Abstract
In some species, a crucial role has been demonstrated for the seed endosperm during germination. The endosperm has been shown to integrate environmental cues with hormonal networks that underpin dormancy and seed germination, a process that involves the action of cell wall remodeling enzymes (CWREs). Here, we examine the cell wall architectures of the endosperms of two related Brassicaceae, Arabidopsis (Arabidopsis thaliana) and the close relative Lepidium (Lepidium sativum), and that of the Solanaceous species, tobacco (Nicotiana tabacum). The Brassicaceae species have a similar cell wall architecture that is rich in pectic homogalacturonan, arabinan, and xyloglucan. Distinctive features of the tobacco endosperm that are absent in the Brassicaceae representatives are major tissue asymmetries in cell wall structural components that reflect the future site of radicle emergence and abundant heteromannan. Cell wall architecture of the micropylar endosperm of tobacco seeds has structural components similar to those seen in Arabidopsis and Lepidium endosperms. In situ and biomechanical analyses were used to study changes in endosperms during seed germination and suggest a role for mannan degradation in tobacco. In the case of the Brassicaceae representatives, the structurally homogeneous cell walls of the endosperm can be acted on by spatially regulated CWRE expression. Genetic manipulations of cell wall components present in the Arabidopsis seed endosperm demonstrate the impact of cell wall architectural changes on germination kinetics.
Angiosperms are a diverse group of seed plants that reproduce by a double fertilization event; the first produces a zygote and the second a specialized nutritive tissue known as the endosperm. The endosperm and the maternally derived testa (seed coat) evolved to protect the embryo until conditions are favorable for germination and establishment of the next generation (Rajjou and Debeaujon, 2008; Linkies et al., 2010). Endosperm from cereals/grasses, such as maize (Zea mays), barley (Hordeum vulgare), and wheat (Triticum aestivum), is vital for human and animal nutrition and is therefore of global economic importance (Olsen, 2007). In many seeds, such as some representatives of the Brassicaceae, the endosperm is entirely absent at seed maturity, the storage reserves having been absorbed by the cotyledons during embryo development. Arabidopsis (Arabidopsis thaliana) and Lepidium (Lepidium sativum) are notable exceptions in that they have retained a thin layer of endosperm tissue in the mature seed (Müller et al., 2006; Linkies and Leubner-Metzger, 2012).
Some seeds exhibit primary dormancy at maturity that has been induced by abscisic acid (ABA; Hilhorst, 1995; Kucera et al., 2005). In its simplest sense, dormancy can be thought of as a block to germination of an intact viable seed under favorable conditions (Hilhorst, 1995; Bewley, 1997). A more sophisticated definition was proposed by Baskin and Baskin (2004), who state that a dormant seed does not have the capacity to germinate in a specified period of time under any combination of normal physical environmental factors that are otherwise favorable for its germination. Seed dormancy can be imposed by the embryo, the seed coat (including the endosperm), or a combination of both depending on the plant species (Bewley, 1997).
The endosperm has been shown to be an important regulator of germination potential in several systems, including tomato (Solanum lycopersicum; Groot et al., 1988; Toorop et al., 2000), tobacco (Nicotiana tabacum; Leubner-Metzger et al., 1995; Petruzzelli et al., 2003), Arabidopsis (Bethke et al., 2007), and Lepidium (Müller et al., 2006; Linkies et al., 2009; Voegele et al., 2011). Arabidopsis continues to be an important model for elucidating the hormonal and genetic networks that regulate dormancy and germination (Kucera et al., 2005; Holdsworth et al., 2008), and new bioinformatic methods are providing insights into the evolutionary conservation of such networks in angiosperms (Bassel et al., 2011). Research using the close relative Lepidium, whose larger size makes it amenable to biomechanical techniques, has given insight into the hormonal control of endosperm weakening during germination and established that the mechanism of control is conserved between Arabidopsis, Lepidium, and tobacco (Müller et al., 2006; Linkies et al., 2009; Voegele et al., 2011). It has been reported that ABA is a key regulator of germination in tobacco, Arabidopsis, and Lepidium, controlling the process of endosperm rupture but not testa rupture (Leubner-Metzger et al., 1995; Petruzzelli et al., 2003; Müller et al., 2006). Microarray analyses of ABA-treated Arabidopsis and Lepidium seeds revealed that many cell wall remodeling enzyme (CWRE) genes are down-regulated upon exogenous application of ABA (Penfield et al., 2006; Linkies et al., 2009). Therefore, it follows that ABA impacts cell wall remodeling, which influences germination kinetics. The endosperm is therefore an important control tissue for seed germination and represents a useful model to investigate cell wall architectures and their remodeling.
Cell walls are robust, multifunctional structures that not only protect cells from biotic and abiotic stresses, but also regulate growth, physiology and development (Albersheim et al., 2010). Cell walls are fibrous composites in which cellulose microfibrils are coextensive with/cross-linked by noncellulosic polysaccharides. In dicotyledonous plants, xyloglucan (XG) is a major polymer that can cross-link cellulose (Cosgrove, 2000). Load-bearing fibrous networks impart tensile strength to cell walls and are embedded in more soluble, gel-like matrices of pectic polysaccharides, glycoproteins, proteins, ions, and water. The constituent pectic polymers are currently classified as homogalacturonan (HG), rhamnogalacturonan I [RG-I; also comprising arabinans and type 1 (arabino)galactans as side branches] and rhamnogalacturonan II, and xylogalacturonan (XGA) (Willats et al., 2001; Caffall and Mohnen, 2009). Pectins are involved in a diverse range of processes, including the regulation of intercellular adhesion/cell separation at the middle lamella, regulating the ionic status, and the porosity of cell walls that influences the access of CWREs to substrates (Willats et al., 2001). Noncellulosic polysaccharides exhibit numerous structural elaborations and differ in their glycan, methyl, and acetyl substitution (Caffall and Mohnen, 2009; Burton et al., 2010). Such modifications have the potential to impact their functionality, including their ability to interact with other wall components and their susceptibility to degradation and modification by CWREs.
Studies using Arabidopsis (Iglesias-Fernández et al., 2011), Lepidium (Morris et al., 2011), and tomato (Groot et al., 1988) have highlighted a role for endo-β-mannanases (EBMs), enzymes that degrade heteromannan polysaccharides, during seed germination. In hard seeds with heteromannan-rich endosperms, such as carob (Ceratonia siliqua), date (Phoenix dactylifera), Chinese senna (Senna obtusifolia), and fenugreek (Trigonella foenum-graecum), however, it has been proposed that thinner walls in the micropylar endosperm (ME) and not EBM activity are responsible for allowing radicle protrusion during germination (Gong et al., 2005). Therefore, enzymatic cell wall remodeling and native cell wall architectural asymmetries both have the potential to impact on germination.
Although studies on the molecular networks controlling germination have indicated a role for several classes of CWREs in endosperm remodeling and the promotion of germination (Penfield et al., 2006; Kanai et al., 2010; Morris et al., 2011), there is a paucity of information relating to the characterization of such changes at the cell wall level and, indeed, cell wall structures themselves. This study focuses on the targets of CWRE genes currently thought to be involved in seed germination (i.e. cellulose, XG, heteromannan, and pectic polysaccharides). We show that all three seeds possess a similar core cell wall architecture containing unesterified HG, arabinan, and XG. In tobacco, the core cell wall architecture is restricted to the ME, whereas in Arabidopsis and Lepidium, this architecture is observed throughout the endosperm. A further unique feature of the tobacco endosperm is abundant heteromannan. We also outline, using Arabidopsis, to what extent cell wall components contribute to the regulation of seed germination.
RESULTS
Seeds of the three main species used in this study are shown in Figure 1A. Our study focused on determining to what extent cell wall structures were conserved between close relatives (Arabidopsis and Lepidium) and different families (Brassicaceae and Solanaceae). The larger size of Lepidium and tobacco seeds makes them particularly amenable to biomechanical analyses. Arabidopsis seeds are too small for such methods; however, the associated molecular genetic resources allow mutant analysis and insights into the roles of cell wall structures and remodeling.
Figure 1.
Seed structure of the three species studied. A, From left to right, mature dry seeds of Arabidopsis, Lepidium, and tobacco. B, Bright-field micrographs of longitudinal medial sections of 3-h-imbibed mature seeds stained with toluidine blue O showing embryo (Em), cotyledons (C), radicle (R), testa (T), ME, PE, CE, and mucilage (M). [See online article for color version of this figure.]
Arabidopsis and Lepidium seeds exhibit a similar morphology. The seeds are classified as Foliate Axil Type 2, according to the phylogeny outlined by Finch-Savage and Leubner-Metzger (2006). The embryos of both seeds are hooked, with the cotyledons lying adjacent to the radicle, whereas tobacco seeds are classified as Linear Axil type and possess a straight embryo (Fig. 1A). The Arabidopsis embryo is surrounded by a single layer of endosperm cells. The endosperm adjacent to the radicle is the ME, the endosperm region adjacent to the cotyledons is the chalazal endosperm (CE), and the region between ME and CE is known as the peripheral endosperm (PE; Fig. 1B). Lepidium seeds are approximately 5 times longer and wider than Arabidopsis seeds yet also possess an endosperm that is a single cell layer (one to two cells thick at the ME apex; Fig. 1B). Both Arabidopsis and Lepidium seeds are myxospermous (i.e. the dry seed extrudes pectin-rich mucilage from mucilage secretory cells in the testa upon imbibition). Tobacco seeds, by contrast, do not release mucilage upon imbibition. The tobacco endosperm comprises three to five cell layers. In Arabidopsis and Lepidium, the endosperm is thickest at the ME, whereas in tobacco, with a much thicker endosperm, the ME is not the thickest region of the endosperm.
The Arabidopsis Seed Endosperm Has a Different Cell Wall Architecture Than the Embryo
Labeling of sections of 3-h-imbibed Arabidopsis seeds with a carbohydrate-binding module (CBM) directed to crystalline cellulose, designated CBM3a (Tormo et al., 1996), revealed cellulose in all Arabidopsis seed cell walls (Fig. 2A). CBM3a binding to endosperm walls was uniform but slightly weaker than to the embryo or testa (Supplemental Fig. S1), whereas Calcofluor White, which binds to cellulose and other β-linked glycans (Herth and Schnepf, 1980), bound uniformly to embryo, endosperm, and testa (Fig. 2B).
Figure 2.
