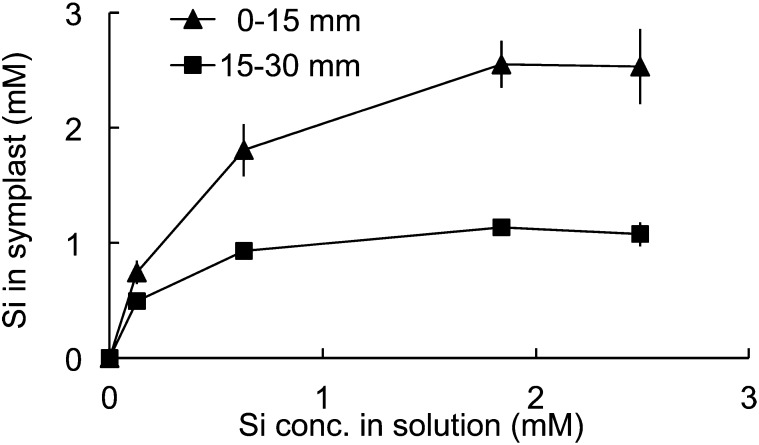
To examine the role of HvLsi6 in Si uptake, a kinetic experiment was conducted with different root segments. At either Si external concentration, Si in the cell sap was higher in the root tip region (0–15 mm) than in the basal root region (15–30 mm; Fig. 5).
Figure 5.
Root spatial kinetics of Si uptake. The seedlings were exposed to a nutrient solution containing different Si concentrations. After 6 h, the root tips (0–15 mm) and the basal root region (15–30 mm) were excised and the cell saps were extracted. Data are means ± sd of three biological replicates.
DISCUSSION
HvLsi6 encodes a plasma membrane-localized transporter for silicic acid (Fig. 1; Supplemental Fig. S2). Unlike HvLsi1, which is only expressed in the roots (Fig. 2; Chiba et al., 2009), HvLsi6 was expressed in various plant tissues in addition to the roots (Fig. 2), suggesting that HvLsi6 plays a role in both Si uptake and distribution in barley.
HvLsi6 Is Responsible for Si Uptake in the Root Tips
HvLsi6 was highly expressed in the root tips compared with the mature root region (Fig. 2B). The protein was polarly localized in epidermis and cortex of the root tip region (Fig. 3A). This expression pattern is different from that of HvLsi1, which is mainly expressed in the mature root zone (Chiba et al., 2009; Fig. 2B). The different expression pattern suggests that HvLsi1 and HvLsi6 play different roles in Si uptake, although both encode for the Si-permeable channel (Chiba et al., 2009; Fig. 1). HvLsi1 is responsible for Si uptake in the root mature region, whereas HvLsi6 is involved in Si uptake in the root tips. This is supported by the findings that Si uptake occurs in both the root tips and mature root region (Chiba et al., 2009). In barley, the Si uptake capacity from the root tips was higher than that from the mature root region (Chiba et al., 2009; Fig. 5). This spatial Si uptake capacity in barley is in contrast to that in rice. In rice, although OsLsi6 and OsLsi1 are also highly expressed in the root tips and mature root zones, respectively, the Si uptake capacity from the root tips was much lower than that from the mature root region (Yamaji and Ma, 2007). Knockout of OsLsi1 resulted in a significant decrease in Si uptake (Ma et al., 2002, 2006). However, knockout of OsLsi6 did not affect Si uptake (Yamaji et al., 2008), indicating that the contribution of OsLsi1 to Si uptake is much larger than that of OsLsi6.
The effect of Si supply on expression also differed between OsLsi6 and HvLsi6. OsLsi6 expression is down-regulated by Si supply (Yamaji et al., 2008), whereas the expression of HvLsi6 was unaffected by Si supply (Fig. 2C). A similar difference was also observed between OsLsi1 (Ma et al., 2006) and HvLsi1 (Chiba et al., 2009); OsLsi1 is down-regulated by Si, whereas HvLsi1 is not. These different responses to Si suggest that rice and barley have different mechanism for regulating Si uptake.
