Abstract
BACKGROUND
Liver ischemia(I)/reperfusion(R) injury(I) is a known risk factor for the postoperative outcome of patients undergoing liver surgery/transplantation. Attempts to protect from organ damage require multidisciplinary strategies and are of emerging interest in view of patients with higher age and ASA-status. Ischemic preconditioning has been successfully applied to prevent from IRI during liver resections/transplantation. Since even short periods of ischemia during preconditioning inevitably lead to hypoxia and formation of anti-inflammatory/ cytoprotective acting adenosine, we reasoned that short non-ischemic hypoxia also protects against hepatic IRI.
METHODS
Mice underwent hypoxic preconditioning(HPC) by breathing 10%-oxygen for 10 minutes, followed by 10 minutes of 21%-oxygen prior to left-liver-lobe-ischemia(45 min) and reperfusion(4 hrs). The interactions of hypoxia->adenosine->adenosine-receptors were tested by pharmacologic antagonism at adenosine receptor(AR) sites in wild type mice and in mice with genetic deletions at the A1-;A2A-;A2B- and A3-ARs. Hepatocellular damage, inflammation and metabolic effects were quantified by enzyme activities, cytokines, liver-myeloperoxidase(MPO), blood adenosine and tissue-adenosinemonophosphate(AMP), respectively.
RESULTS
Hepatoprotection by HPC was significant in wild type and A1-, A2A-and A3 AR-knock-out mice as quantified by lower ALT serum activities, cytokine levels, histological damage-scores, tissue-myeloperoxidase-concentrations and as well as preserved AMP-concentrations. Protection by HPC was blunted in mice pretreated with the A2B-AR-antagonist MRS1754 or in A2B-AR“knock-outs”.
CONCLUSION
Because liver protective effects of HPC are negated when the A2B receptor is non-functional, the "hypoxia->adenosine->A2B receptor" pathway plays a critical role in the prevention of warm ischemia reperfusion injury in vivo. Hypoxic activation of this pathway warrants use of selective A2B-AR-agonists or even intermittent hypoxia (e.g. in deceased organ donors) to protect from liver ischemia/reperfusion injury.
Keywords: hypoxia, murine liver ischemia, preconditioning, hepatoprotection
INTRODUCTION
Liver resections and transplantation has become a routine method to treat otherwise non-curable liver disease of malignant or non-malignant origin. However the organ damage following warm and cold ischemia is aggravated to various degrees following reperfusion. This pathology is defined as ischemia and reperfusion injury (IRI). The underlying mechanisms consist of a disturbed intracellular homeostasis, early immune cell activation, and transmigration, followed by tissue inflammation and evolving organ edema during reperfusion leading to the aggravation of the imbalance between the demand and the achieved nutritional flow to the organ. For example in solid organ transplantation IRI has a direct effect on graft survival and is associated with high morbidity and mortality(1). When organs of marginal quality are transplanted because of donor organ shortage the damaging effects of IRI may be even more critical(2).
Besides general e.g. pharmacological treatments (e.g. corticoids) or anti-cytokine directed approaches (e.g. anti-TNF-α), preconditioning protocols represent alternative non-pharmacological protective approaches. Preconditioning allows organs to adapt to subsequent severe forms of cellular stress caused by ischemic, hypoxic or metabolic insults and confers tolerance and cytoprotection(3–5)). Clinical studies and reports clearly demonstrate benefits of ischemic preconditioning in the course of liver resections (6–8) and also when applied to the donor liver before transplantation(9, 10).
Since ischemia as used for preconditioning inevitably leads to local, i.e. organ hypoxia, we asked whether short, non-ischemic general hypoxia may also protect against hepatic IRI. It has been suggested that the protection by ischemic preconditioning against IRI may be mediated by the action of adenosine, and this may also be true for the protection by hypoxic preconditioning(11, 12). Like ischemia, hypoxia alone results in the hydrolysis of ATP leading to the accumulation of adenosine and its metabolites inosine and hypoxanthine (13). The protective effects of intra- and extracellular formation and release of adenosine are mediated by binding to specific receptors(14). To date, four adenosine receptors A1, A2A, A2B and A3 have been characterised (15, 16). However, the potential benefits of hypoxic preconditioning in vivo and the underlying potentially adenosinergic mechanisms of protection have never been tested so far.
RESULTS
1.) Warm hepatic ischemia/reperfusion injury and the effects of hypoxic preconditioning on liver cell integrity, tissue damage and inflammation
Ischemia (45 minutes) and reperfusion (4 hours) of the left liver lobe caused an increase of serum activities of ALT, AST and LDH. Compared to control mice, serum ALT, AST, and LDH were significantly lower in animals that were pre-treated by hypoxic (10% oxygen) preconditioning (ALT in Fig.1; AST [IU/L] control vs. HPC: 4772±270 vs. 3358±285, p<0.05; LDH [IU/L] control vs. HPC: 16397±1058 vs. 12660±1229;p<0.05). This protective effect was seen already after 1 h of reperfusion (ALT [IU/L] control vs. HPC: 2587±460 vs. 1202±324, p<0.05) as well as after 24 hrs (not shown). The significant decrease of hepatocellular damage was confirmed by histology (Fig.2) as evidenced by a decrease of liver-tissue damage from approximately 75 % of unprotected livers (control) to 50 % (HPC).
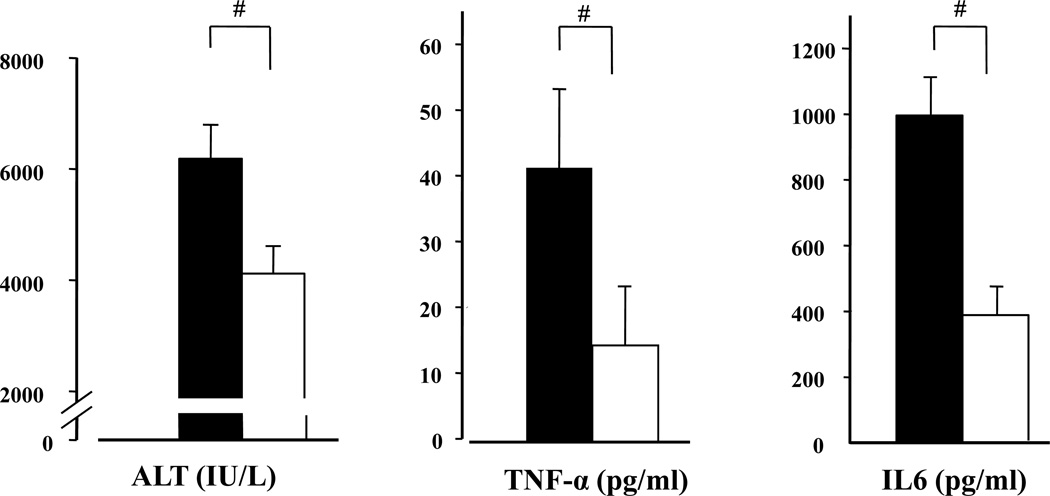
Figure 1. Effect of hypoxic preconditioning (HPC) on hepatic ischemia and reperfusion injury: ALT, TNF-alpha and Il-6 serum concentrations.