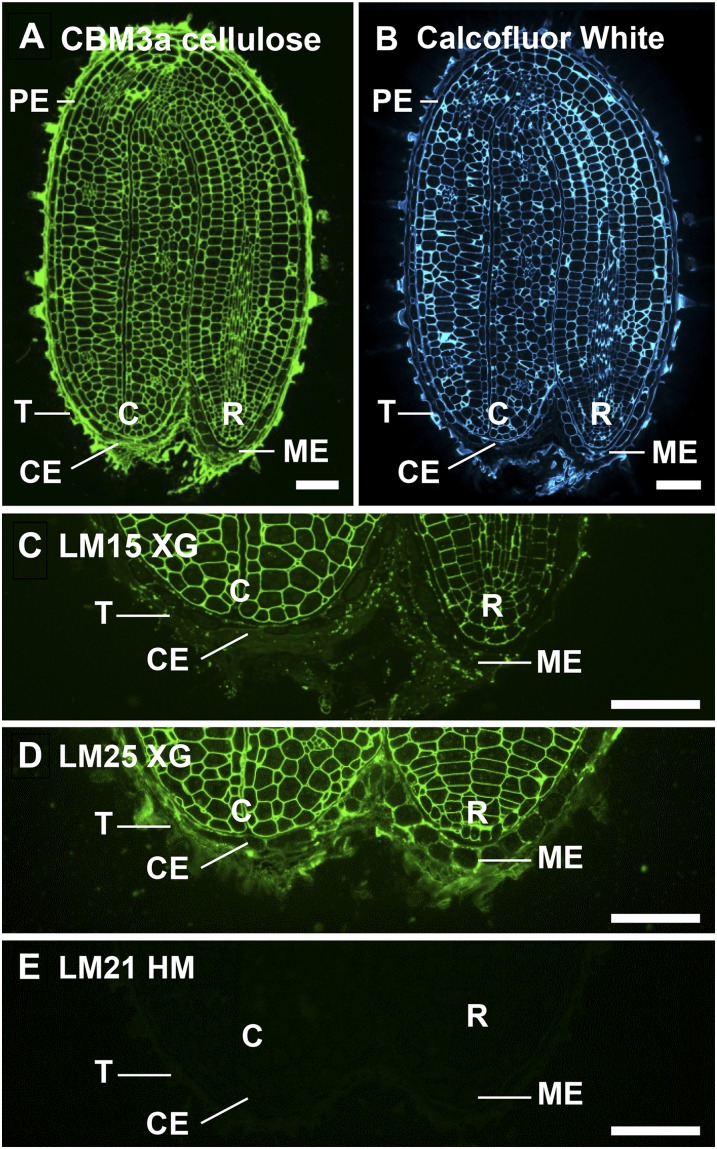
In situ localization of cellulose and noncellulosic polysaccharides in medial longitudinal sections of 3-h-imbibed Arabidopsis seeds. A and B, Whole seed sections labeled with the probes CBM3a and Calcofluor White. CBM3a binding revealed cellulose in all cell walls. C to E, ME and CE seed regions labeled with probes LM15 XG, LM25 XG, and LM21 HM. LM15 XG was detectable in embryo but not endosperm cell walls, whereas LM25 XG was detectable in testa, endosperm, and embryo cell walls. No heteromannan (HM) was detectable in the seed. C, Cotyledons; R, radicle; T, testa. Bars = 50 µm. [See online article for color version of this figure.]
Matrix components of seed cell walls were explored using a range of monoclonal antibodies directed to the expected component cell wall polymers. LM15, a probe directed to a xylosylated epitope of XG (Marcus et al., 2008), bound strongly and uniformly to the embryo (Fig. 2C) but not to endosperm cell walls. A related XG probe, LM25, directed toward a galactosylated XG epitope (Pedersen et al., 2012), bound to embryo cell walls and slightly weaker to endosperm cell walls (Fig. 2D). Notably, the antibody LM21, which binds to heteromannan (Marcus et al., 2010), or the heteromannan-directed carbohydrate-binding module CBM27 (Marcus et al., 2010) did not bind to sections (Fig. 2E and Supplemental Fig. S1, respectively).
The occurrence of HG in seed cell walls was investigated with a panel of probes with specificities for HG with differing degrees and patterns of methyl-esterification. Monoclonal antibody LM19 has a preference for HG with a low degree of methyl-esterification (Verhertbruggen et al., 2009a; Marcus et al., 2010). LM19 bound uniformly but weakly to cell walls of Arabidopsis embryos, mainly at intercellular spaces. The LM19 epitope was particularly abundant in endosperm cell walls and was detectable at the surface of the testa (Fig. 3A). LM20, which has a preference for high methyl-ester HG (Verhertbruggen et al., 2009a; Marcus et al., 2010) bound exclusively to testa columella in Arabidopsis (Supplemental Fig. S1). The relative binding of these and all cell wall probes used in this study are shown in Supplemental Figure S1.
Figure 3.
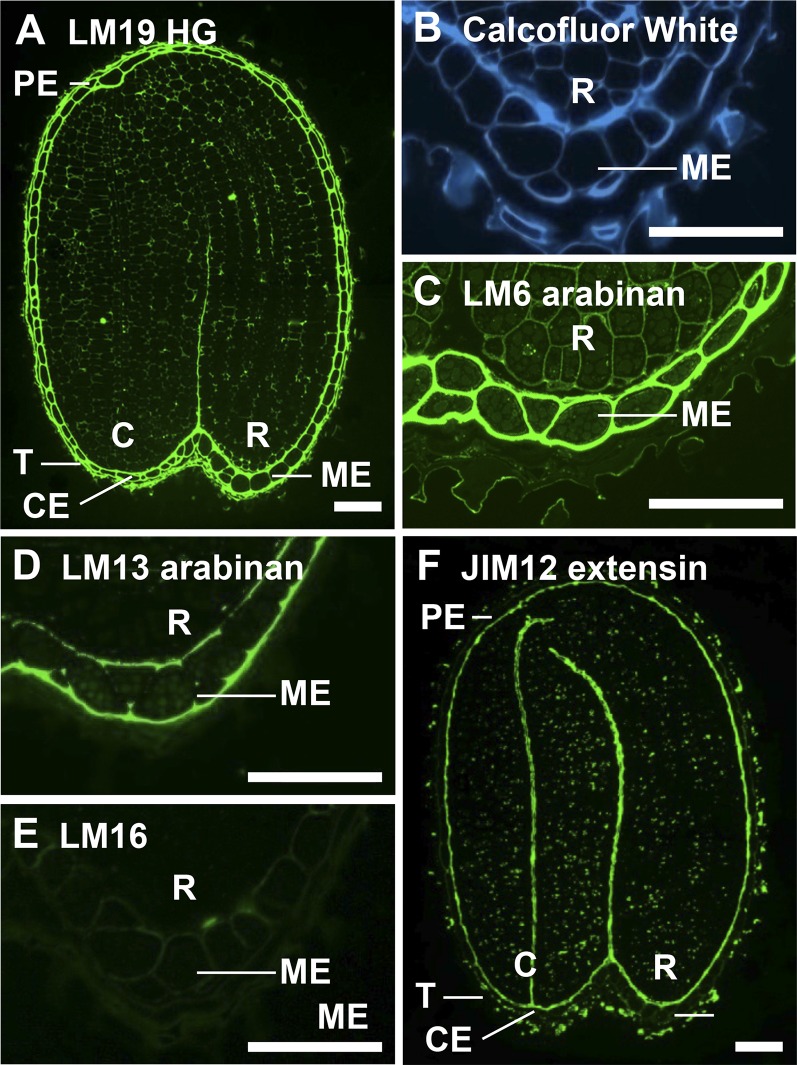
In situ localization of pectic polysaccharides and extensin in medial longitudinal sections of 3-h-imbibed Arabidopsis seeds. A, Immunolabeling with LM19 revealed the abundance of unesterified HG in the endosperm. B, Calcofluor White labeled all cell walls. Labeling with anti-arabinan probes revealed the spatial heterogeneity of arabinan in the endosperm. C, LM6 arabinan was uniformly distributed through endosperm cell walls. D, LM13 bound to the outer walls of the endosperm and weakly to transverse endosperm walls. E, The LM16 epitope was weakly detectable in endosperm walls. F, JIM12 labeling of extensin indicated restricted occurrence at the embryo surface/inner face of the endosperm and testa surface. C, Cotyledons; R, radicle; T, testa. Bars = 50 µm. [See online article for color version of this figure.]
Antibodies LM5 and LM6 bind to (1→4)-β-d-galactan and (1→5)-α-l-arabinan, respectively (Jones et al., 1997; Willats et al., 1998). These polysaccharides are thought to mainly occur as side chains of pectic RG-I (Willats et al., 2001), although (1→5)-α-l-arabinan can exist as a free polymer (Beldman et al., 1997) and has been identified in cytosolic water-soluble heteroglycans in Arabidopsis leaves (Fettke et al., 2005) and in arabinogalactan proteins in Physcomitrella patens (Lee et al., 2005). Immunolabeling of sections with LM5 revealed that galactan was present but not particularly abundant in either embryo or endosperm cell walls (data not shown). The LM6 epitope was present in embryo cell walls and was particularly abundant in the endosperm (Fig. 3C). LM6 binds to short oligoarabinosides and can bind to branched arabinan structures, while a related antiarabinan probe, designated LM13, has a preference for longer linear oligoarabinosides (Verhertbruggen et al., 2009b). LM13 revealed spatial heterogeneity of arabinan structure in the endosperm as the epitope was found to be restricted to the inner and outer walls of the endosperm but absent from transverse cell walls (Fig. 3D).
Arabinans can be processed in cell walls by a variety of arabinan-modifying enzymes, including α-l-arabinofuranosidases (Chávez Montes et al., 2008), and embryo cell wall arabinans have been reported to be metabolized during germination (Gomez et al., 2009). The antibody LM16 is proposed to bind to the residue of α-l-arabinofuranosidase action (Verhertbruggen et al., 2009b). The LM16 epitope was weakly detectable in endosperm cell walls (Fig. 3E).
Extensins are cell wall proteins belonging to the Hyp-rich glycoprotein superfamily (Kieliszewski and Lamport, 1994). A range of probes were used to locate extensin in Arabidopsis. Of those used, LM1 (Smallwood et al., 1995) and JIM12 (Smallwood et al., 1994) bound to Arabidopsis sections, both probes exhibited the same binding profile, and the abundance of the JIM12 epitope at the embryo surface is shown in Figure 3F.
In summary, the in situ cell wall epitope detection study suggests that Arabidopsis endosperm cell walls comprise cellulose, unesterified HG, arabinan, and XG polysaccharides. This architecture is distinct from embryo cell walls, which are more cellulose and XG rich, with lesser amounts of unesterified HG and arabinan.