HvLsi6 Is Implicated in Xylem Unloading and Intervascular Transfer of Si
HvLsi6 in the leaf was localized to the parenchyma cells in the vascular bundles (Fig. 3). This cell specificity of localization is similar to but slightly different from that of OsLsi6 and ZmLsi6 (Yamaji et al., 2008; Mitani et al., 2009b). OsLsi6 and ZmLsi6 were only detected in the parenchyma cells adjacent to the xylem vessels in leaf. By contrast, HvLsi6 was also detected in the outer parenchyma cells surrounding the phloem area in addition to the xylem parenchyma cells (Fig. 3, E and G). In rice, knockout of OsLsi6 affects the silica deposition pattern in the leaf blades and sheaths. In the OsLsi6 knockout line, a fraction of abaxial epidermal cells were also silicified, an infrequent occurrence in the wild-type leaf blades (Yamaji et al., 2008). Furthermore, excretion of Si in the guttation fluid in the knockout line increased. These changes contributed to the altered pathway of Si. Since the knockout line of HvLsi6 is not available, it is not possible to investigate the effect of the disruption of HvLsi6 on the Si distribution pattern in barley leaves. However, based on the cell specificity of the localization, similar to OsLsi6 (Fig. 3; Yamaji et al., 2008), HvLsi6 is probably involved in xylem unloading, which release silicic acid out of the xylem in the leaf, although an additional role of the HvLsi6 expression around the phloem remains to be examined.
Among different organs, the highest Si is accumulated in the husk of rice and barley grains (Sangster et al., 2001). This accumulation is important for sustainable and high grain yield by protecting the grains from excessive transpiration and pathogen infection (Ma and Takahashi, 2002). Grains have a low transpiration compared with leaf blades; therefore, a transporter-mediated rather than transpiration-dependent distribution of Si is required. A final diverging point of Si distribution is node I, which connects with panicle and flag leaf. Recently, OsLsi6 expressed in rice node I was demonstrated to be involved in the intervascular transfer of Si at the nodes (Yamaji and Ma, 2009). OsLsi6 is localized at the xylem transfer cells with polarity facing toward the xylem vessel. These cells are located at the outer boundary region of the enlarged vascular bundles and characterized by a large surface area due to cell wall ingrowth (Busby and O’Brien, 1979; Chonan et al., 1985). Knockout of OsLsi6 decreased Si accumulation in the panicles but increased Si accumulation in the flag leaf (Yamaji and Ma, 2009). Therefore, OsLsi6 is required for the transfer of Si from the enlarged vascular bundles coming from the roots to the diffuse vascular bundles connected to the panicles. Similar to OsLsi6, HvLsi6 is also polarly localized at the xylem transfer cells (Fig. 4), indicating its involvement in the intervascular transfer of Si.
Furthermore, we found that HvLsi2 is also highly expressed in the nodes at the reproductive stage (Fig. 2F). HvLsi2 is an efflux transporter of silicic acid for uptake and localized at the root endodermis without polarity (Mitani et al., 2009a). In node I, HvLsi2 is localized at the outer parenchyma cell layer adjacent to the xylem transfer cells (Fig. 4, C and D) with opposite polarity of HvLsi6 (Fig. 4D). These results suggest that HvLsi2 probably plays a role in releasing Si into the diffuse vascular bundles. Coupling of HvLsi2 and HvLsi6 is probably required for the efficient intervascular transfer of Si.
The importance of the intervascular transfer of nutrients between enlarged vascular bundles and diffuse vascular bundles through the “parenchyma cell bridge” was suggested by anatomical and tracer studies (Kawahara et al., 1974; Obata and Kitagishi, 1980). However, the molecular mechanisms for this transport are still poorly known. HvLsi6 and HvLsi2 identified in this study are, to our knowledge, the first example of a functional pair of transporters for intervascular transfer at the nodes. HvLsi6 and HvLsi2 showed different polar localization (Fig. 4), but the mechanisms underlying polar localization at different cells remain to be examined in the future.
In conclusion, HvLsi6 is a plasma membrane-localized transporter for silicic acid. It is probably involved in Si uptake in the root tips, xylem unloading in the leaves, and intervascular transfer in the nodes of barley. Furthermore, there is a coupling system mediated by HvLsi6 and HvLsi2 for the intervascular transfer in barley nodes.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Barley (Hordeum vulgare ‘Haruna-Nijo’) was used for all experiments. Seeds were soaked in deionized water for 2 h and then incubated overnight on moist paper in the dark at 20°C. Germinated seeds were transferred to a net floated on a continuously aerated solution containing 0.5 mm CaCl2 (pH 5.6) in the dark. Seedlings were then transferred into the aerated one-fifth-strength Hoagland solution (pH 6.0) as described by Chiba et al. (2009) for further growth.
Phylogenetic Analysis of HvLsi6
Peptide sequence alignment was analyzed by ClustalW using default settings (http://clustalw.ddbj.nig.ac.jp/). The phylogenetic tree was constructed using the neighbor-joining algorithm with MEGA4 software (Tamura et al., 2007; http://megasoftware.net/) after ClustalW alignment with 1,000 bootstrap trials.