Mice were subjected to ischemia (45 min) and liver enzymes were determined after 4 hours of reperfusion. As compared with control mice (filled bars) breathing normal air over the whole experimental period, mice subjected to hypoxic preconditioning (HPC) prior to ischemia-reperfusion (open bars) showed liver enzyme activities that were reduced significantly by more than 30%. Hypoxic preconditioning was achieved by subjecting mice to 10% oxygen for 10 minutes followed by breathing of normal air (21% O2) for another 10 minutes prior to start of ischemia. (Means ± SEM, T-test: #p< 0.05 vs. “No-HPC”; n= 15 per group). TNF-α and IL-6 concentrations determined in serum after ischemia (45 min) and reperfusion (4 h) were significantly reduced by hypoxic preconditioning. Sham animals were treated accordingly (No-HPC with 21% O2, HPC with 10% O2,), underwent laparotomy followed by left liver lobe clamping but, with immediate unclamping to only induce the trauma, but no relevant liver ischemia and reperfusion (Means ± SEM “No-HPC-sham”: ALT 113±6,6 IU/L.; AST 200±0, LDH 340± 120; “HPC-sham”: ALT 1003±0 IU/L; AST 193±17, LDH 962 ± 174). TNF-α and IL-6 concentration in sham –treated mice were below the detection threshold of 5 pg/ml (Means ± SEM, #: p<0.05 vs. “No HPC”, t-Test, n=8 per group).
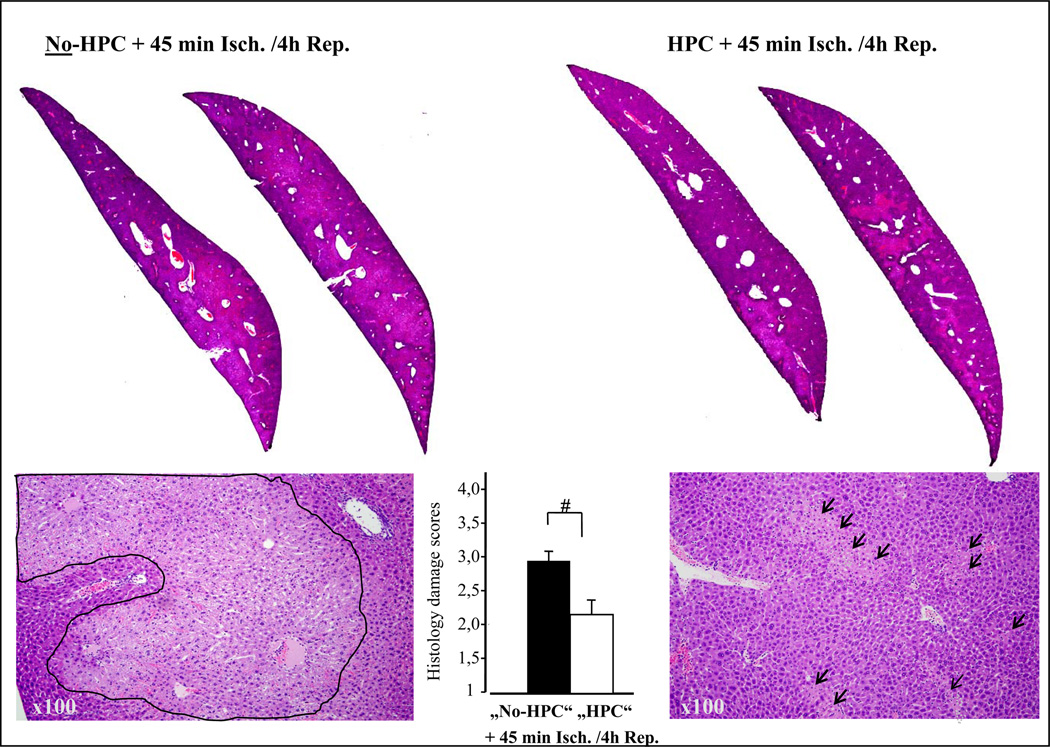
Figure 2. Effect of HPC on hepatic ischemia and reperfusion injury: Histology.
Staining of non damaged liver tissue with H&E results in an eosinophilic, homogenous staining indicating viable/undamaged cells which appear almost as black in the black & white print. However, after 45 minutes of ischemia and 4 hours of reperfusion major parts of the left liver lobe sections (upper left two slides) turned homogenously pale into confluent "grey" areas (see line-marked areas in lower left 100× magnification) indicating severe tissue damage (hepatocyte degeneration and necrosis). In contrast, when the mouse was treated by hypoxic preconditioning prior to ischemia and reperfusion (HPC, two upper right slices) areas of damage became less pronounced and showed focally (arrow marked areas in lower left 100× magnification) rather than confluent pattern of tissue damage.
TNF-α serum concentrations increased from undetectable values in sham-operated groups to mean values of 40pg/ml after liver ischemia and reperfusion. When hypoxic preconditioning preceded liver ischemia TNF-α concentrations were significantly reduced (Fig.1). Similar changes were obtained for the effects of HPC on ischemia-reperfusion induced IL-6 release. IL-6 mean values after sham operation remained low in the range of the detection threshold of the assays (data not shown).
2.) Effects of 10 minutes of 10 % oxygen for hypoxic preconditioning on arterial oxygen tension, plasma purines, tissue damage and inflammatory responses
As compared to normoxic conditions (control) breathing hypoxic gas mixture for 10 minutes during hypoxic preconditioning decreased arterial oxygen tension (paO2) from ~75 mm Hg (21%oxygen) to 40 mmHg (10%oxygen) in anesthetized, spontaneously breathing mice. Inversely to the drop of paO2, adenosine plasma concentrations increased during hypoxia (21%O2 vs. 10%O2: 436±70 nM vs. 664±42 nM; n=7, p<0.01). Accordingly, the adenosine degradation products inosine and hypoxanthine were elevated (inosine 21% O2 vs. 10% O2: 38±17 vs. 261±131nM, p=0.07; hypoxanthine 21% O2 vs. 10% O2 : 226±132 vs. 711±103nM, p=0.03).