Lepidium and Arabidopsis Endosperm Cell Wall Architectures Are Similar But with Structural Distinctions
Lepidium and Arabidopsis are closely related species that, with the exception of their size, exhibit similar seed structures (Linkies and Leubner-Metzger, 2012). To determine whether this similarity extends to cell wall architecture, analyses of 3-h-imbibed resin-embedded Lepidium seeds were performed. As with Arabidopsis, Calcofluor White bound strongly to all cell walls of the embryo, endosperm, and testa (Fig. 4A). The binding profile was the same for CBM3a (data not shown).
Figure 4.
In situ localization of cell wall epitopes in medial longitudinal sections of 3-h-imbibed Lepidium seeds. A, Calcofluor White labeling of Lepidium showing cotyledons (C), radicle (R), testa (T), ME, CE, and PE. B, The LM15 XG epitope was uniformly distributed in embryo cell walls. C, LM19 bound strongly and uniformly to endosperm, testa, and mucilage. D, The LM8 epitope was restricted to the inner face of the endosperm and surface of the testa. E and F, The LM15 XG and CCRCM1 XG epitopes were restricted to the inner wall in the endosperm. G, A related XG probe LM25 bound more extensively than LM15 and CCRCM1. CCRCM1 and LM25 both bound strongly to embryo cell walls. H and I, Localization of cell wall arabinans using LM6 and LM13 revealed spatial heterogeneity; the LM6 epitope was most abundant at the inner face of the endosperm, while LM13 bound more uniformly to endosperm walls. J, LM16 strongly labeled the testa surface and weakly labeled endosperm cell walls. I, Extensin recognized by JIM20 was abundant in the endosperm and at the inner face of the ME. Bars = 50 µm. [See online article for color version of this figure.]
Immunolocalization of XG in Lepidium sections revealed the LM15 XG epitope to be abundant in embryo cell walls, at the surface of the seed testa, and, in contrast with Arabidopsis, abundant in endosperm cell walls (Fig. 4B). Interestingly, LM15 labeling of endosperm cell walls revealed, to our knowledge, a hitherto unseen spatial distribution of XG polysaccharides; rather than being uniformly distributed though the cell wall, the LM15 epitope was most abundant at inner cell wall regions (Fig. 4E). The galactosylated XG probe LM25 bound more extensively than LM15 but still preferentially to inner cell wall regions (Fig. 4G). The binding profile of CCRCM1, directed to fucosylated XG (Puhlmann et al., 1994), was similar to that of LM15 (Fig. 4F). As with Arabidopsis, Lepidium endosperm lacked detectable heteromannan; however, heteromannan was abundant at both the testa surface and in seed mucilage (Supplemental Fig. S2).
Probing of sections with the antibody LM19 revealed that the endosperm and testa cell walls and mucilage of Lepidium seeds contain abundant low methyl-ester HG and the embryo radicle to a lesser extent (Fig. 4C). LM20 did not bind to either the embryo or endosperm but bound strongly to the testa and mucilage, indicating both high and low methyl-ester HG is abundant in Lepidium mucilage (data not shown). LM8 binds to XGA, a HG substituted at C3 with single β-d-Xyl residues (Willats et al., 2004) most often associated with cells undergoing cell detachment. LM8 did not bind to Arabidopsis sections; however, binding was abundant in Lepidium where it was restricted to the inner face of the endosperm and the surface of the testa (Fig. 4D).
Labeling of sections with probes directed to arabinans revealed spatial heterogeneity within the endosperm that was to some extent distinct from the profiles observed in Arabidopsis. In the Lepidium endosperm, the LM6 epitope has an asymmetric distribution being more abundant at the inner face of the endosperm adjacent to the embryo (Fig. 5H). The linear arabinan epitope recognized by LM13 was uniformly distributed through endosperm cell walls and at an inner region of the cell wall at the ME (Fig. 5I). The LM16 antibody bound strongly to the surface of the testa and weakly to cell walls of the ME (Fig. 5J).
Figure 5.
In situ localization of cell wall epitopes in medial longitudinal sections of 3-h-imbibed tobacco seeds. A, Calcofluor White labeling showing cotyledons (C), radicle (R), testa (T), ME, CE, and PE. B, CBM3a cellulose labeled the embryo alone. C and D, LM15 XG and LM25 XG epitopes were uniformly distributed in embryo cell walls and restricted to the ME in the endosperm. E to G, LM15 XG binds to intercellular spaces and middle lamellar regions in the ME. H, LM21 HM binds to abundant heteromannans in the endosperm. I, LM5 galactan is restricted to the PE and CE. J, LM6 arabinan epitopes are abundant in embryo and endosperm cell walls. K, Extensin recognized by JIM20 was restricted to the ME. Bars = 50 µm. [See online article for color version of this figure.]
Immunolocalization with antibodies LM1 and JIM20 (Smallwood et al., 1994) revealed that extensins are a readily detected component of Lepidium endosperm cell walls (Supplemental Fig. S1 and Fig. 4K, respectively). Extensins were detected in all endosperm cell walls but were more abundant at the inner face of the endosperm and thus colocalized with the LM6 arabinan and LM8 XGA epitopes (Fig. 4K).
In summary, Lepidium endosperm cell walls contain cellulose, XG, unesterified HG, and arabinan, indicating conserved architectures between these two members of the Brassicaceae. Lepidium embryo cell wall composition is broadly the same as Arabidopsis, being relatively more cellulose and XG rich than the endosperm cell walls. In contrast with Arabidopsis, the endosperm cell walls of Lepidium are also extensin rich. Despite the ME region being the thickest part of the endosperm, this difference did not coincide with any detectable cell wall architectural asymmetry in either Arabidopsis or Lepidium endosperms.
Tobacco Endosperm Is Heteromannan Rich and Exhibits Tissue-Level Cell Wall Architectural Asymmetry
Arabidopsis and Lepidium are both endospermic Brassicaceae exhibiting broadly similar endosperm cell wall molecular architectures. As the hormonal regulation of endosperm rupture is conserved between Arabidopsis, Lepidium, and tobacco, we investigated to what extent cell wall architecture was conserved. Analysis of sections of tobacco seeds (imbibed for 3 h) with Calcofluor White revealed that the embryo has a distinct cell wall architecture and that the ME has a cell wall architecture that is different to that of the PE and CE (Fig. 5A), indicating a tissue level asymmetry in cell wall structures. CBM3a labeled the embryo uniformly but did not bind to the endosperm (Fig. 5B).
XG was abundant in embryo cell walls, as evidenced by labeling of sections with antibodies LM15 and LM25 (Fig. 5, C and D, respectively) but was only present in the cell walls of the ME. Analysis of antibody binding to the ME at higher magnification revealed that the LM15 XG epitope was restricted to middle lamellae and intercellular regions (Fig. 5, F and G). The most striking feature of tobacco endosperm cell wall architecture that was distinct from Arabidopsis and Lepidium was the abundance of heteromannan (Fig. 5H). Both LM21 and CBM27 bound strongly and uniformly to endosperm but not to embryo cell walls (Fig. 5H and Supplemental Fig. S1, respectively).
RG-I-associated (1→4)-β-d-galactan recognized by LM5 was restricted to an inner region of the cell walls of the PE and CE and absent from the embryo and ME (Fig. 5I); arabinan recognized by LM6 was detected in a complementary manner and while detected in all cell walls was most abundant in ME cell walls (Fig. 5J). Extensin recognized by JIM20 was restricted to the ME alone (Fig. 5K). As observed with Arabidopsis and Lepidium, tobacco embryo cell walls were relatively more cellulose and XG rich than endosperm walls and contained reduced levels of HG and arabinan as indicated by antibody binding.
To investigate further the differential detection between species of heteromannan in endosperm cell walls and patterns of polysaccharide occurrence as revealed by in situ immunofluorescence analyses, neutral monosaccharide linkage analysis was performed on cell wall extracts from Lepidium and tobacco endosperms with Lepidium embryo cell walls for comparison. The results (Table I) indicate that Lepidium endosperm and embryo cell walls contain low levels of heteromannan (2.8% and 3.5% of neutral polysaccharides, respectively), whereas tobacco endosperm contains abundant heteromannan (64.6%), corroborating our in situ observations with probes LM21 and CBM27. Linkage composition for other neutral monosaccharides supported the in situ analyses and indicated, in Lepidium endosperm, the presence of cellulose, XG, and arabinan, with the latter being particularly abundant. The monosaccharide linkage analysis also confirmed the presence of arabinan in the tobacco seed endosperm. The presence in Lepidium endosperm samples of 4-linked xylosyl residues indicative of substituted xylans, albeit at a relatively low level (5.8%), was unexpected. In situ analysis with a range of probes directed to unsubstituted and substituted xylans did not reveal any insight into its location.
Table I. Monosaccharide linkage analysis of neutral sugars in cell walls of Lepidium endosperm and embryo tissues and tobacco endosperm.
Figures are molar percentages and are means of three assessments. f, Furanose; p, pyranose.