Si Influx Transport Activity Assay
The ORF of HvLsi6 cDNA was amplified by RT-PCR with the following primers: 5′-GAAAGATCTATGTCGGTGACTTCCAACACGCCG-3′ and 5′-GAAAGATCTTCAGACATGGTCGAACTCGTCGAC-3′. The fragment containing the ORF was inserted into the BglII site of a Xenopus laevis oocyte expression vector, pXbG-ev1 (Preston et al., 1992). cRNA preparation, microinjection into oocytes, and Si influx transport activity assay were performed as described previously (Mitani et al., 2008).
Expression Pattern
Seedlings (10 d old) grown hydroponically were separated into roots and shoots and then subjected to RNA extraction. To investigate the effect of Si supply on gene expression, seedlings (10 d old) were cultured in a one-strength Hoagland solution containing 0 or 1 mm silicic acid. Silicic acid was prepared by passing K2SiO3 through a cation-exchange column (Ma et al., 2001). The roots were sampled at 0, 1, 4, and 7 d after the addition of silicic acid. Different root regions (0–15 and 15–30 mm from the apex) were also sampled from the roots (5 d old). At the reproductive stage, different organs including the roots, leaf blade and sheath, awn, and nodes I, III, and V were sampled. All samples were immediately frozen in liquid nitrogen. Total RNA was extracted from frozen plant samples by using the RNeasy plant mini kit (Qiagen) and then converted to cDNA followed by DNase I treatment using the protocol attached to SuperScript II (Invitrogen). Specific cDNAs were amplified by SYBR premix ExTaq (Takara) and real-time RT-PCR (ABI Prism 7500; Applied Biosystems) with the following primer sets: 5′-TTATGCGTGTGCGTGTGTGT-3′ and 5′-TGAACAGAGCGAGAGAGAGCA-3′ for HvLsi1, 5′-GACTCTGGTCATGGTGTCAGC-3′ and 5′-GGCTGGAAGAGGACCTCAGG-3′ for Actin, 5′-CCTGTCGTGTCGTCGGTCTAAA-3′ and 5′-ACGCAGATCCAGCAGCCTAAAG-3′ for Cyclophilin, 5′-GTCCTAGCCTCCGTTTTCGT-3′ and 5′-CCATCTCCCTCTCCCTCTCTT-3′ for HvLsi2, and 5′-GCGTGAGTACATGGCTGGTG-3′ and 5′-GACGCAGTACAAAGCGAAAGAG-3′ for HvLsi6. All experiments were replicated three times.
Construction and Transient Expression Analysis of an HvLsi1 GFP Fusion
The ORF of the HvLsi6 cDNA fragment was amplified using the primers 5′-GATGTACAAGATGTCGGTGACTTCCAACACGCCGAC-3′ and 5′-GATGTACAGTCAGACATGGTCGAACTCGTCG-3′. The HvLsi6 fragment was ligated to the 3′ end of GFP and placed under the control of the cauliflower mosaic virus 35S promoter in pBluescript SK−. Gold particles with a diameter of 1 µm, coated with pGFP-HvLsi6 or GFP control, were introduced into onion (Allium cepa) epidermal cells using particle bombardment (PDS-1000/He Particle Delivery System; Bio-Rad) with 1,100 ψ pressure disks. GFP fluorescence was observed with Axio Imager with Apotome (Carl Zeiss) using appropriate filters.
Immunohistological Staining
Antibody against HvLsi6 was prepared by immunizing rabbits with the synthetic peptide C-QRSMAVDEFDHV (positions 289–300 of HvLsi6). Antibody against HvLsi2 has been described by Mitani et al. (2009a). HvLsi6 immunostaining was performed in the roots, leaf sheaths and blades, and node I according to Yamaji and Ma (2007). Immunostaining with HvLsi2 was also performed in node I. A sliced section 100 µm thick and Axio Imager with Apotome (Carl Zeiss) were used for observation.
Spatial Kinetic Uptake Experiment
Seedlings (5 d old) were exposed to a solution containing 0, 0.1, 0.6, 1.8, and 2.5 mm silicic acid. Silicic acid was prepared according to Ma et al. (2001). After 6 h, the roots were washed with distilled water three times and then tips (0–15 mm) and the basal root region (15–30 mm) were excised with a razor. Three root segments were poured together for one sample, and three samples were prepared for each region. The samples were put in an Ultra free-MC centrifugal filter unit (Millipore) and centrifuged at 2,000g for 15 min at 4°C to remove apoplastic solution. After being frozen at −80°C overnight, the samples were thawed at room temperature for 30 min and the root cell sap solution was obtained by centrifuging at 2,000g for 15 min at 4°C. Si concentration in the root cell sap was determined by the colorimetric molybdenum blue method (Mitani and Ma, 2005).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AB447484.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Alignment and phylogenetic tree of HvLsi6.