Exposure to hypoxia for 10 minutes alone had no effect on markers of liver tissue damage.
Parameters of hepatocellular integrity (ALT, AST, LDH), histology or inflammatory cytokines (IL-6, TNF-α) indicated no signs of liver damage or inflammation, respectively (data not shown).
To identify effects of hypoxia on inflammatory reactions, we have additionally tested distinct innate and adaptive immune responses in mice subjected to 10 minutes of hypoxia alone using the following set-up:
Mice were treated in the same way as mice in the control group of the study protocol but were not subjected to surgery. No effects could be observed on immunological reactions determined ex vivo immediately or 45 minutes after hypoxic precondition, respectively. The capability of murine granulocytes to generate H2O2 was not affected by hypoxic pretreatment as evidenced by unchanged resting or stimulated H2O2 production, respectively. Similarly, CD11b expression on murine granulocytes remained unaffected. When murine spleen T-cells were activated ex vivo by anti-CD3mAb for 24 h, no differences were observed between hypoxic and normoxic pre-treated mice with respect to stimulated interferon-gamma production respectively (SDC Tab. 1).
3.) Hypoxic preconditioning and the role of adenosine receptors
All four adenosine receptors were shown to be expressed in murine liver tissue. The relative expression levels of A1-, A2A-, A2B- and A3-receptor-specific mRNA was almost equivalently distributed with a preference for the A2B adenosine receptors (38%, n=2). To test which subtype of the different adenosine receptors mediates HPC effects, WT mice pre-treated with antagonists to specific adenosine receptors or mice with genetic deficiencies were preconditioned by breathing hypoxic air and the extent of protection against ischemia/reperfusion was determined.
Role of A2A -and A2B adenosine receptors (AR) in hypoxic preconditioning
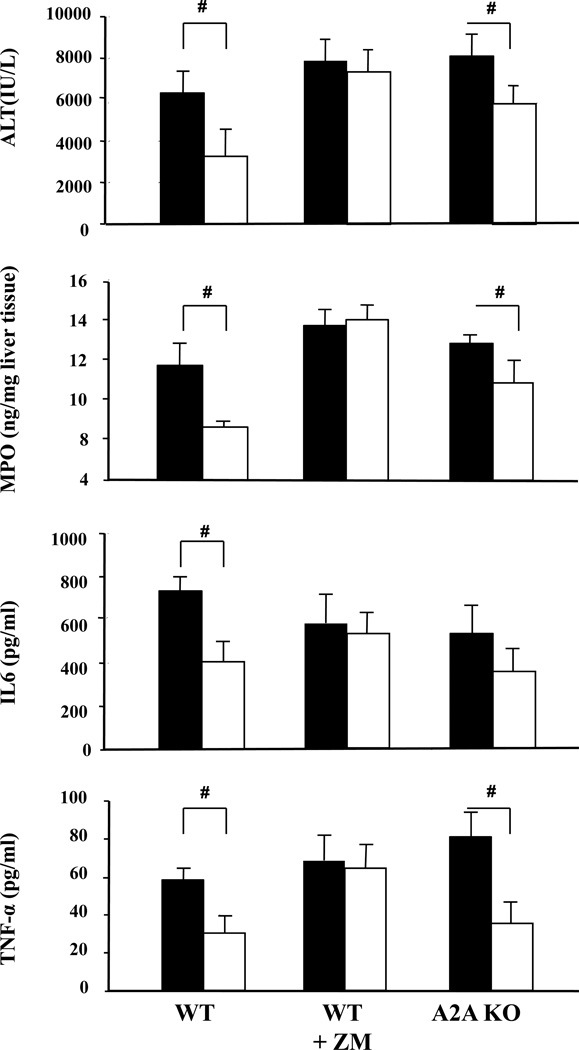
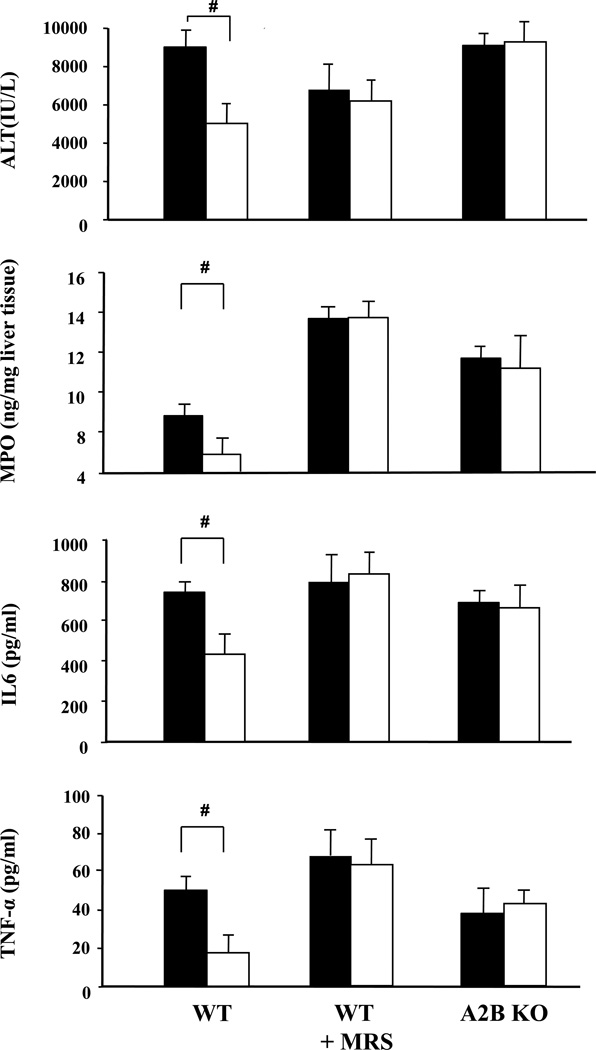
In contrast to controls (Fig.1, Fig.3), HPC was no longer hepatoprotective in WT mice pre-treated with the adenosine receptor (AR) antagonist ZM241385, known to be antagonistic at both A2A and A2B AR sites (Fig. 3). When WT mice were treated with the selective A2B antagonist MRS1754 prior to hypoxic preconditioning, the protection in the HPC-group was nullified as shown by parameters of liver cell integrity (ALT, Fig. 4) and histology (SDC Tab. 2), sequestration of myeloid cells (MPO, Fig. 4) and inflammatory responses (IL6, TNF-α, Fig. 4). Also pharmacologic antagonism at the A2B AR receptor with either MRS1754 or ZM241385 was tested in A2A AR knock out mice and was also shown to reverse the effects of HPC (e.g. ALT: HPC A2A AR-KO vs. HPC A2A AR-KO+MRS1754 vs. HPC A2A AR-KO+ZM241385; 4853±823 vs. 5825±877 vs. 6466±1138 [IU/L]; IL6: 343±74 vs. 465±133 vs. 580±150 (pg/ml), n=5–9, A2B AR antagonists treated vs. untreated, n.s.). Testing for the effects of HPC in A2B AR knockout mice showed also full reversal of the protective effects of HPC as verified on all biochemical parameters analysed (ALT, MPO, IL6, TNF-α; Fig. 4) as well a by histology (SDC Tab. 2).