| Monosaccharide | Lepidium |
Tobacco |
|
|---|---|---|---|
| Endosperm | Embryo | Endosperm | |
| Ara | |||
| t-Ara (f) | 15.4 | 14.9 | 2.5 |
| 1,2-Ara (f) | 3.1 | 0.3 | 0.2 |
| 1,3-Ara (f) | 0.8 | 0.3 | 0.0 |
| 1,5-Ara (f) | 18.8 | 10.6 | 3.8 |
| 1,3,5-Ara (f) | 7.2 | 16.0 | 4.1 |
| 1,2,5-Ara (f) | 2.9 | 0.6 | 0.0 |
| Total | 48.3 | 42.8 | 10.6 |
| Fuc | |||
| t-Fuc (f) | 0.7 | 1.4 | 0.0 |
| Total | 0.7 | 1.4 | 0.0 |
| Xyl | |||
| t-Xyl (p) | 4.0 | 5.9 | 0.0 |
| 1,3-Xyl (p) | 0.0 | 0.2 | 0.0 |
| 1,2-Xyl (p) | 1.1 | 1.5 | 0.0 |
| 1,4-Xyl (p) | 1.7 | 1.1 | 0.0 |
| 1,2,4-Xyl (p) | 1.6 | 0.2 | 0.0 |
| 1,2,3,4-Xyl (p) | 2.5 | 0.5 | 0.0 |
| Total | 10.9 | 9.5 | 0.0 |
| Rha | |||
| 1,2-Rha (p) | 0.4 | 0.4 | 0.2 |
| 1,2,4-Rha (p) | 0.8 | 1.2 | 0.0 |
| Total | 1.2 | 1.6 | 0.2 |
| Man | |||
| t-Man (p) | 0.1 | 0.5 | 0.3 |
| 1,4-Man (p) | 0.7 | 1.4 | 60.2 |
| 1,2,4-Man (p) | 0.0 | 0.0 | 0.4 |
| 1,4,6-Man (p) | 1.2 | 1.0 | 3.7 |
| 1,3,6-Man (p) | 0.7 | 0.4 | 0.0 |
| 1,2,3,6-Man (p) | 0.0 | 0.2 | 0.0 |
| Total | 2.8 | 3.5 | 64.6 |
| Glc | |||
| t-Glc (p) | 0.3 | 0.2 | 0.5 |
| 1,3-Glc (p) | 0.1 | 0.2 | 0.0 |
| 1,4-Glc (p) | 20.6 | 23.9 | 19.2 |
| 1,3,4-Glc (p) | 0.2 | 0.2 | 0.0 |
| 1,2,4-Glc (p) | 0.4 | 0.4 | 0.0 |
| 1,4,6-Glc (p) | 2.9 | 8.4 | 0.7 |
| 1,3,4,6-Glc (p) | 0.2 | 0.2 | 0.0 |
| Total | 24.7 | 33.5 | 20.4 |
| Gal | |||
| t-Gal (p) | 3.4 | 2.3 | 3.3 |
| 1,3-Gal (p) | 2.5 | 0.7 | 0.0 |
| 1,2-Gal (p) | 2.0 | 2.7 | 0.0 |
| 1,6-Gal (p) | 0.4 | 0.3 | 0.0 |
| 1,3,6-Gal (p) | 3.2 | 1.7 | 0.8 |
| Total | 11.5 | 7.7 | 4.1 |
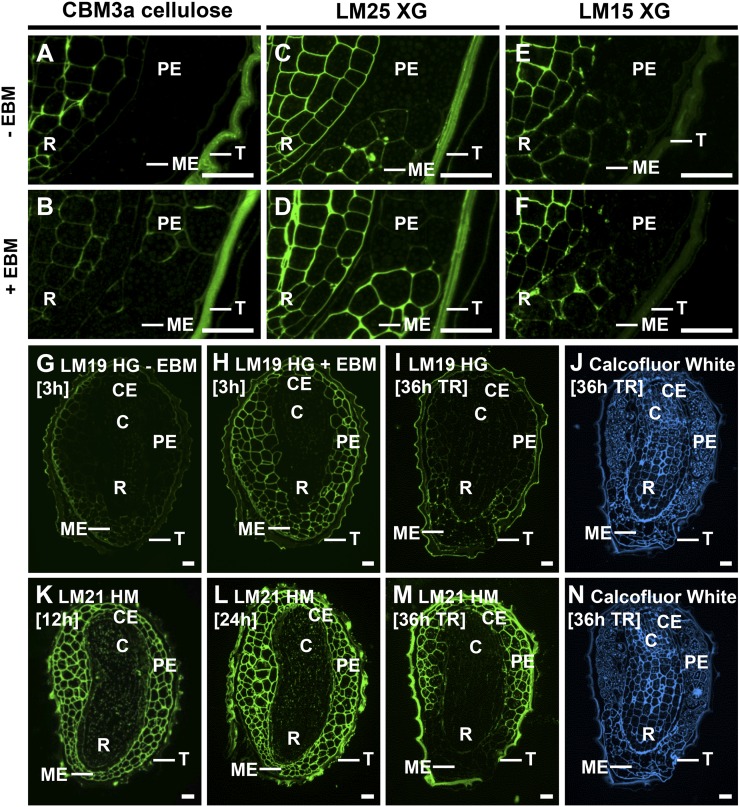
We previously reported the masking of polysaccharides by HG in a number of parenchyma systems (Marcus et al., 2008, 2010). To investigate whether the abundant heteromannans were capable of masking other cell wall polymers in the endosperm of tobacco seeds, sections were treated with EBM prior to immunolabeling. CBM3a binding was revealed in the PE and CE following EBM treatment but not in the ME (Fig. 6, A and B). EBM treatment of sections had some effect on XG detection, and this was particularly the case for the LM25 XG epitope, which increased at the ME (Fig. 6, C and D). Less impact was observed on the LM15 XG epitope, which is more restricted to the intercellular matrices at the ME (Fig. 6, E and F).
Figure 6.
Medial longitudinal sections of tobacco seeds with enzymatic deconstruction and during germination. Three-hour-imbibed mature tobacco seed sections were treated with EBM or a buffer control prior to immunolabeling. A and B, CBM3a cellulose was unmasked in PE and CE cell walls following EBM treatment. C and D, LM25 XG epitopes were unmasked in ME following EBM treatment. E and F, LM15 XG epitopes were not substantially unmasked in ME following EBM treatment. G and F, LM19 HG was weakly detectable in the endosperm of 3-h-imbibed seeds and was unmasked in all endosperm cell walls by EBM treatment. I, The LM19 epitope was unmasked in the ME at testa rupture (TR) at 36 h. K to M, Immunolabeling of germinating tobacco seeds with LM21 HM revealed the specific degradation of heteromannan (HM) at the ME at testa rupture. R, Radicle; C, cotyledons; T, testa. Bars = 50 µm. [See online article for color version of this figure.]
In untreated sections of tobacco seeds, the LM19 HG epitope was detected weakly in the ME and the outer region of the PE and was abundant at the testa surface (Fig. 6G). The LM19 HG epitope was masked by heteromannan, as following EBM treatment, LM19 bound to PE and CE and more strongly to the ME (Fig. 6H). Taken together, this indicates that heteromannans are able to block probe access to LM25 XG, LM19 HG, and cellulose in the endosperm but not intercellular matrix-located LM15 XG. These findings have implications for the timing of CWRE action during endosperm cell wall remodeling as these data indicate that the porosity of the cell wall can be affected by heteromannans.
No Heteromannan Detected in Arabidopsis and Lepidium Endosperms after Cell Wall Deconstructions
Heteromannans were detectable in the mucilage of whole-mount preparations of Arabidopsis seeds (Supplemental Fig. S2) where they have low abundance (Walker et al., 2011) but were not detected in resin-embedded, sectioned material. Heteromannan was retained at the testa surface and to a lesser degree in the mucilage of Lepidium seeds following sectioning (Supplemental Fig. S2). To confirm the absence of heteromannan from cell walls of Arabidopsis and Lepidium endosperms, a series of enzymatic deconstructions were performed aimed at the removal of the most abundant components of the endosperm cell wall, namely, HG, arabinan, and XG. Sections were treated with pectate lyase, α-l-arabinofuranosidase, and xyloglucanase alone and in combination (Supplemental Fig. S2). No heteromannan epitopes were detected in Arabidopsis sections after the enzyme deconstructions nor in the Lepidium endosperm; however, the LM21 epitope was weakly detectable in Lepidium embryo cell walls following enzyme deconstruction (Supplemental Fig. S2).
Heteromannans Are Degraded in the ME during Tobacco Germination
Expression of EBM during seed germination has been well studied for the Solanaceous species tomato, Datura ferox, and Datura stramonium (Sánchez et al., 1990; Nomaguchi et al., 1995). In Datura spp. seeds, cell wall material was degraded at the inner face of the ME during germination without a concurrent decrease in heteromannan in cell wall extracts (Sánchez et al., 1990).
To determine whether EBM remodeling of tobacco endosperm cell walls occurs during germination, we analyzed cell wall architectures in nongerminated, testa-ruptured, and germinated endosperm-ruptured seeds. In situ analyses of seed sections revealed that heteromannan recognized by LM21/CBM27 was specifically degraded in the ME at testa rupture but not at earlier time points (Fig. 6, K–M). Furthermore, in testa-ruptured seeds, LM19 bound to the ME, which indicates germination-associated unmasking of HG by EBM-mediated degradation of heteromannan (Fig. 6I). These data indicate a significant difference between tobacco and the Brassicaceae representatives used in this study as, in addition to their lack of endosperm cell wall heteromannan, in situ analyses of Arabidopsis and Lepidium seeds at the time points of testa rupture and endosperm rupture revealed no endosperm cell wall dynamics.
Differential Mechanics of Endosperm Weakening during Germination in Lepidium and Tobacco
Distinct cell wall molecular architectures are likely to result in varying cell wall properties, such as porosity, and may also reflect mechanical properties. The data presented here indicate that the presence of heteromannan in tobacco endosperm cell walls probably impacts cell wall porosity. During germination, the radicle pushes against endosperm tissues, which are breached at the point of germination. To explore the functional significance of the differing molecular architectures of Lepidium and tobacco, direct measurements of tissue weakening were made on isolated endosperms using the puncture force method (Müller et al., 2006). Endosperm halves from tobacco and Lepidium were used to calculate puncture force values at defined time points during germination. Values for ME and CE (or PE in the case of Lepidium) were determined (Fig. 7). During tobacco germination, a decline in puncture force was observed that was more pronounced in ME than CE samples (Fig. 7A). A similar decline in puncture force was observed in Lepidium ME samples (Fig. 7B), indicating that ME-focused endosperm weakening occurs in both species during germination. In Lepidium, the force required to rupture the PE does not change significantly after testa rupture (from 7 to 14 h); by contrast, tobacco CE shows a significant decrease after testa rupture (from 36 to 60 h). The decrease in puncture force of tobacco ME is associated with a decrease in detectable heteromannan in seed sections (Fig. 6M), suggesting that heteromannans and their degradation contribute to this aspect of cell wall mechanical properties in tobacco.
Figure 7.
Puncture force analyses of tobacco and Lepidium endosperms during germination. A, Puncture force measurements of ME and CE from tobacco. B, Puncture force measurements of ME and PE from Lepidium. Tobacco seeds exhibit testa rupture (TR) at 36 h, and endosperm rupture (ER) begins at 60 h. Lepidium seeds exhibit testa rupture at 7 h, and endosperm rupture begins at 14 h. Pre-endosperm rupture tobacco and Lepidium seeds were selected for the 60- and 14-h measurements, respectively.
Arabidopsis Cell Wall Mutants Display Altered Germination Characteristics
Next, we aimed to investigate to what extent cell walls and their composition contribute to the regulation of seed germination using mutants with defects in XG, arabinan, and HG. The genotypes used included XG-deficient xxt1 xxt2, which lacks detectable XG (Cavalier et al., 2008), the arabinan-deficient mutant arad1 arad2 (Harholt et al., 2012), and the putative pectin methyltransferase mutant qua2, which has been shown to have 50% reduction in HG without effects on other wall polysaccharides (Mouille et al., 2007). Seeds of the xxt1 xxt2, arad1 arad2, and qua2 mutants were immunolabeled with appropriate probes to confirm defects in seed cell walls (Fig. 8A).
