Abstract
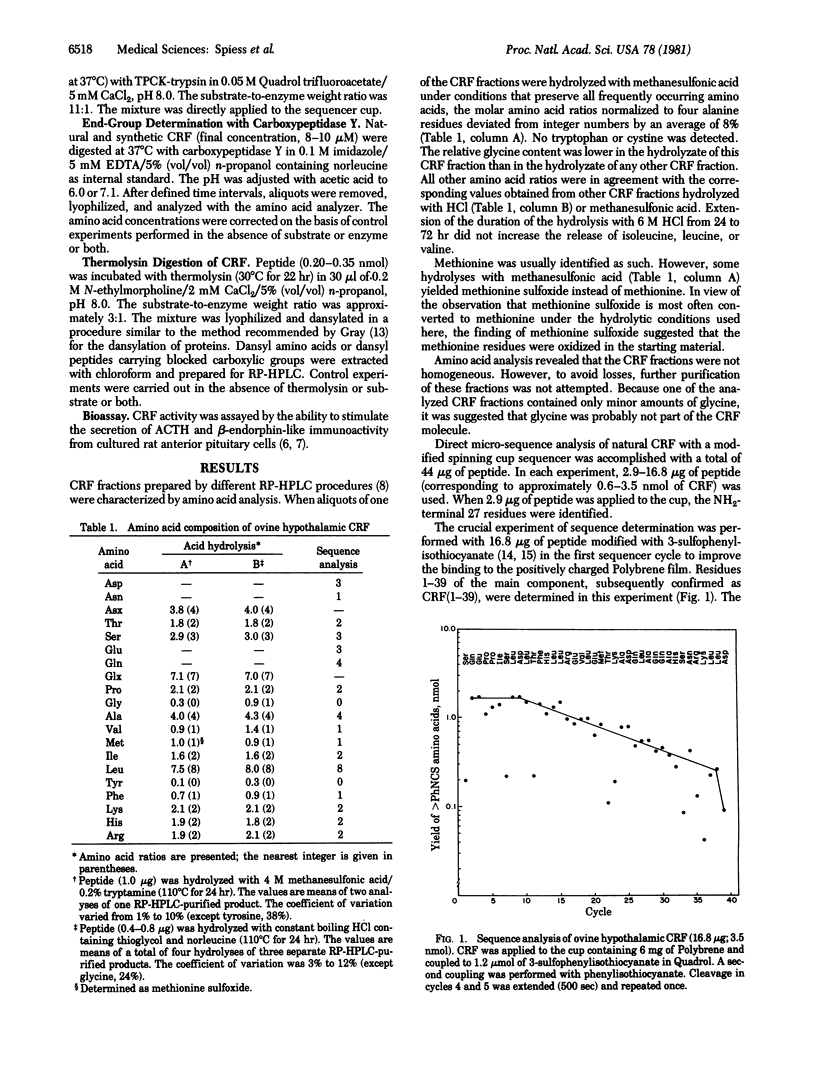
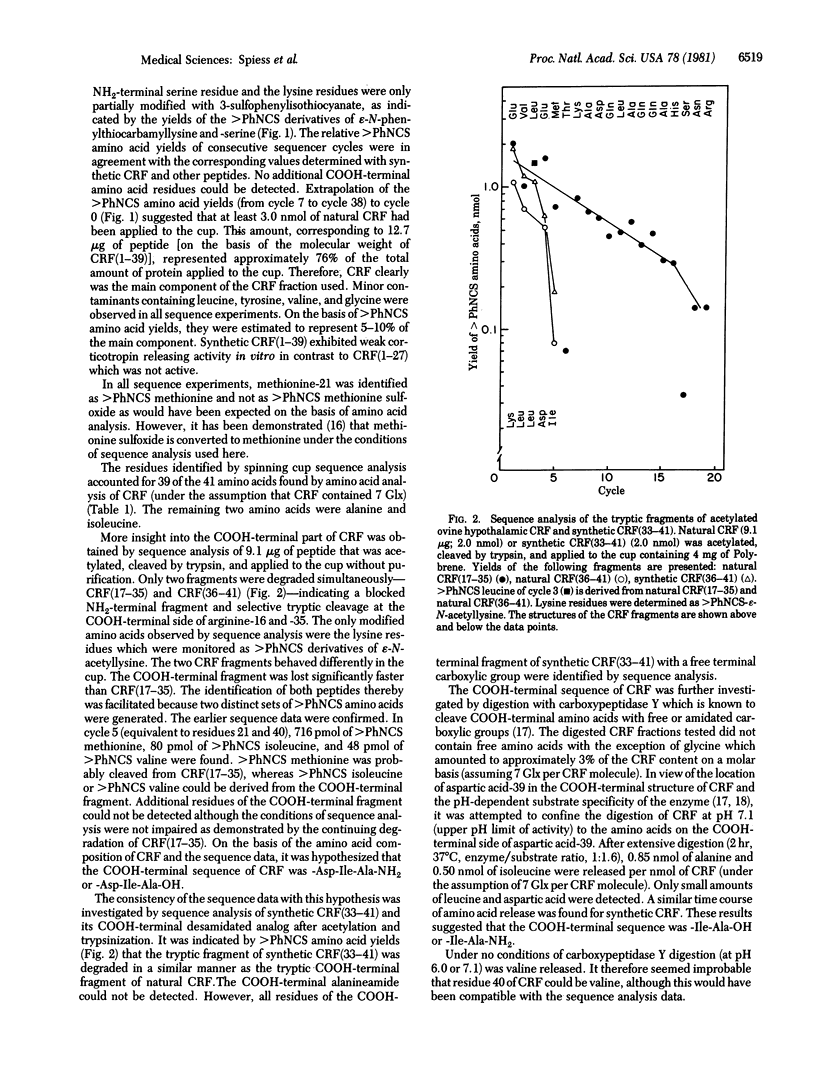
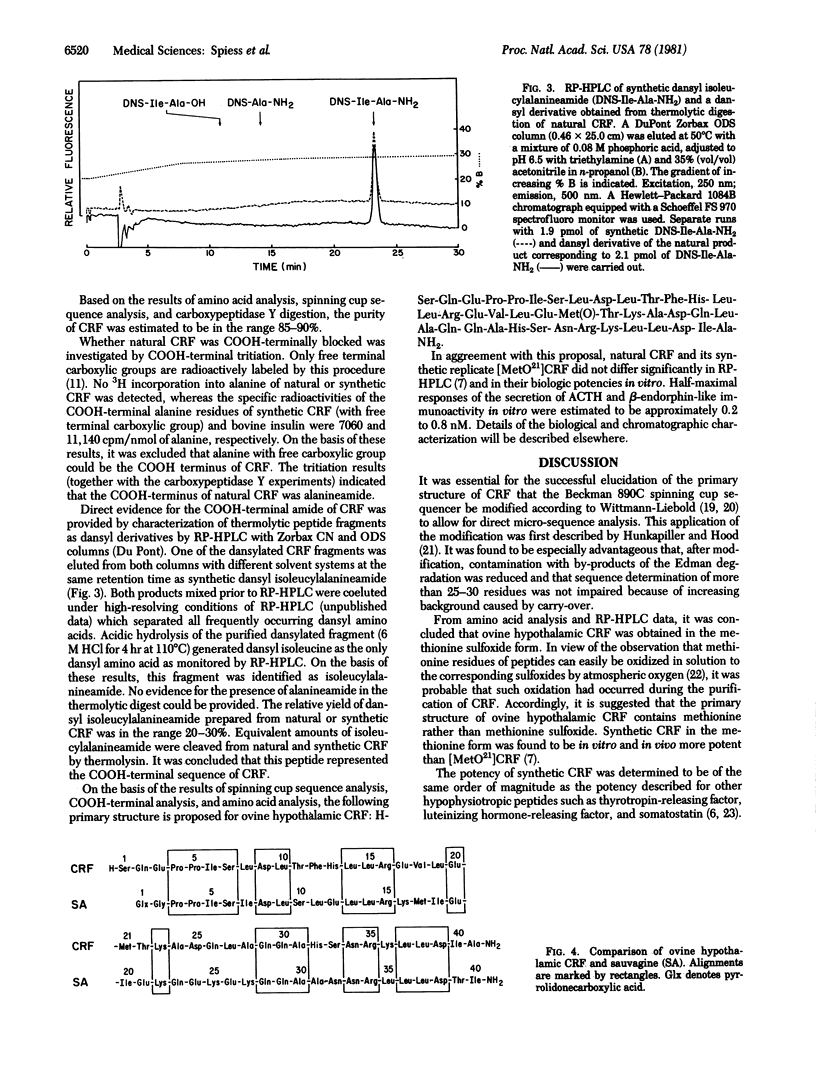
Sequence analysis was performed of an ovine hypothalamic 41-residue polypeptide that had been postulated to be a putative corticotropin-releasing factor (CRF) because of its high intrinsic corticotropin releasing activity. The NH2-terminal 39 residues of CRF were determined by Edman degradation of 0.6-3.5 nmol of peptide in a Wittmann-Liebold modified Beckman 890C spinning cup sequencer with reverse-phase high-pressure liquid chromatography for the identification of amino acid phenylthiohydantoins (direct micro-sequence analysis). Evidence for residue 40 (isoleucine) was provided by direct micro-sequence analysis of 2.0 nmol of acetylated CRF selectively cleaved at its arginine residues by trypsin prior to analysis. The thermolytic COOH-terminal fragment isoleucyl-alanineamide was characterized as its dansyl derivative. Based on the analytical data, the following primary structure is proposed for ovine hypothalamic CRF: H-Ser-Gln-Glu-Pro-Pro-Ile-Ser-Leu-Asp-Leu-Thr-Phe-His-Leu-Leu-Arg-Glu-Val-Leu-Glu-Met-Thr-Lys-Ala-Asp-Gln-Leu-Ala-Gln-Gln-Ala-His-Ser-Asn-Arg-Lys-Leu-Leu-Asp -Ile-Ala-NH2. In agreement with this proposal, the synthetic replicate of CRF is highly potent in stimulating secretion of both corticotropin and beta-endorphin-like immunoactivities.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braunitzer G., Schrank B., Ruhfus A. Zum vollständigen und autoatischen Abbau von Peptiden nach der Quadrolmethode. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1589–1590. [PubMed] [Google Scholar]