Figure 3. Role of the adenosine A2A receptor (A2A AR) in the protection by HPC from hepatic ischemia-reperfusion injury as assessed by liver enzymes, tissue myeloperoxidase, Interleukin 6 -and TNF-α serum concentration.
A2A AR-wild type (WT) mice, WT mice pre-treated with the adenosine A2 receptor antagonist ZM241385 or mice genetically lacking in A2A adenosine receptors (AR knockout, KO) were subjected to ischemia (45 min) and reperfusion (4 hours) without (filled bars) or with (open bars) prior hypoxic preconditioning. Serum activities of alanine- amino-transferase (ALT) were determined after 4 hours of reperfusion. Comparison of A2A+/+ (A2A WT ± ZM241385) and A2A−/− (A2A KO); Means ± SEM, n = 7–11 per group (#: p<0.05 vs. “No HPC”, T-Test).
Figure 4. A2B adenosine receptors (A2B AR) mediate the protective effects of HPC after hepatic ischemia-reperfusion injury as assessed by liver enzyme tissue myeloperoxidase, Interleukin 6- and TNF-α serum concentrations.
A2B AR wild type (WT) mice, WT mice pre-treated with the selective adenosine A2B receptor antagonist MRS1754 or mice genetically lacking in A2B adenosine receptors (knockout, KO) were subjected to ischemia (45 min) and reperfusion (4 hours) without (filled bars) or with (open bars) prior hypoxic preconditioning. Serum activities of alanine- amino-transferase (ALT) were determined after 4 hours of reperfusion. Comparison of A2B+/+ (A2A WT ± MRS1754) and A2B−/− (A2B AR KO); Means ± SEM, n = 9–10 per group, 8–9 for MPO (#: p<0.05 vs. “No HPC”, T-Test).
Because ischemia is known to activate the cytosolic 5´ AMP nucleotidase, which dephosphorylates AMP to adenosine, we tested for AMP concentration in freeze clamped liver tissues as a function of the decrease in tissue energy charge(17, 18). As compared to sham treated mice without ischemia, AMP tissue concentrations decreased by two thirds when the liver was subjected to 45 min of ischemia. Pre-treatment by 10 min of hypoxic preconditioning could significantly attenuate this decrease and AMP values remained close to those as observed in the sham group. Confirmatively to the effects described for liver integrity and inflammation, the MRS pretreatment significantly reverted this protective effect of HPC, and the AMP tissue concentrations remained low after ischemia (p<0.05, Tab. 1).
Table 1.
Tissue adenosinmonophosphate (AMP) concentrations after hypoxic preconditioning (HPC) and pharmacological antagonism at the A2B receptor site (MRS1754).
| AMP | ||
|---|---|---|
| ng/mg liver wet weight | n | |
| Sham | 1318 ± 241 | 7 |
| No-HPC + 45 min Ischemia | 584 ± 65* | 4 |
| HPC + 45 min Ischemia | 1024 ± 126#+ | 4 |
| MRS Sham | 1502 ± 296 | 6 |
| MRS No-HPC + 45 min Ischemia | 598 ± 51* | 4 |
| MRS HPC + 45 min Ischemia | 718 ± 139 | 4 |
Adenosine monophosphate in the tissue of liver which were quantified either from a) sham operated mice, from ii) mice subjected to 45 min of ischemia without prior hypoxic preconditioning or iii) with prior hypoxic precondition. Liver tissues were further assessed under the same experimental conditions but in mice pretreated with the selective A2B antagonist MRS1754.
Means ± SEM, two sided T-Test, #: p<0.05 "No-HPC", n = 4–7 per group).
Role of the A1 and A3 adenosine receptors in hypoxic preconditioning
Hypoxic preconditioning significantly reduced serum levels of liver enzymes and histological tissue damage scores in both wild type and adenosine A1 AR and A3 AR knockout mice, e.g. ALT [IU/L] in A1 AR-KO: 5390±1333 (control) vs. #2713±853 (HPC); ALT[IU/L] in A3-KO: 7036±1049 (control) vs. #4817±846 (HPC); Histology damage score A1 AR-KO: 1,75±0,025 (control) vs. #1,0±0,0 (HPC); Histology score in A3 AR-KO: 2,0±0,15 (control) vs. #1,4±0,18 (HPC); #p<0.05 vs. respective controls). These findings were also confirmed by a decrease in inflammatory cytokines in hypoxic preconditioned A1 AR–KO and A3 AR-KO mice. In addition, pharmacological antagonism at the A1 receptor site by pre-treatment with the selective adenosine A1 antagonist DPCPX (2.5 mg/kg) did not compromise the protective effects of HPC as shown by improved liver cell integrity, liver tissue damage and inflammatory responses (not shown).
DISCUSSION
Organ injury by ischemia and reperfusion is associated with high morbidity and mortality(19). This has initiated numerous attempts to achieve better tissue protection against ischemia/reperfusion injury of the liver(1) e.g. through ischemic preconditioning (IPC)(6–8, 20, 21). Also in the course of liver transplantation some reports indicate the benefits of IPC in livers of deceased donors that were conditioned in situ and resulted in attenuated inflammation and reduced tissue damage to the allograft(9, 10). These anti-inflammatory and tissue protective effects of IPC were confirmed also in genomic responses e.g. in liver allografts (22). Besides the efficiency of ischemic as well as pharmacologic preconditioning(23, 24), the concept of protection by hypoxia was introduced more recently and was shown to exert cytoprotective effects for cerebral (25–27) and cardiac preconditioning(4, 28). However, there have been no earlier attempts to test hypoxic preconditioning as a hepatoprotective measure in vivo. Since hypoxia is a well known and extremely potent stimulus for the formation of adenosine(16), and each of the four adenosine receptor subtypes has been implicated in the phenomenon of preconditioning, we investigated whether a brief period of in vivo hypoxic preconditioning i) can protect the liver from IRI and reduce inflammatory mediators, ii) can induce formation of adenosine, and asked iii) which of the four adenosine receptor subtypes to provide hepatic protection is involved, and iv) if this effect is due to metabolic protection of parenchymal cells of the liver or to decreased myeloid cell activation and function, respectively.