Supplemental Figure S2. Subcellular localization of HvLsi6.
Supplementary Material
Glossary
- Si
silicon
- ORF
open reading frame
- cRNA
complementary RNA
- RT
reverse transcription
References
- Busby CH, O’Brien TP. (1979) Aspects of vascular anatomy and differentiation of vascular tissues and transfer cells in vegetative nodes of wheat. Aust J Bot 27: 703–711 [Google Scholar]
- Chiba Y, Mitani N, Yamaji N, Ma JF. (2009) HvLsi1 is a silicon influx transporter in barley. Plant J 57: 810–818 [DOI] [PubMed] [Google Scholar]
- Chonan N, Kawahara H, Matsuda T. (1985) Ultrastructure of elliptical and diffuse bundles in the vegetative nodes of rice. Jpn J Crop Sci 54: 393–402 [Google Scholar]
- Cotterill JV, Watkins RW, Brennon CB, Cowan DP. (2007) Boosting silica levels in wheat leaves reduces grazing by rabbits. Pest Manag Sci 63: 247–253 [DOI] [PubMed] [Google Scholar]
- Datnoff LE, Rodrigues FA. (February, 2005) The role of silicon in suppressing rice diseases. APSnet Features. http://dx.doi.org/10.1094/APSnetFeature-2005-0205
- Epstein E. (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50: 641–664 [DOI] [PubMed] [Google Scholar]
- Fauteux F, Rémus-Borel W, Menzies JG, Bélanger RR. (2005) Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol Lett 249: 1–6 [DOI] [PubMed] [Google Scholar]
- Grégoire C, Rémus-Borel W, Vivancos J, Labbé C, Belzile F, Bélanger RR. (2012) Discovery of a multigene family of aquaporin silicon transporters in the primitive plant Equisetum arvense. Plant J (in press) [DOI] [PubMed] [Google Scholar]
- Kawahara H, Chonan N, Matsuda T. (1974) Studies on morphogenesis in rice plants. 7. The morphology of vascular bundles in the vegetative nodes of the culm. Jpn J Crop Sci 43: 389–401 [Google Scholar]
- Ma JF. (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50: 11–18 [Google Scholar]
- Ma JF, Goto S, Tamai K, Ichii M. (2001) Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiol 127: 1773–1780 [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Takahashi E. (2002) Soil, Fertilizer, and Plant Silicon Research in Japan. Elsevier, Amsterdam
- Ma JF, Tamai K, Ichii M, Wu GF. (2002) A rice mutant defective in Si uptake. Plant Physiol 130: 2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. (2006) A silicon transporter in rice. Nature 440: 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11: 392–397 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. (2007) An efflux transporter of silicon in rice. Nature 448: 209–212 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani-Ueno N. (2011) Transport of silicon from roots to panicles in plants. Proc Jpn Acad Ser B Phys Biol Sci 87: 377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani N, Chiba Y, Yamaji N, Ma JF. (2009a) Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 21: 2133–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani N, Ma JF. (2005) Uptake system of silicon in different plant species. J Exp Bot 56: 1255–1261 [DOI] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ago Y, Iwasaki K, Ma JF. (2011) Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J 66: 231–240 [DOI] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ma JF. (2008) Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch Eur J Physiol 456: 679–686 [DOI] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ma JF. (2009b) Identification of maize silicon influx transporters. Plant Cell Physiol 50: 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, Kitagishi K. (1980) Investigation on pathway of Zn in vegetative node of rice plants by autoradiography: behavior of zinc in rice plants (III). Jpn J Soil Sci Plant Nutr 51: 297–301 [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256: 385–387 [DOI] [PubMed] [Google Scholar]
- Sangster AG, Hodson MJ, Tubb HJ. (2001) Silicon deposition in higher plants. In LE Datonoff, GH Snyder, GH Korndörfer, eds, Silicon in Agriculture. Elsevier Science, New York, pp 85–114
- Savant NK, Snyder GH, Datnoff LE. (1997) Silicon management and sustainable rice production. In DL Sparks, ed, Advances in Agronomy, Vol 58. Academic Press, San Diego, pp 151–199
- Tamura K, Dudley J, Nei M, Kumar S. (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Yamaji N, Mitatni N, Ma JF. (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20: 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF. (2007) Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol 143: 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF. (2009) A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 21: 2878–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.