Abstract
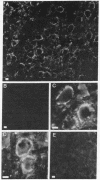
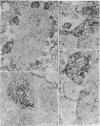
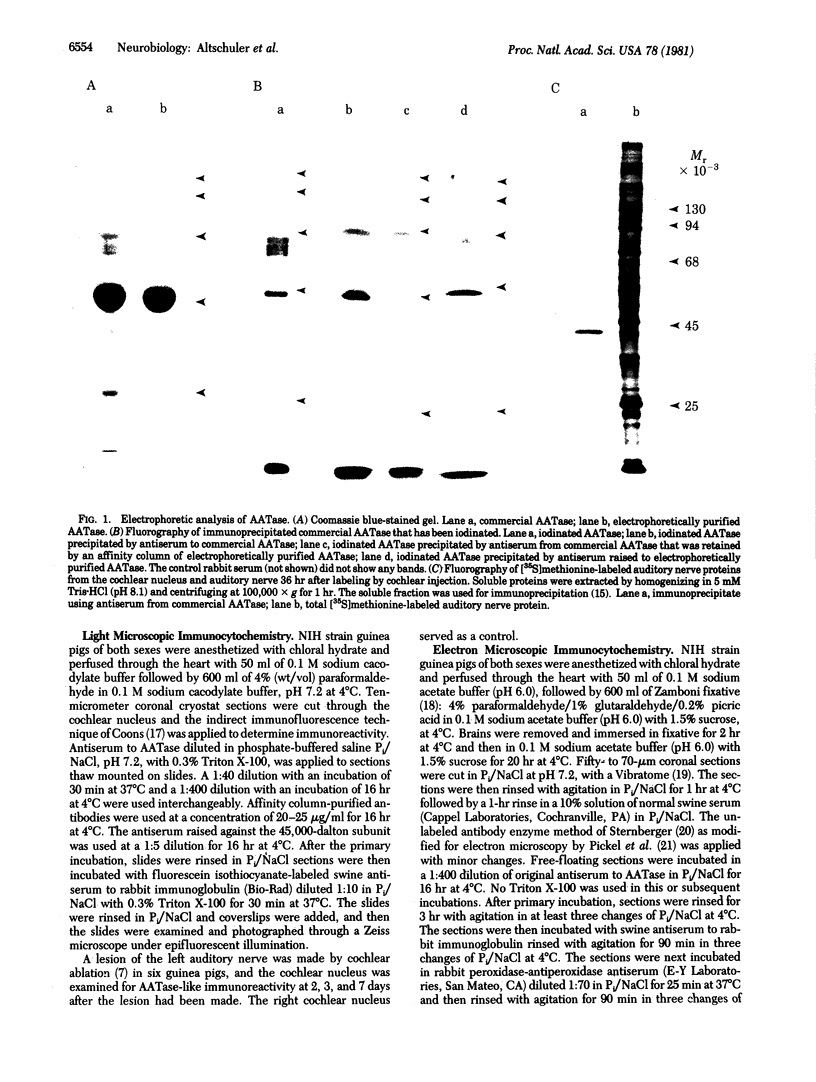
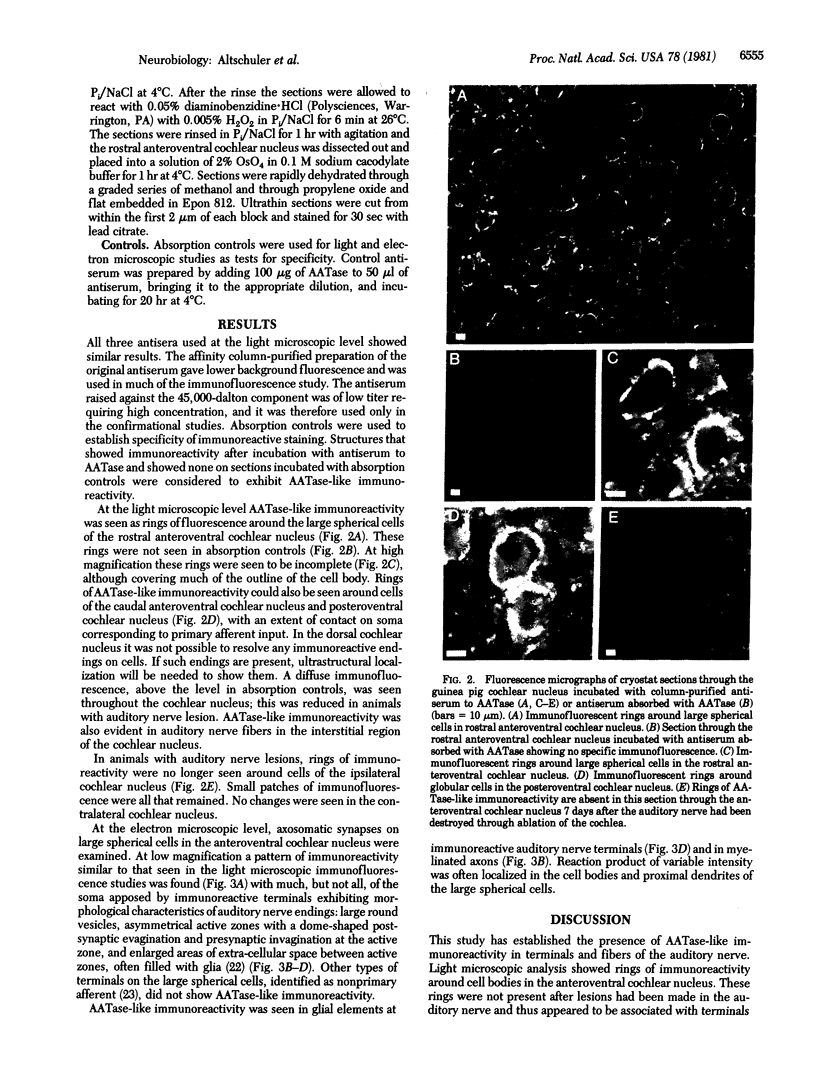
There is substantial evidence supporting the role of aspartate or glutamate as the neurotransmitter of the auditory nerve. The concentration of aspartate aminotransferase (L-aspartate:2-oxoglutarate aminotransferase, EC 2.6.1.1), an enzyme associated with the metabolism of these amino acids, is high in axons and terminals of the auditory nerve. Antibodies were raised against aspartate aminotransferase and used in immunocytochemical studies to determine its localization in the cochlear nucleus of the guinea pig. Indirect immunofluorescence techniques were used for light microscopic localization of aspartate aminotransferase-like immunoreactivity in normal guinea pigs and guinea pigs with auditory nerve lesions. Fluorescent rings of aspartate aminotransferase-like immunoreactivity were seen around spherical cells in the anteroventral cochlear nucleus. In animals with auditory nerve lesions, rings were no longer seen in the ipsilateral cochlear nucleus. Immunoreactivity was also seen on cells in the posteroventral cochlear nucleus and in auditory nerve fibers. Ultrastructural studies were done in the rostral anteroventral cochlear nucleus, using the peroxidase-antiperoxidase technique. Aspartate aminotransferase-like immunoreactivity was seen at axosomatic synapses on large spherical cells in terminals with the morphological characteristics of auditory nerve terminals. Other classes of terminals on the soma of large spherical cells showed no immunoreactivity. It was concluded that aspartate aminotransferase-like immunoreactivity is present in axons and terminals of the auditory nerve. These findings indicate that aspartate aminotransferase-like immunoreactivity may serve as a marker at terminals where aspartate or glutamate is a neurotransmitter.
Full text
PDF
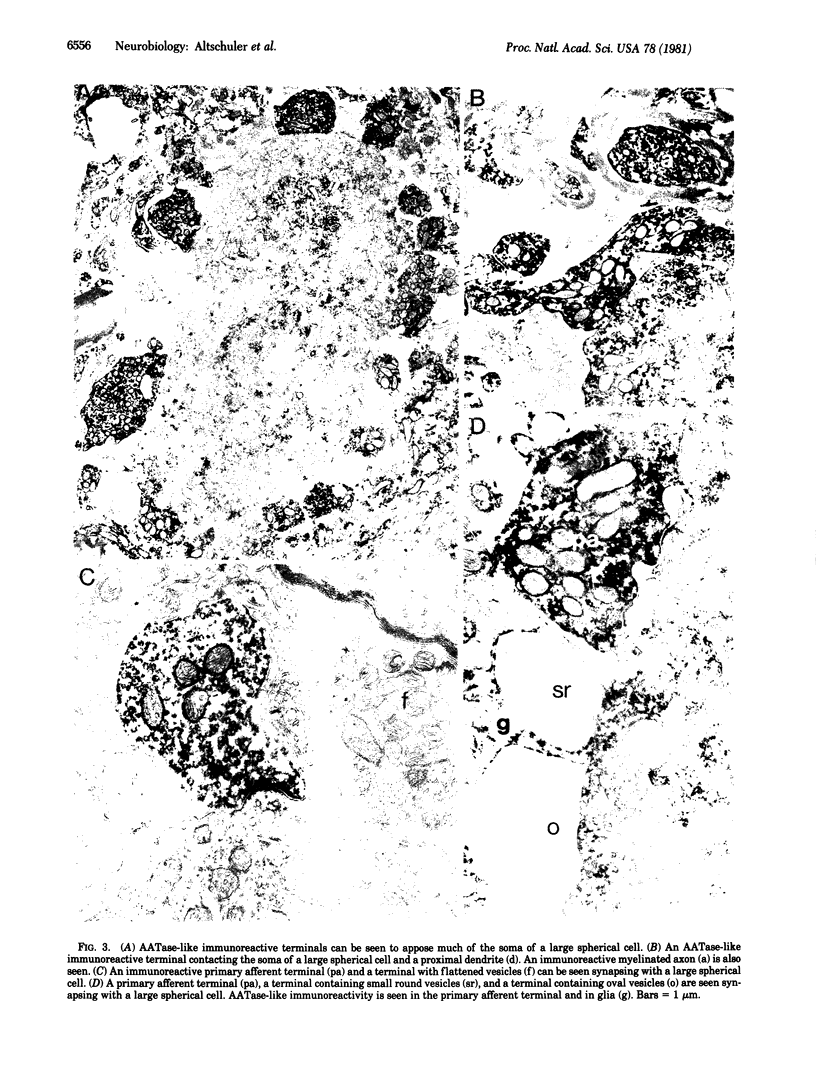