Hypoxic preconditioning (HPC) attenuates hepatic ischemia-reperfusion injury in vivo
To the best of our knowledge; the results of our experiments provide evidence for the first time that the liver can be protected against ischemia/reperfusion injury (IRI) by hypoxic preconditioning. HPC by breathing 10% oxygen for ten minutes prior to prolonged liver ischemia(I)-and reperfusion(R) can provide significant protection against IRI as shown by the reduction in the extent of liver damage, the amount of sequestered myeloid cells, IL-6 and TNF-α serum concentrations, respectively. The lower concentration of myeloid cells in the liver tissue together with lower level of TNF-α mRNA transcripts (not shown) and IL-6 and TNF-α serum protein concentrations indicate less inflammatory reactions(29). Such an association between liver protection and the reduction of TNF-α serum protein concentrations were also reported very recently by Wang(30).
Hypoxia (10% O2) increases plasma purine concentrations
The purine nucleoside adenosine is produced under conditions that favour ATP-degradation like ischemia and hypoxia(16), involving extracellular located NTPDase (ATP diphosphohydrolase, ecto/CD39) and ecto as well as cytosolic 5'-nucleotidase with the latter enzymes known to dephosphorylate AMP to adenosine. Moreover, it was shown in a heart model that hypoxia leads to the accumulation of adenosine by the inhibition of the enzyme adenosine kinase which under physiological conditions recycles adenosine by phosphorylation to AMP(31). In agreement with earlier studies, demonstrating an increase in extracellular tissue concentrations of adenosine after a hypoxic challenge in the heart muscle(11) or in the hippocampus(12), adenosine plasma concentrations doubled after 10 minutes of breathing 10%oxygen for hypoxic preconditioning. Increased levels of adenosine are consistent with the parallel rise in its degradation products inosine and hypoxanthine(7). Since under physiological conditions plasma levels of adenosine are much lower than interstitial concentrations due to fast reuptake and degradation by the vascular endothelial cell layer and circulating erythrocytes, it is highly likely that the increase of adenosine in liver tissue was even greater than that in plasma.
HPC is not mediated by A2A adenosine receptors
Binding of adenosine to adenosine A2 receptors has been shown to be critically involved in the hypoxic preconditioning of rat hepatocytes against subsequent prolonged hypoxic stress in vitro(32).
Because exposition to hypoxia for 10 minutes increases plasma levels of adenosine, we tested whether adenosine A2 receptors mediate the hepatoprotective action by HPC also in vivo. While the beneficial effect of HPC was fully antagonized by the A2 receptor antagonist ZM 241385, a similar experiment in A2A AR-KO mice revealed that the hypoxic preconditioning phenomenon was not selectively dependent on the presence of a functional A2A adenosine receptor site, as HPC still significantly protected liver tissue against ischemia/reperfusion injury in mice with a genetic lack in A2A AR sites. This result was reproducible in several experiments despite the observation that A2A AR-knockout mice exhibited more tissue damage as compared to wild type littermates, confirming previous results by Linden(33). Since ZM241385, however, is a mixed A2A/B adenosine receptor antagonist which competitively inhibits the action of adenosine not only at A2A but also at A2B adenosine receptor sites(34, 35), the results in A2A knock-out mice pre-treated with ZM241385 indicate that it was most likely the antagonism at the remaining A2B receptor sites(36) which abolished the hepatoprotective effects of HPC.
A2B adenosine receptors mediate the protective effects of HPC
Using quantitative real-time RT-PCR, we demonstrated the presence of mRNA of all adenosine receptor subtypes in liver tissue, the A2B receptor mRNA being the most abundant. Because endogenous adenosine is acting only briefly in the course of in vivo hypoxic preconditioning, one wishes to have hydrolysable and hence short acting selective A2A or A2B receptor agonists to test for pharmacologic preconditioning. However, such short-lived agonists are not available and the currently used selective A2A or A2B agonists have a much longer half-life after i.v. injection, affording liver protection rather by their anti-inflammatory effects than by preconditioning. In order to overcome these problems, wild type mice, A2A and A2B receptor knockout mice were pretreated by either the selective A2B-receptor antagonist MRS1754(37) or its vehicle only, to test for the proposed HPC induced endogenous adenosine->A2B mediated action. Most importantly, when WT mice were pretreated with the potent A2B receptor antagonist MRS1754, the hepatoprotective effect of hypoxic preconditioning was fully antagonized. This effect of antagonism at the A2B receptor site was verified at the level of liver cell integrity markers, of overall tissue necrosis (histology), the infiltration of the liver tissue by myeloid cells (MPO) and humoral parameters of inflammation in the blood (IL-6, TNF-α). The final proof of this critical protective transient hypoxia->adenosine->A2B receptor site signaling pathway in hypoxic preconditioning was provided by A2B receptor knockout mice, in which the protection by the hypoxia->adenosine->A2B receptor site signaling pathway was blocked to the same degree as seen in the MRS1754 pre-treated WT mice.
Are the liver protective effects of HPC resulting from an increased metabolic resistance of hepatocytes to ischemia or are they due to the decreased capability of myeloid cell to become activated during reperfusion?
From the data presented on the effects of HPC to protect from liver damage one can conclude that this procedure is efficient to reduce liver damage in the course of hepatic ischemia and reperfusion.