- Burgus R., Amoss M., Brazeau P., Brown M., Ling N., Rivier C., Rivier J., Vale W., Villarreal J. Isolation and characterization of hypothalamic peptide hormones. Curr Top Mol Endocrinol. 1976;3:355–372. doi: 10.1007/978-1-4684-2598-7_19. [DOI] [PubMed] [Google Scholar]
- Cappugi G., Nassi P., Treves C., Ramponi G. Use of amino acid-analyzer for identification of 3H labelled C-terminal amino acid. Experientia. 1971 Feb 15;27(2):237–239. doi: 10.1007/BF02145918. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Gurd F. R. A comparison of sulfonated phenylisothiocyanates for reducing losses of lysine-containg peptides during automated sequencing. Anal Biochem. 1976 Dec;76(2):530–538. doi: 10.1016/0003-2697(76)90345-6. [DOI] [PubMed] [Google Scholar]
- GUILLEMIN R., ROSENBERG B. Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 1955 Nov;57(5):599–607. doi: 10.1210/endo-57-5-599. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Bai Y., Hata T. Kinetic studies of carboxypeptidase Y. I. Kinetic parameters for the hydrolysis of synthetic substrates. J Biochem. 1975 Jan 1;77(1?):69–79. [PubMed] [Google Scholar]
- Hayashi R. Carboxypeptidase Y in sequence determination of peptides. Methods Enzymol. 1977;47:84–93. doi: 10.1016/0076-6879(77)47010-1. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- SAFFRAN M., SCHALLY A. V. The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol. 1955 May;33(3):408–415. [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, KAHN J. R., LENTZ K., SHUMWAY N. P. The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med. 1957 Sep 1;106(3):439–453. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savige W. E., Fontana A. Interconversion of methionine and methionine sulfoxide. Methods Enzymol. 1977;47:453–459. doi: 10.1016/0076-6879(77)47045-9. [DOI] [PubMed] [Google Scholar]
- Spiess J., Rivier J. E., Rodkey J. A., Bennett C. D., Vale W. Isolation and characterization of somatostatin from pigeon pancreas. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2974–2978. doi: 10.1073/pnas.76.6.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Villarreal J., Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981 Mar 31;20(7):1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- Vale W., Brazeau P., Rivier C., Brown M., Boss B., Rivier J., Burgus R., Ling N., Guillemin R. Somatostatin. Recent Prog Horm Res. 1975;31:365–397. doi: 10.1016/b978-0-12-571131-9.50014-1. [DOI] [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B. Amino acid sequence studies on ten ribosomal proteins of Escherichia coli with an improved sequenator equipped with an automatic conversion device. Hoppe Seylers Z Physiol Chem. 1973 Oct-Nov;354(10-11):1415–1431. doi: 10.1515/bchm2.1973.354.2.1415. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Graffunder H., Kohls H. A device coupled to a modified sequenator for the automated conversion of anilinothiazolinones into PTH amino acids. Anal Biochem. 1976 Oct;75(2):621–633. doi: 10.1016/0003-2697(76)90117-2. [DOI] [PubMed] [Google Scholar]