Figure 8.
Alterations in seed cell wall structure affect seed dormancy and germination characteristics in Arabidopsis. A, Immunofluorescence micrographs of cell wall mutants showing alterations in cell wall architecture. xxt1 xxt2 seed cell walls contain no detectable LM25 XG epitope. arad1 arad2 endosperm cell walls contain reduced levels of LM13 arabinan epitope. qua2 seeds contain less LM19 HG epitope. R, Radicle. Bars = 50 µm. B, Seed germination kinetics were assessed for after-ripened wild-type and mutant seeds. qua2 and arad1 arad2 germination speeds were similar to wild-type seeds. xxt1 xxt2 germinates significantly more slowly than the wild type. Means of germination speeds shown in tabular form. [See online article for color version of this figure.]
We analyzed whether germination speed was affected by the altered cell wall structures. Fully after-ripened, nondormant seeds of all lines showed a final germination percentage close to 100%; thus, all batches showed a high viability. The analysis indicated that xxt1 xxt2 germinated significantly more slowly than the wild type, whereas qua2 and arad1 arad2 germination kinetics did not differ from the wild type (Fig. 8B). As seed germination rates can differ between seed batches, due to a range of factors, the delayed germination phenotype of xxt1 xxt2 was confirmed in a third independently grown batch of seeds. These data suggest that XG polymers and/or their respective CWREs play a role in regulating germination kinetics in Arabidopsis seeds.
DISCUSSION
Tobacco seed endosperm cell walls contain abundant heteromannan, whereas Arabidopsis and Lepidium endosperms do not. Moreover, in tobacco, this heteromannan overlays a tissue-level asymmetry in cell wall architecture as evidenced by the staining of the ME region by Calcofluor White (Fig. 5). Such asymmetries in probe labeling of endosperm cell walls were not observed for any polymer class in either Arabidopsis or Lepidium. We also immunodetected abundant heteromannan in tomato seed endosperm (Supplemental Fig. S2), supporting previous work (Groot et al., 1988; Dahal et al., 1997). Endosperm cell wall architectural asymmetry has also previously been demonstrated for tomato (Nonogaki et al., 2007). The process of tomato seed endosperm weakening during germination has been assessed by puncture force analyses, and these have indicated the involvement of EBM and other CWREs (Toorop et al., 2000; Wu et al., 2001). This work indicates that cell wall architecture in tobacco seed endosperm is similar to that observed in tomato; moreover, these sets of observations point to a clear distinction in cell wall molecular architectures of seed endosperms between the Brassicaceae and the Solanaceae families. Such knowledge has consequences for understanding CWRE action and its role in seed germination.
Tobacco and tomato belong to two different subgroups within the Solanaceae based on morphological and molecular criteria, the Cestroideae and Solanoideae, respectively. Tobacco seed germination has two steps (i.e. testa rupture followed by endosperm rupture). In tomato seeds, by contrast, the testa and MPE are adhered to each other and rupture together (Petruzzelli et al., 2003). Endosperm weakening in tomato is biphasic (Toorop et al., 2000). ABA-insensitive expression of EBM and other proteins occurs in the early phase and has been shown to be necessary but not sufficient for the completion of germination. The late phase is critical, as it controls the final step of radicle emergence (Wu et al., 2001). The spatiotemporal expression patterns of CWRE genes in the MPE have been elucidated from in situ hybridization analyses of germinating tomato seeds. Within 12 h of imbibition, xyloglucan endotransglycosylase/hydrolase (XTH; LeXET4) and expansin (LeEXPA4) expression was detected. After 12 h of imbibition, cellulase (LeCel55) expression, EBM (LeMAN2), polygalacturonase (LeXPG1), and β-1,3-glucanase (LeGluB) genes were expressed (for review, see Nonogaki et al., 2007). These data suggest that CWRE action on the cellulose-XG network to allow limited expansion of the wall precedes bulk cell wall disassembly during tomato seed germination; therefore, a similar pattern of CWRE gene expression might occur during tobacco germination.
The evolution of the seed endosperm in species such as Arabidopsis and Lepidium is thought to have been a reduction from a multicellular layer to a single or two-celled layer with reduced capacity for storage of reserves (Linkies and Leubner-Metzger, 2012). It is possible that this has been associated with loss of abundant heteromannan polysaccharides and an associated increase in the level of cellulose and cell wall architecture more typical of vegetative organ cell walls, albeit arabinan rich.
Heteromannans May Have Distinct Roles in Brassicaceae and Solanaceae Seeds
A major distinctive feature of the tobacco seed endosperm, relative to Arabidopsis and Lepidium, is its inherent spatial heterogeneity, or asymmetry, in terms of cell wall architecture. This spatial heterogeneity does not involve detectable heteromannan but asymmetries in cell structure/Calcofluor White binding and specific RG-I structures (arabinan), XG, and extensin occurrence at the ME that broadly reflects the cell wall architecture of the entire Arabidopsis and Lepidium endosperms. In tobacco endosperm, heteromannan is abundantly and evenly detectable prior to germination, and here we have shown that heteromannan degradation in tobacco is clearly spatially dynamic and focused on the ME. We propose that this heteromannan degradation results in exposure of the HG, XG, and arabinan components of the cell wall that can also then be acted upon by other CWREs. It is of considerable interest in this regard that our experimental enzymatic intervention can unmask arabinan, cellulose, and LM25 XG but not LM15 XG. The LM15 form of XG is located in intercellular/middle lamellae regions and does not appear to be subject to masking, presumably because of its restricted occurrence in the intercellular matrix. The distinct cell wall locations of XG epitopes (inner cell wall in Lepidium and cell wall/intercellular matrix in tobacco) indicates the considerable potential for structural heterogeneity of location of cell wall components between species, even for the same polymer class in the same tissue. Such observations point to distinct functions for XG ranging from mechanics to cell adhesion, which has previously been proposed for XG in Solanaceous systems (Marcus et al., 2008; Ordaz-Ortiz et al., 2009).
The role of heteromannan as structural and storage carbohydrates is well established (Meier and Reid, 1982), and the data we present here suggest that they can function as a structural polysaccharide in tobacco endosperms. Heteromannans are also proposed to act as regulators of growth and development (Auxtová et al., 1995; Bilisics et al., 2004; Benová-Kákosová et al., 2006). The heteromannan backbone is synthesized by cellulose synthase-like A (CSLA) glycan synthases (Liepman et al., 2005, 2007; Suzuki et al., 2006). CSLA7 has been shown to synthesize mannan in vitro (Liepman et al., 2005) and to synthesize stem glucomannan in vivo (Goubet et al., 2009). Disrupting the CSLA7 gene resulted in defective pollen tube growth and embryo lethality (Goubet et al., 2003). Following enzymatic deconstruction of seed sections, we were able to detect heteromannan in Lepidium embryo cell walls, albeit at very low quantities, but were not able to detect heteromannan in Arabidopsis embryos. However, regulated expression of EBMs is evident in Lepidium seeds (Morris et al., 2011), and analysis of Arabidopsis EBM mutants demonstrated that such mutations have a negative impact on seed germination (Iglesias-Fernández et al., 2011). We show here that heteromannans are present in Arabidopsis and Lepidium mucilage but are only detectable at low levels in Lepidium embryo cell walls following enzymatic deconstruction. Heteromannans may either be absent from Arabidopsis/Lepidium endosperms or present at a level below the detection limit. If heteromannans are absent from endosperms, it is possible that EBMs produced by the embryo and/or endosperm are required to act on heteromannans in the mucilage to generate signaling oligosaccharides, which have growth-promoting effects on the seedling.
Conserved, Multifunctional Roles for Pectic Arabinans
An important insight from both the in situ and monosaccharide linkage analyses is that the three species studied all possess an arabinan-rich endosperm. Tomato endosperm cell wall neutral sugars comprise up to 10% Ara (Dahal et al., 1997) which is equivalent to that observed in tobacco endosperm cell walls (Table I). Endosperm cell walls are distinct from embryo cell walls, the latter being more cellulose and XG rich. Pectic arabinans can occur as side chains of RG-I but may also exist as free arabinans (Beldman et al., 1997). The monosaccharide composition of the side chains of RG-I show extreme variability, and RG-I (particularly arabinan substructure) is extensively developmentally regulated (Willats et al., 2001; Caffall and Mohnen, 2009; Verhertbruggen et al., 2009b; Yapo, 2011).
The functional significance of abundant arabinan within cell walls is not clear, but an association with maintaining cell wall flexibility/elasticity has been documented. Studies of the resurrection plant Myrothamnus flabellifolia revealed that its leaf cell walls have unusually high arabinan content that is proposed to stop HG forming irreversible associations during drying and thereby maintaining wall flexibility (Moore et al., 2008). Furthermore, arabinans have been demonstrated to maintain cell wall elasticity in stomatal guard cells where they are proposed to modulate HG interactions (Jones et al., 2003). Arabinans have been shown to be metabolized during embryo development and germination (Gomez et al., 2009). AtBX3 is a bifunctional xylosidase/arabinosidase hydrolase expressed specifically in the endosperm at the globular stage of embryo development. bx3 mutant seeds exhibited reduced size, but not weight, and delayed germination (Minic et al., 2006). The delayed germination phenotype of bx3 seeds may be a result of incorrect arabinan processing during endosperm development. Therefore, seed arabinans may be multifunctional and can act as storage carbohydrates, polymers that maintain wall integrity during seed rehydration and also contribute to endosperm mechanical properties (tissue elasticity) that impact seed germination.
A Core Cell Wall Architecture, Common to Endosperms, Is a Target for CWREs to Effect Endosperm Rupture
In Arabidopsis, Lepidium, and tobacco, the seed endosperm has to be able to stretch to allow initial embryo expansion during water uptake before endosperm rupture takes place. In one sense, germination progress can be thought of as a function of endosperm cell wall elasticity prior to rupture and embryo expansion driven by turgor pressure. The puncture force data highlight a key difference between the multicellular endosperm of tobacco and the thin endosperm of Lepidium. The endosperms of both species exhibit an ME-focused decrease in puncture force during germination; however, in Lepidium, the PE shows no weakening, while in tobacco, the puncture force for the CE decreases. We propose that the final decrease in force required to rupture the endosperm, in both Lepidium and tobacco, is the result of CWRE action on a core ME cell wall architecture that is XG, HG, and arabinan rich. We propose that all three species have this similar core architecture in their ME cell walls and that this will have a similar inherent elasticity. In the case of tobacco, this core architecture is restricted to the ME and encased in abundant heteromannan. It is the occurrence of the abundant heteromannan that gives the tobacco endosperm increased strength: Degradation of heteromannan by EBM at the ME during germination is correlated with a decrease in the puncture force of the endosperm. Heteromannan could possibly act as a control polysaccharide in tobacco that is degraded to allow CWREs unconstrained access to the polymers of the core cell wall architecture. Our proposal is that the endosperm core cell wall architecture is acted on by XG, pectic, and related enzymes and in the case of Arabidopsis and Lepidium, unlike tobacco, no evidence for a clear hierarchy or sequence of polymer degradation has yet been obtained.