Interestingly, the extent of hepatocellular damage was decreased as early as after 1h after liver reperfusion. At this very early point in time cytokine levels were not increased (data not shown) indicating that HPC mediates initial prevention of tissue damage through other than inflammatory reactions, but potentially through a cytoprotective pathway. Because tissue ischemia is known to activate the cytosolic 5´AMP nucleotidase as a proportional function of the tissue energy charges´ decrease(17) we tested for the liver AMP concentration which – to our hypothesis - is increasingly degraded under conditions of non-preconditioned liver tissue during ischemia, but could be less subjected to degradation when the liver is pre-conditioned by hypoxic preconditioning, respectively. Accordingly, AMP tissue concentrations decreased by two thirds when the liver was subjected to 45 min of ischemia while the pre-treatment by hypoxic preconditioning could significantly prevent from this effect and AMP values were close as high as observed in the sham group. Confirmatively to the effects described for liver integrity and inflammation, pretreatment with the selective A2B receptor antagonist abolished the protective effect of HPC also at the level of AMP tissue concentrations. These experimental results may allow concluding that hypoxic preconditioning and the adenosine A2B receptor likely affect metabolic processes. This might induce an initial state of hepatocellular protection and therefore seems to be one of the leading mechanisms to reduce - not necessarily primarily but secondarily- the inflammatory consequences of the ischemic insult. However, further experiments are warranted to investigate also the role of the A2B receptor during reperfusion, as inflammatory cascades during reperfusion are likely to be susceptible to regulation by A2B receptor sites(38, 39). Finally, hypoxia alone (10% for 10 min) seemed not to reduce or affect distinct functions of leukocyte functions. When granulocytes or splenocytes were taken from mice subjected to hypoxic preconditioning, functional assays for innate or adaptive immune responses revealed no suppression even after 45 min, i.e. the point in time when reperfusion of ischemic livers would have taken place. These findings of a lack of any effect of HPC on cellular reactivity of neither granulocytes nor splenocytes (T-cells) further substantiates the conclusion that HPC provides protection by conferring metabolic resistance to ischemic stress than by primarily attenuating early inflammatory reactions.
Adenosine A1 and A3 receptors do not mediate liver protective effects of hypoxic preconditioning
Whereas adenosine A1 and A3 receptors have been shown to play an important physiological role in the preconditioning of various tissues (e.g. heart(40, 41), kidney(42)) their role in the preconditioning of the liver has not been investigated. To evaluate a potential contribution of the A1 and A3 AR to hypoxic preconditioning, we tested the effects of HPC in mice with genetic deficiencies of the adenosine A1 and A3 receptors and observed that the protective effect of hypoxic preconditioning was maintained in A1 AR or A3 AR knockout mice, as well as in WT mice pretreated with the A1 AR selective antagonist DPCPX. These findings clearly rule out a significant role of these receptor subtypes in the protection by hypoxic preconditioning.
In summary, this report provides evidence that in vivo hypoxic preconditioning provides an efficient tool to protect the liver from subsequent ischemia and reperfusion injury. Moreover, the hypoxia->adenosine->A2B receptor pathway plays an important role in this particular form of preconditioning. This conclusion is based not only on the reversal of HPC by non-selective and selective A2B receptor antagonists in WT mice as well as in A2A KO mice, but most importantly also supported by genetic evidence obtained in A2B adenosine receptor knockout mice (SDC Fig. 1). HPC seems to induce rather cytoprotective metabolic effects than to down-regulate damaging inflammatory reactions during the early phase of reperfusion. Thus, the decrease in parameters of cellular und humoral inflammation determined 4 h after reperfusion most likely reflects secondary responses to the ischemically induced liver injury, which is less pronounced in hypoxic preconditioned livers and is therefore of less inflammation stimulating capacity.
As ventilation with hypoxic inspiratory oxygen concentrations even for short durations is no therapeutic option in patients (yet), the pharmacological recruitment of the protective A2B adenosine-signalling pathway may warrant further investigation in experimental liver surgery with Pringle maneuver or total vascular exclusion. In addition, 10% hypoxic ventilation for preconditioning might be applicable and of benefit in deceased organ donors. Both means of liver protection either by highly selective adenosine A2B receptor agonists or by hypoxia, however, clearly need to be further tested in large animal models of liver transplantation and thereafter might become applicable for transplantation medicine.
MATERIAL AND METHODS
Mouse strains
The experiments were conducted in accordance with the regulation for the use of animals in research (http://oacu.od.nih.gov/regs/USGovtPrncpl.htm) as approved by the NIH Animal Care and Use Committee. All mice used were on an at least partial C57BL/6 background. The A2A AR−/−, A2B AR−/− (A2A-and A2B adenosine receptor knockout (KO)) and A3 AR−/− (A3 AR-KO) mice were backcrossed at least 10 times while the A1 AR−/− (A1 AR-KO) mice were on a mixed 129sv/C57BL/6-background. Phenotypic comparisons were made between AR KO mice with their corresponding wild type littermates (WT) or between antagonist to ARs and placebo treated mice. All mice used in the experiments were age-matched males of nine- to twelve weeks age. Animal experiments were repeated at least 3 times.
Model for hepatic ischemia and reperfusion
Anaesthesia was induced by i. p. injection of ketamine (100 mg/kg) and xylazine (4 mg/kg) and maintained by ketamine. After abdominal midline incision, clamping the left lower lobe with a microvascular clip (Fine Science Tools, Foster City, CA) induced ischemia in one third of total liver mass. Thereafter the abdomen was closed temporarily until re-opened to remove the vascular clamp after 45 minutes to induce reperfusion. Finally, the peritoneum and skin were closed by a running nylon suture. During the entire surgical procedure the body temperature of the mouse was automatically maintained at 37° C±0.3° C by a homeothermic blanket and temperature control system with a rectal probe (Stoelting Co., Wood Dale, IL). After surgery all mice received sterile heparinized saline (20 µl/g BW, heparin 5 I.U./ml) subcutaneously to compensate for fluid loss. In sham-operated mice the hilus of the left liver lobe was clamped for ~10 seconds and de-clamped to mimic the trauma to the vessels without inducing ischemia/reperfusion injury.
Hypoxic Preconditioning
Twenty minutes prior to liver clamping for induction of left lobe ischemia, the anesthetized mouse was breathing spontaneously either normoxic (21%oxygen, “control”-group) or hypoxic (10%oxygen, hypoxic preconditioning “HPC”-group) gas mixtures at a constant gas flow (1,0 l/min) through a nose cone for 10 minutes. Thereafter, the nose cone was removed and all mice respirated room air for the rest of the experiment.
Pre-treatment with A2 receptor antagonists
The adenosine A2A and A2B AR antagonist ZM241385 (Tocris, MO) was injected subcutaneously (10 mg/kg BW) 1.5hours prior to the start of hypoxic- or sham-preconditioning. The selective A2B receptor antagonist MRS1754 (Sigma RBI, MO) was injected subcutaneously (3 mg/kg BW) 1.5 hours prior to the start of hypoxic-or sham-preconditioning. Control animals received the same volume of the vehicle solution.