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Gulley R. L., Landis D. M., Reese T. S. Internal organization of membranes at end bulbs of Held in the anteroventral cochlear nucleus. J Comp Neurol. 1978 Aug 15;180(4):707–741. doi: 10.1002/cne.901800405. [DOI] [PubMed] [Google Scholar]
- Gulley R. L., Wenthold R. J., Neises G. R. Changes in the synapses of spiral ganglion cells in the rostral anteroventral cochlear nucleus of the waltzing guinea pig following hair cell loss. Brain Res. 1978 Dec 15;158(2):279–294. doi: 10.1016/0006-8993(78)90675-3. [DOI] [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Martin M. R., Adams J. C. Effects of DL-alpha-aminoadipate on synaptically and chemically evoked excitation of anteroventral cochlear nucleus neurons of the cat. Neuroscience. 1979;4(8):1097–1105. doi: 10.1016/0306-4522(79)90191-x. [DOI] [PubMed] [Google Scholar]
- Martin M. R. The effects of iontophoretically applied antagonists on auditory nerve and amino acid evoked excitation of anteroventral cochlear nucleus neurons. Neuropharmacology. 1980 Jun;19(6):519–528. doi: 10.1016/0028-3908(80)90021-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrion M., Turano C., Chiancone E., Bossa F., Giartosio A., Riva F., Fasella P. Isolation and characterization of multiple forms of glutamate-asparate aminotransferase from pig heart. J Biol Chem. 1967 May 25;242(10):2397–2409. [PubMed] [Google Scholar]
- Martinez-Hernandez A., Bell K. P., Norenberg M. D. Glutamine synthetase: glial localization in brain. Science. 1977 Mar 25;195(4284):1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- McLennan H. The autoradiographic localization of L-[3h]glutamate in rat brain tissue. Brain Res. 1976 Oct 8;115(1):139–144. doi: 10.1016/0006-8993(76)90828-3. [DOI] [PubMed] [Google Scholar]
- Norenberg M. D., Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979 Feb 2;161(2):303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Reis D. J. Monoamine-synthesizing enzymes in central dopaminergic, noradrenergic and serotonergic neurons. Immunocytochemical localization by light and electron microscopy. J Histochem Cytochem. 1976 Jul;24(7):792–306. doi: 10.1177/24.7.8567. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Reis D. J. Ultrastructural localization of tyrosine hydroxylase in noradrenergic neurons of brain. Proc Natl Acad Sci U S A. 1975 Feb;72(2):659–663. doi: 10.1073/pnas.72.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. M., Gulley R. L. Non-primary afferents to the principal cells of the rostral anteroventral cochlear nucleus of the guinea pig. Am J Anat. 1978 Dec;153(4):489–508. doi: 10.1002/aja.1001530402. [DOI] [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J., Iversen L. L. Uptake of [3H]Glutamic acid in excitatory nerve endings: light and electronmicroscopic observations in the hippocampal formation of the rat. Neuroscience. 1979;4(9):1237–1253. doi: 10.1016/0306-4522(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Tytell M., Gulley R. L., Wenthold R. J., Lasek R. J. Fast axonal transport in auditory neurons of the guinea pig: a rapidly turned-over glycoprotein. Proc Natl Acad Sci U S A. 1980 May;77(5):3042–3046. doi: 10.1073/pnas.77.5.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold R. J. Glutamate and aspartate as neurotransmitters for the auditory nerve. Adv Biochem Psychopharmacol. 1981;27:69–78. [PubMed] [Google Scholar]
- Wenthold R. J. Glutamic acid and aspartic acid in subdivisions of the cochlear nucleus after auditory nerve lesion. Brain Res. 1978 Mar 31;143(3):544–548. doi: 10.1016/0006-8993(78)90365-7. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J. Glutaminase and aspartate aminotransferase decrease in the cochlear nucleus after lesion of the auditory nerve. Brain Res. 1980 May 19;190(1):293–297. doi: 10.1016/0006-8993(80)91183-x. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J., Gulley R. L. Aspartic acid and glutamic acid levels in the cochlear nucleus after auditory nerve lesion. Brain Res. 1977 Dec 9;138(1):111–123. doi: 10.1016/0006-8993(77)90787-9. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J. Release of endogenous glutamic acid, aspartic acid and GABA from cochlear nucleus slices. Brain Res. 1979 Feb 23;162(2):338–343. doi: 10.1016/0006-8993(79)90294-4. [DOI] [PubMed] [Google Scholar]