Candidate cell wall remodeling factors active on this core cell wall architecture would include expansins and XTHs acting on the XG/cellulose network within the wall. Expansin expression is correlated with endosperm weakening in tomato (Chen and Bradford, 2000) and Lepidium (Voegele et al., 2011). Both expansin and XTH expression increases markedly in the endosperm during Arabidopsis and Lepidium seed germination and are down-regulated upon exogenous application of ABA or paclobutrazol (an inhibitor of GA synthesis; Penfield et al., 2006; Voegele et al., 2011). Pectic CWREs acting on endosperm cell walls will include polygalacturonase, pectin methylesterase, pectate lyases, and arabinofuranosidases (Sitrit et al., 1999; Ren and Kermode, 2000; González-Carranza et al., 2007).
This study indicates that extensins are abundant in the Lepidium endosperm, restricted to the ME in tobacco endosperm, and appear to be restricted to the embryo and seed surface in Arabidopsis, although it is possible that in Arabidopsis endosperms, they possess distinct structural features not recognized by available probes. Extensins are proposed to function in cell wall assembly (Cannon et al., 2008). Extensin gene expression has been correlated with tissues that have to withstand tensile and osmotic stress, such as root hairs (Bucher et al., 1997), hypocotyls (Shirsat et al., 1996), and seed coats (Cassab et al., 1985). The endosperm has to withstand both tensile and osmotic stress during seed dehydration and imbibition, and the extensin network likely contributes to cell wall mechanical properties.
Reactive oxygen species (ROS; e.g. superoxide, hydrogen peroxide, and hydroxyl radicals) have recently emerged as important factors in seed dormancy (Bailly et al., 2008) and have previously been shown to be synthesized by seed coats of Raphanus sativus in response to germination stimuli (Schopfer et al., 2001). ROS are implicated in cell wall loosening through the cleavage of cell wall polysaccharides (Fry, 1998) and wall stiffening by the cross-linking of cell wall components, including extensins (Hohl et al., 1995; Ros-Barcelo et al., 2002). ROS play a role in cell expansion processes (e.g. root elongation; Liszkay et al., 2004; Renew et al., 2005). In Lepidium, radicle and endosperm cell walls ROS are produced during germination, and this process has been shown to be promoted by GA and inhibited by ABA (Müller et al., 2009). Moreover, ROS production was associated with endosperm weakening, suggesting scission of cell wall polysaccharides by ROS is an important aspect of cell wall remodeling during germination (Müller et al., 2009). How ROS are integrated with specific components of what we propose as the core endosperm cell wall architecture has yet to be elucidated.
Arabidopsis Cell Wall Mutants and Germination Control?
XTHs are implicated in XG remodeling, and XG oligosaccharides have been shown to have cell expansion promoting effects (McDougall and Fry, 1990). Stress/strain assays analyzing the properties of xxt1 xxt2 walls revealed them to be more extensible than wild-type walls, thereby supporting a reinforcing role for XG; however, xxt1 xxt2 walls were less extensible in creep assays mediated by α-expansin (Park and Cosgrove, 2012). Such observations, including the fact that xxt1 xxt2 plants are nearly wild-type in appearance yet contain no detectable XG, presents a confusing picture regarding the exact role of XG in cell walls. Our analyses of xxt1 xxt2 seed germination kinetics suggests that XG, and the associated CWREs, are functional components of the seed germination network. Interestingly, germination kinetic analysis of xth31 xtr8 mutants, an endosperm-specific XTH, revealed that these seeds germinate faster than the wild type, suggesting a cell wall strengthening role for these gene products (Endo et al., 2012). Further studies on associated gene expression changes and responses of these seeds to stress during germination may give us a clearer understanding of why such changes in cell wall architecture and in what specific tissues, embryo or endosperm, impact the control of seed germination in this manner.
CONCLUSION
Prior to this study, in situ analyses of seed cell wall architectures had been limited and had not been performed to the level of resolution presented here. The panel of monoclonal antibody and CBM probes currently available for cell wall biology is considerable, and their use in conjunction with chemical and enzymatic cell wall disassembly is revealing hitherto unexpected complexities in cell wall compositions, heterogeneities, and diversity. We suggest that for the endosperm tissue of the species examined, there is a core architecture consisting of XG, unesterified HG, and arabinan that are all or in part remodeled during seed germination. In tobacco endosperm, this core architecture is restricted to the ME and all endosperm cell walls contain abundant heteromannan that is effectively degraded at the ME during seed germination. The core cell wall architecture is the abundant type in Arabidopsis and Lepidium endosperms, and there is no detectable inherent structural cell wall asymmetry or heterogeneity in the seed endosperm prior to germination. This also has the consequence of no major ME-focused cell wall architectural dynamics during germination as seen in tobacco, but CWRE action on abundant HG, arabinan, XG, and the action of expansins at the ME that leave no readily detectable cell wall architectural traces. It could be argued that the spatial cell wall dynamics in Arabidopsis and Lepidium do not involve any bulk change in cell wall polymers but in CWRE gene expression. By contrast, the tobacco seed endosperm has inherent spatial cell wall heterogeneity and presents a clear bulk polymer dynamic in the selective removal of heteromannan during radicle emergence.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) seeds ecotype Columbia were germinated in 10-cm2 tissue culture petri dishes (Sterilin) on 0.7% (w/v) agarose (Sigma-Aldrich) under continuous light at 22°C. Cell wall mutants were germinated under the same growth conditions. Seeds of xxt1 xxt2 (Cavalier et al., 2008) were obtained from the European Arabidopsis Stock Centre (Nottingham, UK), arad1 arad2 (Harholt et al., 2012) seeds were a generous gift from Jesper Harholt, and qua2 (Mouille et al., 2007) seeds were a generous gift from Grégory Mouille. Lepidium (Lepidium sativum) ‘Gartenkresse, einfache’ (Juliwa) seeds were germinated in 10-cm2 petri dishes containing 6 mL of distilled water and two layers of 3MM filter paper (Scientific Laboratory Supplies) under continuous light at 24°C. Tobacco (Nicotiana tabacum ‘Havana 425’) seeds were germinated in 10-cm2 petri dishes containing 6 mL of one-tenth Murashige and Skoog (Murashige and Skoog, 1962) basal medium without hormones or vitamins (Duchefa), adjusted to pH 7.0, and two layers of 3MM filter paper under continuous light at 24°C.
Preparation of Plant Materials for Microscopy
For sectioned material, seeds were harvested at the following time points: 3 h of imbibition, testa rupture, and endosperm rupture. The testa was punctured using a fine needle and the seeds immediately fixed in PEM buffer (50 mm PIPES, 5 mm EGTA, and 5 mm MgSO4, pH 6.9) containing 4% (w/v) paraformaldehyde under vacuum (1 h at room temperature). Seeds were washed twice in phosphate-buffered saline (PBS; 0.01 m phosphate buffer, 0.0027 m potassium chloride, and 0.137 m sodium chloride, pH 7.4), dehydrated through a graded ethanol series, and then infiltrated with LR White resin (London Resin Company) in a series consisting of 3:1, 2:1, 1:1, 1:2, and 1:3 (v/v) in absolute ethanol (1 × 24 h at 4°C) followed by 100% resin (3 × 24 h at 4°C). The samples were polymerized in gelatin capsules for 5 d at 37°C. Sections were cut to a thickness of 0.5 µm using a diamond knife on an Ultracut microtome (Reichart-Jung), and sections were collected on multiwell slides (ICN Biomedicals) coated with Vectabond reagent (Vector Laboratories).
For toluidine blue O staining, sections were incubated in a solution of 1% (w/v) toluidine blue O containing 1% (w/v) sodium borate for 5 min, washed in water (3 × 5 min), mounted in glycerol, and observed using an Olympus BX61 microscope. Micrographs were taken using a Hamamatsu Orca 285 camera with Volocity 4 software (Perkin-Elmer).
Immunofluorescence Microscopy
For LM, JIM, and CCRC series antibodies, sections were incubated in PBS containing 3% (w/v) milk protein (MP/PBS; Marvel, Premier Beverages) and a 5-fold dilution of antibody hybridoma supernatant for 1 h. Samples were washed in PBS (3 × 5 min) and incubated with a 100-fold dilution of anti-rat IgG or anti-mouse IgG (whole molecule) linked to fluorescein isothiocyanate (Sigma-Aldrich) in MP/PBS for 1 h in darkness. For CBM labeling, sections were incubated in MP/PBS containing 10 μg mL−1 CBM for 1 h. Samples were washed in PBS (3 × 5 min) and incubated with a 1,000-fold dilution of mouse anti-HIS (Sigma-Aldrich) for 1 h, washed in PBS (3 × 5 min), and then incubated in a 100-fold dilution of anti-mouse IgG fluorescein isothiocyanate. Following antibody or CBM labeling, the samples were washed in PBS (3 × 5 min) counterstained for 5 min with the fluorochrome Calcofluor White M2R (fluorescent brightener 28; Sigma-Aldrich) at 0.25 µg mL−1 in PBS, washed in PBS (3 × 5 min), mounted in a PBS-based antifade solution (Citifluor AF3; Agar Scientific), and observed using an Olympus BX61 microscope equipped with epifluorescence irradiation. Micrographs were taken using a Hamamatsu Orca 285 camera with Volocity 4 software.
For unmasking of cell wall polysaccharides, samples were treated with 0.1 m sodium carbonate for 2 h at room temperature followed by 50 µg mL−1 pectate lyase (Pel10A; Brown et al., 2001) in 50 mm CAPS, 2 mm CaCl2 buffer, pH 10, for 2 h at room temperature; 100 μg mL−1 Bacillus spp. EBM (Megazyme International) in 0.1 m Gly, pH 8.8, for 2 h at 37°C; 50 µg mL−1 Aspergillus niger α-l-arabinofuranosidase (Megazyme International) in 50 mm sodium acetate, pH 4.0, for 2 h at 37°C; or 50 µg mL−1 Clostridium thermocellum xyloglucanase (NZYTech) in 50 mm sodium phosphate buffer, pH 7.0, for 2 h at 37°C, alone or sequentially with washes between treatments.