Liver damage related enzymes
Blood was collected from the carotid artery to determine serum activities of ALT, AST and LDH after 4 hours. In selected experiments this blood collection was performed at 1 hr after reperfusion. Serum analyses were performed with an automated analyzer (Synchron LX20, Brea, CA)
Histology
Liver samples were taken 4h after reperfusion and tissue was fixed in 10% neutral buffered formalin, embedded in paraffin, and sections stained with hematoxylin and eosin (H&E) according to standard protocols. Stained tissue slices were analyzed in duplicates or triplicates in a blinded fashion by an experienced veterinary pathologist (JMW). The degree of tissue damage was scored on a scale from 0 to 4 with zero defining unaffected tissue, and 1, 2, 3, or 4 showing degeneration of less than 25% of the liver section, of 26%–50% of the liver section, of 51%–75% of the liver section, and of >75 % of the section, respectively.
Serum cytokines
Interleukin 6 (IL-6) and tumour necrosis factor-alpha (TNF-α) were quantified from serum by ELISA (R&D systems, MN).
Tissue myeloperoxidase (MPO)
Quantification of murine MPO in liver homogenates (ng/mg liver-homogenate) was performed in accordance to the manufacturers´ instructions (HyCult Biotechnology, The Netherlands).
Plasma concentrations of purines and paO2
Blood samples were taken from the carotid artery of anesthetized mice breathing either 21% oxygen or 10 % oxygen for 10 minutes to determine blood purine concentrations. Because of the short half-life of the purine nucleoside adenosine in biological fluids, syringes were pre-filled with ice-cold physiological saline containing dipyridamole (2×10−4M), EHNA (erythro-9-(2-hydroxy-3-nonyl)adenine 2×10−5M), DL-a-glycerophosphate (2×10−2M), EDTANa (2×10−2M), EGTA (2×10−2M) to inhibit nucleoside transporters, adenosine deaminase, non-specific phosphatases and ecto-nuclotidases. The samples were processed and plasma concentrations of adenosine, inosine and hypoxanthine were determined by HPLC as previously described (43). Arterial blood samples were collected at the same time into heparinised capillaries for analyses of the blood oxygen tension (Rapidlab 248,Chiron Diagnostics,UK).
Adenosine receptor-specific mRNA levels in liver tissue
The relative distribution of adenosine A1, A3, A2A and A2B receptor specific mRNA in liver tissue of wild type mice was assessed by real time PCR as previously described (44). The relative mRNA expression was standardized to the levels of ribosomal protein L32 mRNA.
Hypoxia (10 min) and leukocyte activation
C57Bl/6 mice were treated in the same way as mice in the study protocols described above but were not subjected to surgery. Blood samples were taken after 10 minutes of breathing of either hypoxic (10%O2) or normoxic gas mixtures over a period of 10 minutes. Another blood sample was taken after another 45 minutes to get insight in the reactivity of immune cells at a time point at which in animals subjected to ischemia reperfusion would have been started. The effect of hypoxia on some immune cell functions were representatively assessed on PMNs´ respiratory burst activity in either resting state or after stimulation with TNF/fMLP (N-formyl-leucyl-phenylalanin at 10−6M)) as measured by dyihydrorhodamin (DHR) fluorescence by flow cytometry (488 nm laser) and by the expression of CD11b (β2-integrine) using activation protocol as described elsewhere (45). Lymphocytes´ responsiveness was analyzed by stimulation of T-cells separated from spleen of the mice subjected to the same conditions as described above. Splenocytes were stimulated by anti-CD3mAb ((0.1 µg/ml; 145-2C11, BD Biosciences) for 24 hrs (37°C, normoxia) and interferon-gamma was determined in the supernatants thereafter by ELISA (R&D).
AMP measurements
Adenosine monophosphate was measured in murine liver tissues obtained either from sham operated mice and from mice subjected to 45 min of ischemia without prior hypoxic preconditioning or with prior preconditioning, respectively. Because of the shown importance of the A2B receptor, we have tested for the effects of pretreatment of mice by the selective A2B antagonist MRS in the experimental settings described above. The liver tissue samples were taken after 45 minutes of ischemia, before reperfusion. For analyses, mice had been sacrificed and a small piece of liver tissue was weighed (50–60 mg), washed with 500 µl cold PBS, transferred to a centrifuge tube containing 500 µl of water, and boiled for 4 minutes (for enzyme inactivation) and the tissue was homogenized. The homogenate was centrifuged at 14.000 rpm for 5 minutes and the supernatant centrifuged for a second time. The resulting supernatant was loaded onto centrifugal filter devices (Biomax-30, Millipore) and filtered to remove proteins. The filtrate was diluted 1:200 in water and an internal standard (adenine-α-9-D arabinofuranoside) was added to a final concentration of 10pg/µl.
All samples were analyzed with an LCMS assay as previously described (46). Briefly the assay was developed using a Thermofinnigan HPLC system coupled to a Thermofinnigan LCQ Duo ion trap mass spectrometer equipped with an electrospray ionization source (ESI). The mass spectrometer was operated in the ESI positive ion mode. The analytes were monitored using single ion monitoring: for adenine 9-α-D arabinofuranoside (internal standard) the m/z was 268 and for AMP m/z was 348. The amount of AMP (in ng/µl) was calculated using a standard curve and finally expressed as ng/mg of tissue based on the liver tissue mass homogenized.
STATISTICS
All data were normally distributed as assessed by Kolmogorov-Smirnov-test (p >0.05). Data were tested by paired T-test for detection of in-between differences and are presented as means ± standard error of the mean (SEM). P-values below 0.05 were considered to be statistically significant. All statistical analyses were performed by SPSS 14.0 program (SPSS Inc.,Chicago, IL).
Supplementary Material
Acknowledgements
The authors thank Dr. G. Scako, Pearl Powers and T. Anderson (NIH, Clinical Centre, Bethesda, MD) for quantification of liver enzymes and Dr. Alex Salam, Sandra Matzel, Marion Hörl and Stefan Meindl (Department of Anesthesiology, University of Munich, Germany) for discussion and for technical support of purine quantification, respectively. We are also very thankful to Dr. Y. Yuang (NIH, NIDDK) who helped to provide A1 knock-outs for some experiments. We are very grateful to Dr. Martina Choukèr (University of Munich, Germany), Heidi Unkle (Rockville, MD), and to Dr. W. Paul (NIH, NIAID, Laboratory of Immunology) for their continuous support. This research work was supported, in part by the Intramural Research Program of the NIH, NIAID, John E. Fogarty research grant awarded to AC, by the Department of Anesthesiology of the LMU, Munich, and by NIH grants (DK079307, HL109002, DK068575) awarded to EKJ.