All epifluorescence and light microscopy analyses were performed on at least three seeds for each time point and treatment.
Monosaccharide Linkage Analysis
Three replicates of 40 Lepidium seeds and 80 tobacco seeds were dissected after approximately 6 h of imbibition to separate endosperm and embryo tissues. Tissue was snap frozen in liquid N2 and macerated using a TissueLyser LT (Qiagen). Cell walls were prepared as described previously (Zablackis et al., 1995). Linkage analysis was performed as described previously (Sims and Bacic, 1995). A complete description of the methods can be found in Pettolino et al. (2012).
Germination Analyses
The different genotypes were grown in a growth cell (16 h of light/8 h of dark) set at 22°C and 70% humidity. Plants were grown on rockwool blocks (Grodan) and placed on a flooding table, ensuring equal watering of all plants. Plants were watered with Hyponex nutrient solution (1 g/L). For all lines, three to four replicates (each consisting of a bulk three plants) were collected for germination experiments and stored under identical conditions. Cell wall mutant germination phenotypes were confirmed with seeds from two independent harvests.
For germination tests, seeds were sown on two layers of blue filter paper (Blue Blotter Paper; Anchor Paper Company) in transparent plastic trays (DBP Plastics) with in total 49 mL of deionized water. The plastic trays were piled and wrapped in a plastic bag. Each pile contained two trays at the bottom and two trays at the top with 25 mL of water to prevent unequal evaporation. The trays were incubated in a germination cabinet (Snijders Scientific) set at 22°C with continuous lighting. Trays were photographed at multiple time points after imbibition. The images were used for automated scoring of germination using the Germinator software package (Joosen et al., 2010; http://www.pph.wur.nl/UK/seedlab/resources/germinator/). The data points were used to fit a curve from which we determined germination speed (= T50; i.e. the time taken for 50% of the seeds to germinate) (Joosen et al., 2010).
Puncture Force Measurements
Puncture force experiments were performed as described previously (Müller et al., 2006) with a modified custom-made machine. Imbibed seeds were split in half, the embryo fully removed, and the endospermic tissue placed in a sample holder. A rounded metal pin was driven into the sample while force and displacement were recorded simultaneously. For Lepidium, a pin with 0.3-mm diameter was used and a speed of 0.7 mm min−1. For tobacco, a 0.2-mm pin was used with a speed of 0.35 mm min−1. The puncture force was measured for Lepidium at two time points before (3 and 7 h) and one after (14 h) testa rupture. Tobacco measurements were performed at two time points before (3 and 12 h) and two after (36 and 72 h) testa rupture.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Cell wall epitope detection summary.
Supplemental Figure S2. Immunodetection of heteromannans in enzymatic deconstructed sections of Arabidopsis and Lepidium seeds and in untreated section of tomato seeds.
Supplementary Material
Acknowledgments
We thank Susan Marcus and Marieke van Bolderen-Veldkamp for technical assistance. K.J.D.L., B.D., T.S., A.B., L.B., G.L.-M., and J.P.K. designed the research. K.J.D.L., B.D., T.S., and C.T.W. performed the research. K.J.D.L. wrote the article with critical input from B.D., A.B., L.B., G.L.-M., and J.P.K.
Glossary
- ABA
abscisic acid
- CWRE
cell wall remodeling enzyme
- XG
xyloglucan
- HG
homogalacturonan
- XGA
xylogalacturonan
- RG-I
rhamnogalacturonan I
- EBM
endo-β-mannanase
- ME
micropylar endosperm
- CE
chalazal endosperm
- PE
peripheral endosperm
- CBM
carbohydrate-binding module
- XTH
xyloglucan endotransglycosylase/hydrolase
- ROS
reactive oxygen species
- PBS
phosphate-buffered saline
- MP
milk protein
References
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. (2010) Plant Cell Walls: From Chemistry to Biology. Garland Science, New York [Google Scholar]
- Auxtová O, Lišková D, Kákoniová D, Kubačková M, Karácsonyi Š, Bilisics L. (1995) Effect of galactoglucomannan-derived oligosaccharides on elongation growth of pea and spruce stem segments stimulated by auxin. Planta 196: 420–424 [Google Scholar]
- Bailly C, El-Maarouf-Bouteau H, Corbineau F. (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331: 806–814 [DOI] [PubMed] [Google Scholar]
- Baskin JM, Baskin CC. (2004) A classification system for seed dormancy. Seed Sci Res 14: 1–16 [Google Scholar]
- Bassel GW, Lan H, Glaab E, Gibbs DJ, Gerjets T, Krasnogor N, Bonner AJ, Holdsworth MJ, Provart NJ. (2011) Genome-wide network model capturing seed germination reveals coordinated regulation of plant cellular phase transitions. Proc Natl Acad Sci USA 108: 9709–9714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldman G, Schols HA, Pitson SM, Searle-van Leeuwen MJF, Voragen AGJ. (1997). Arabinans and arabinan degrading enzymes. In RJ Sturgeon, ed, Advances in Macromolecular Carbohydrate Research, Vol 1. JAI Press, Stamford, CT, pp 1–64 [Google Scholar]
- Benová-Kákosová A, Digonnet C, Goubet F, Ranocha P, Jauneau A, Pesquet E, Barbier O, Zhang Z, Capek P, Dupree P, Lisková D, Goffner D. (2006) Galactoglucomannans increase cell population density and alter the protoxylem/metaxylem tracheary element ratio in xylogenic cultures of zinnia. Plant Physiol 142: 696–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Libourel IG, Aoyama N, Chung YY, Still DW, Jones RL. (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143: 1173–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. (1997) Seed germination and dormancy. Plant Cell 9: 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilisics L, Vojtassák J, Capek P, Kollárová K, Lisková D. (2004) Changes in glycosidase activities during galactoglucomannan oligosaccharide inhibition of auxin induced growth. Phytochemistry 65: 1903–1909 [DOI] [PubMed] [Google Scholar]
- Brown IE, Mallen MH, Charnock SJ, Davies GJ, Black GW. (2001) Pectate lyase 10A from Pseudomonas cellulosa is a modular enzyme containing a family 2a carbohydrate-binding module. Biochem J 355: 155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M, Schroeer B, Willmitzer L, Riesmeier JW. (1997) Two genes encoding extension-like proteins are predominantly expressed in tomato root hair cells. Plant Mol Biol 35: 497–508 [DOI] [PubMed] [Google Scholar]
- Burton RA, Gidley MJ, Fincher GB. (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6: 724–732 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D. (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Cassab GI, Nieto-Sotelo J, Cooper JB, van Holst GJ, Varner JE. (1985) A developmentally regulated hydroxyproline-rich glycoprotein from the cell walls of soybean seed coats. Plant Physiol 77: 532–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MC, Terneus K, Hall Q, Tan L, Wang Y, Wegenhart BL, Chen L, Lamport DTA, Chen Y, Kieliszewski MJ. (2008) Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci USA 105: 2226–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M, Raikhel NV, Keegstra K. (2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20: 1519–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez Montes RA, Ranocha P, Martinez Y, Minic Z, Jouanin L, Marquis M, Saulnier L, Fulton LM, Cobbett CS, Bitton F, et al. (2008) Cell wall modifications in Arabidopsis plants with altered α-l-arabinofuranosidase activity. Plant Physiol 147: 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Bradford KJ. (2000) Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol 124: 1265–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38: 109–124 [DOI] [PubMed] [Google Scholar]
- Dahal P, Nevins DJ, Bradford KJ. (1997) Relationship of endo-β-d-mannanase activity and cell wall hydrolysis in tomato endosperm to germination rates. Plant Physiol 113: 1243–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A, Tatematsu K, Hanada K, Duermeyer L, Okamoto M, Yonekura-Sakakibara K, Saito K, Toyoda T, Kawakami N, Kamiya Y, Seki M, Nambara E. (2012) Tissue-specific transcriptome analysis reveals cell wall metabolism, flavonol biosynthesis and defense responses are activated in the endosperm of germinating Arabidopsis thaliana seeds. Plant Cell Physiol 53: 16–27 [DOI] [PubMed] [Google Scholar]
- Fettke J, Eckermann N, Tiessen A, Geigenberger P, Steup M. (2005) Identification, subcellular localization and biochemical characterization of water-soluble heteroglycans (SHG) in leaves of Arabidopsis thaliana L.: distinct SHG reside in the cytosol and in the apoplast. Plant J 43: 568–585 [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. (2006) Seed dormancy and the control of germination. New Phytol 171: 501–523 [DOI] [PubMed] [Google Scholar]
- Fry SC. (1998) Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J 332: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez LD, Steele-King CG, Jones L, Foster JM, Vuttipongchaikij S, McQueen-Mason SJ. (2009) Arabinan metabolism during seed development and germination in Arabidopsis. Mol Plant 2: 966–976 [DOI] [PubMed] [Google Scholar]
- Gong X, Bassel GW, Wang A, Greenwood JS, Bewley JD. (2005) The emergence of embryos from hard seeds is related to the structure of the cell walls of the micropylar endosperm, and not to endo-β-mannanase activity. Ann Bot (Lond) 96: 1165–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Carranza ZH, Elliott KA, Roberts JA. (2007) Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J Exp Bot 58: 3719–3730 [DOI] [PubMed] [Google Scholar]
- Goubet F, Barton CJ, Mortimer JC, Yu X, Zhang Z, Miles GP, Richens J, Liepman AH, Seffen K, Dupree P. (2009) Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J 60: 527–538 [DOI] [PubMed] [Google Scholar]
- Goubet F, Misrahi A, Park SK, Zhang Z, Twell D, Dupree P. (2003) AtCSLA7, a cellulose synthase-like putative glycosyltransferase, is important for pollen tube growth and embryogenesis in Arabidopsis. Plant Physiol 131: 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, Kieliszewska-Rokicka B, Vermeer E, Karssen CM. (1988) Gibberellin-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta 174: 500–504 [DOI] [PubMed] [Google Scholar]
- Harholt J, Jensen JK, Verhertbruggen Y, Søgaard C, Bernard S, Nafisi M, Poulsen CP, Geshi N, Sakuragi Y, Driouich A, Knox JP, Scheller HV. (2012) ARAD proteins associated with pectic Arabinan biosynthesis form complexes when transiently overexpressed in planta. Planta 236: 115–128 [DOI] [PubMed] [Google Scholar]
- Herth W, Schnepf E. (1980) The fluorochrome, Calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 105: 129–133 [Google Scholar]
- Hilhorst HWM. (1995) A critical update on seed dormancy. I. Primary dormancy. Seed Sci Res 5: 61–73 [Google Scholar]
- Hohl M, Greiner H, Schopfer P. (1995) The cryptic growth response of maize coleoptiles and its relationship to H2O2 dependent cell wall stiffening. Physiol Plant 94: 491–498 [Google Scholar]
- Holdsworth MJ, Bentsink L, Soppe WJ. (2008) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 179: 33–54 [DOI] [PubMed] [Google Scholar]
- Iglesias-Fernández R, Rodríguez-Gacio MC, Barrero-Sicilia C, Carbonero P, Matilla A. (2011) Three endo-β-mannanase genes expressed in the micropylar endosperm and in the radicle influence germination of Arabidopsis thaliana seeds. Planta 233: 25–36 [DOI] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ. (2003) Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci USA 100: 11783–11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Seymour GB, Knox JP. (1997) Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-d-galactan. Plant Physiol 113: 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosen RVL, Kodde J, Willems LAJ, Ligterink W, van der Plas LHW, Hilhorst HW. (2010) GERMINATOR: a software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J 62: 148–159 [DOI] [PubMed] [Google Scholar]
- Kanai M, Nishimura M, Hayashi M. (2010) A peroxisomal ABC transporter promotes seed germination by inducing pectin degradation under the control of ABI5. Plant J 62: 936–947 [DOI] [PubMed] [Google Scholar]
- Kieliszewski MJ, Lamport DTA. (1994) Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J 5: 157–172 [DOI] [PubMed] [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15: 281–307 [Google Scholar]
- Lee KJD, Sakata Y, Mau S-L, Pettolino F, Bacic A, Quatrano RS, Knight CD, Knox JP. (2005) Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens. Plant Cell 17: 3051–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leubner-Metzger G, Fründt C, Vögeli-Lange R, Meins F., Jr (1995) Class I β-1,3-glucanases in the endosperm of tobacco during germination. Plant Physiol 109: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Nairn CJ, Willats WG, Sørensen I, Roberts AW, Keegstra K. (2007) Functional genomic analysis supports conservation of function among cellulose synthase-like a gene family members and suggests diverse roles of mannans in plants. Plant Physiol 143: 1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Wilkerson CG, Keegstra K. (2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA 102: 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkies A, Graeber K, Knight C, Leubner-Metzger G. (2010) The evolution of seeds. New Phytol 186: 817–831 [DOI] [PubMed] [Google Scholar]
- Linkies A, Leubner-Metzger G. (2012) Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep 31: 253–270 [DOI] [PubMed] [Google Scholar]
- Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, et al. (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21: 3803–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszkay A, van der Zalm E, Schopfer P. (2004) Production of reactive oxygen intermediates O2·–, H2O2, and ·OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136: 3114–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SE, Blake AW, Benians TAS, Lee KJD, Poyser C, Donaldson L, Leroux O, Rogowski A, Petersen HL, Boraston A, et al. (2010) Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J 64: 191–203 [DOI] [PubMed] [Google Scholar]
- Marcus SE, Verhertbruggen Y, Hervé C, Ordaz-Ortiz JJ, Farkas V, Pedersen HL, Willats WGT, Knox JP. (2008) Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ, Fry SC. (1990) Xyloglucan oligosaccharides promote growth and activate cellulase: evidence for a role of cellulase in cell expansion. Plant Physiol 93: 1042–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier H, Reid JSG. (1982). Reserve polysaccharides other than starch in higher plants. In FA Loewus, W Tanner, eds, Encyclopedia of Plant Physiology, Vol 13A. Springer, Berlin, pp 418–471 [Google Scholar]
- Minic Z, Do CT, Rihouey C, Morin H, Lerouge P, Jouanin L. (2006) Purification, functional characterization, cloning, and identification of mutants of a seed-specific arabinan hydrolase in Arabidopsis. J Exp Bot 57: 2339–2351 [DOI] [PubMed] [Google Scholar]
- Moore JP, Farrant JM, Driouich A. (2008) A role for pectin-associated arabinans in maintaining the flexibility of the plant cell wall during water deficit stress. Plant Signal Behav 3: 102–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K, Linkies A, Müller K, Oracz K, Wang X, Lynn JR, Leubner-Metzger G, Finch-Savage WE. (2011) Regulation of seed germination in the close Arabidopsis relative Lepidium sativum: a global tissue-specific transcript analysis. Plant Physiol 155: 1851–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouille G, Ralet MC, Cavelier C, Eland C, Effroy D, Hématy K, McCartney L, Truong HN, Gaudon V, Thibault JF, et al. (2007) Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J 50: 605–614 [DOI] [PubMed] [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. (2009) In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol 150: 1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Tintelnot S, Leubner-Metzger G. (2006) Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol 47: 864–877 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nomaguchi M, Nonogaki H, Morohashi Y. (1995) Development of galactomannan-hydrolyzing activity in the micropylar endosperm tip of tomato seed prior to germination. Physiol Plant 94: 105–109 [Google Scholar]
- Nonogaki H, Chen F, Bradford KJ. (2007). Mechanisms and genes involved in germination sensu stricto. In KJ Bradford, H Nonogaki, eds, Seed Development, Dormancy and Germination. Blackwell Publishing, Oxford, UK, pp 264–304 [Google Scholar]
- Olsen OA. (2007) Endosperm: Developmental and Molecular Biology. Springer-Verlag, Berlin, Heidelberg [Google Scholar]
- Ordaz-Ortiz JJ, Marcus SE, Knox JP. (2009) Cell wall microstructure analysis implicates hemicellulose polysaccharides in cell adhesion in tomato fruit pericarp parenchyma. Mol Plant 2: 910–921 [DOI] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ. (2012) Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol 158: 465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen HL, Fangel JU, McCleary B, Ruzanski C, Rydahl MG, Ralet M-C, Farkas V, von Schantz L, Marcus SE, Andersen MCF, et al. (2012) Versatile high-resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J Biol Chem (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Li Y, Gilday AD, Graham S, Graham IA. (2006) Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18: 1887–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L, Müller K, Hermann K, Leubner-Metzger G. (2003) Distinct expression patterns of β-1,3-glucanases and chitinases during the germination of Solanaceous seeds. Seed Sci Res 13: 139–153 [Google Scholar]
- Pettolino FA, Walsh C, Fincher GB, Bacic A. (2012) Determining the polysaccharide composition of plant cell walls. Nat Protoc 7: 1590–1607 [DOI] [PubMed] [Google Scholar]
- Puhlmann J, Bucheli E, Swain MJ, Dunning N, Albersheim P, Darvill AG, Hahn MG. (1994) Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1→2)-linked fucosyl-containing epitope. Plant Physiol 104: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajjou L, Debeaujon I. (2008) Seed longevity: survival and maintenance of high germination ability of dry seeds. C R Biol 331: 796–805 [DOI] [PubMed] [Google Scholar]
- Ren CW, Kermode AR. (2000) An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol 124: 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renew S, Heyno E, Schopfer P, Liszkay A. (2005) Sensitive detection and localization of hydroxyl radical production in cucumber roots and Arabidopsis seedlings by spin trapping electron paramagnetic resonance spectroscopy. Plant J 44: 342–347 [DOI] [PubMed] [Google Scholar]
- Ros-Barcelo A, Pomar F, Lopez-Serrano M, Martinez P, Pedreno MA. (2002) Developmental regulation of the H2O2-producing system and of a basic peroxidase isoenzyme in the Zinnia elegans lignifying xylem. Plant Physiol Biochem 40: 325–332 [Google Scholar]
- Sánchez RA, Sunell L, Labavitch JM, Bonner BA. (1990) Changes in the endosperm cell walls of two Datura species before radicle protrusion. Plant Physiol 93: 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P, Plachy C, Frahry G. (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125: 1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirsat AH, Bell A, Spence J, Harris JN. (1996) The Brassica napus extA extensin gene is expressed in regions of the plant subject to tensile stresses. Planta 199: 618–624 [Google Scholar]
- Sims IM, Bacic A. (1995) Extracellular polysaccharides from suspension cultures of Nicotiana plumbaginifolia. Phytochemistry 38: 1397–1405 [Google Scholar]
- Sitrit Y, Hadfield KA, Bennett AB, Bradford KJ, Downie AB. (1999) Expression of a polygalacturonase associated with tomato seed germination. Plant Physiol 121: 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M, Beven A, Donovan N, Neill SJ, Peart J, Roberts K, Knox JP. (1994) Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant J 5: 237–246 [Google Scholar]
- Smallwood M, Martin H, Knox JP. (1995) An epitope of rice threonine- and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta 196: 510–522 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Li L, Sun YH, Chiang VL. (2006) The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiol 142: 1233–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo J, Lamed R, Chirino AJ, Morag E, Bayer EA, Shoham Y, Steitz TA. (1996) Crystal structure of a bacterial family-III cellulose-binding domain: a general mechanism for attachment to cellulose. EMBO J 15: 5739–5751 [PMC free article] [PubMed] [Google Scholar]
- Toorop PE, van Aelst AC, Hilhorst HWM. (2000) The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J Exp Bot 51: 1371–1379 [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP. (2009a) An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr Res 344: 1858–1862 [DOI] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Verhoef R, Schols HA, McCleary BV, McKee L, Gilbert HJ, Knox JP. (2009b) Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J 59: 413–425 [DOI] [PubMed] [Google Scholar]
- Voegele A, Linkies A, Müller K, Leubner-Metzger G. (2011) Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. J Exp Bot 62: 5131–5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Tehseen M, Doblin MS, Pettolino FA, Wilson SM, Bacic A, Golz JF. (2011) The transcriptional regulator LEUNIG_HOMOLOG regulates mucilage release from the Arabidopsis testa. Plant Physiol 156: 46–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, Marcus SE, Knox JP. (1998) Generation of monoclonal antibody specific to (1→5)-α-l-arabinan. Carbohydr Res 308: 149–152 [DOI] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP. (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9–27 [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Steele-King CG, Marcus SE, Mort A, Huisman M, van Alebeek G-J, Schols HA, Voragen AGJ, Le Goff A, et al. (2004) A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta 218: 673–681 [DOI] [PubMed] [Google Scholar]
- Wu CT, Leubner-Metzger G, Meins F, Jr, Bradford KJ. (2001) Class I β-1,3-glucanase and chitinase are expressed in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol 126: 1299–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapo BM. (2011) Rhamnogalacturonan-I: a structurally puzzling and functionally versatile polysaccharide from plant cell walls and mucilages. Pol Rev 51: 391–413 [Google Scholar]
- Zablackis E, Huang J, Müller B, Darvill AG, Albersheim P. (1995) Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol 107: 1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.