Abbreviations
- ADO
Adenosine
- ALT
Alanine transaminase
- AMP
Adenosine monophosphate
- AR
Adenosine receptor
- AST
Aspartate aminotransferase
- BW
Body weight
- DPCPX
(8-cyclopentyl-1,3-dipropylxanthine)
- IRI
Ischemia/Reperfusion injury
- H&E
Hematoxylin and eosin staining
- HPC
Hypoxic Preconditioning
- IPC
Ischemic Preconditioning
- LDH
Lactate dehydrogenase
- MRS 1754
8-[4-[((4-Cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)xanthine
- PaO2
Arterial blood oxygen tension
- PMN
polymorphonuclear leukocyte
- WT
wild type
- ZM241385
(4-(2-[7-amino-2-(2-furyl[1,2,4]-triazolo[2,3-a[1,3,5]triazin-5-yl-aminoethyl)phenol)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors specific contributions:
Alexander Choukèr
Participated in research design, participated in the writing of the paper, participated in the performance of the research, participated in data analysis
Akio Ohta, André Martignoni, Dmitriy Lukashev, Lefteris C Zacharia, Edward K Jackson, Jerrold M Ward,
Participated in the writing of the paper, participated in the performance of the research, contributed new reagents or analytic tools, participated in data analysis
Jürgen Schnermann
Participated in the writing of the paper, participated in the performance of the research, contributed new reagents or analytic tools
Michail V Sitkovsky, Manfred Thiel
Participated in research design, participated in the writing of the paper, participated in data analysis
Brenda Klaunberg
Participated in the writing of the paper, contributed new reagents or analytic tools
André Martignoni, Ines Kaufmann
Participated in research design, participated in the writing of the paper, participated in data analysis
BIBLIOGRAPHY
- 1.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125(3):917. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 2.Singhal AK, Sheng X, Drakos SG, Stehlik J. Impact of donor cause of death on transplant outcomes: UNOS registry analysis. Transplant Proc. 2009;41(9):3539. doi: 10.1016/j.transproceed.2009.06.192. [DOI] [PubMed] [Google Scholar]
- 3.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res. 2003;13(5):385. doi: 10.1038/sj.cr.7290184. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Wu X, Cai L, Tang C, Su J. Hypoxic preconditioning of cardiomyocytes and cardioprotection: phophorylation of HIF-1alpha induced by p42/p44 mitogen-activated protein kinases is involved. Pathophysiology. 2003;9(4):201. doi: 10.1016/s0928-4680(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 5.Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109(24):3042. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 6.Chouker A, Martignoni A, Schauer R, et al. Beneficial effects of ischemic preconditioning in patients undergoing hepatectomy: the role of neutrophils. Arch Surg. 2005;140(2):129. doi: 10.1001/archsurg.140.2.129. [DOI] [PubMed] [Google Scholar]
- 7.Chouker A, Martignoni A, Schauer RJ, et al. Ischemic preconditioning attenuates portal venous plasma concentrations of purines following warm liver ischemia in man. Eur Surg Res. 2005;37(3):144. doi: 10.1159/000085961. [DOI] [PubMed] [Google Scholar]
- 8.Chouker A, Schachtner T, Schauer R, et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93(2):204. doi: 10.1093/bja/aeh195. [DOI] [PubMed] [Google Scholar]
- 9.Franchello A, Gilbo N, David E, et al. Ischemic preconditioning (IP) of the liver as a safe and protective technique against ischemia/reperfusion injury (IRI) Am J Transplant. 2009;9(7):1629. doi: 10.1111/j.1600-6143.2009.02680.x. [DOI] [PubMed] [Google Scholar]
- 10.Jassem W, Fuggle SV, Cerundolo L, Heaton ND, Rela M. Ischemic preconditioning of cadaver donor livers protects allografts following transplantation. Transplantation. 2006;81(2):169. doi: 10.1097/01.tp.0000188640.05459.37. [DOI] [PubMed] [Google Scholar]
- 11.Mei DA, Nithipatikom K, Lasley RD, Gross GJ. Myocardial preconditioning produced by ischemia, hypoxia, and a KATP channel opener: effects on interstitial adenosine in dogs. J Mol Cell Cardiol. 1998;30(6):1225. doi: 10.1006/jmcc.1998.0687. [DOI] [PubMed] [Google Scholar]
- 12.Zhang WL, Lu GW. Changes of adenosine and its A(1) receptor in hypoxic preconditioning. Biol Signals Recept. 1999;8(4–5):275. doi: 10.1159/000014598. [DOI] [PubMed] [Google Scholar]
- 13.Cherqui D, Benoist S, Malassagne B, Humeres R, Rodriguez V, Fagniez PL. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135(3):302. doi: 10.1001/archsurg.135.3.302. [DOI] [PubMed] [Google Scholar]
- 14.Thiel M, Caldwell CC, Sitkovsky MV. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect. 2003;5(6):515. doi: 10.1016/s1286-4579(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 15.Olah ME, Stiles GL. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu Rev Pharmacol Toxicol. 1995;35:581. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- 16.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 17.Itoh R. Regulation of cytosol 5'-nucleotidase by adenylate energy charge. Biochim Biophys Acta. 1981;659(1):31. doi: 10.1016/0005-2744(81)90268-0. [DOI] [PubMed] [Google Scholar]
- 18.Itoh R, Oka J, Ozasa H. Regulation of rat heart cytosol 5'-nucleotidase by adenylate energy charge. Biochem J. 1986;235(3):847. doi: 10.1042/bj2350847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paugam-Burtz C, Albuquerque M, Baron G, et al. Plasma proteome to look for diagnostic biomarkers of early bacterial sepsis after liver transplantation: a preliminary study. Anesthesiology. 2010;112(4):926. doi: 10.1097/ALN.0b013e3181d049f0. [DOI] [PubMed] [Google Scholar]
- 20.Clavien PA, Selzner M, Rudiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238(6):843. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39(1):55. doi: 10.1016/s0168-8278(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 22.Jassem W, Fuggle S, Thompson R, et al. Effect of ischemic preconditioning on the genomic response to reperfusion injury in deceased donor liver transplantation. Liver Transpl. 2009;15(12):1750. doi: 10.1002/lt.21936. [DOI] [PubMed] [Google Scholar]
- 23.Astarcioglu I, Cursio R, Reynes M, Gugenheim J. Increased risk of antibody-mediated rejection of reduced-size liver allografts. J Surg Res. 1999;87(2):258. doi: 10.1006/jsre.1999.5734. [DOI] [PubMed] [Google Scholar]
- 24.Howell JG, Zibari GB, Brown MF, et al. Both ischemic and pharmacological preconditioning decrease hepatic leukocyte/endothelial cell interactions. Transplantation. 2000;69(2):300. doi: 10.1097/00007890-200001270-00017. [DOI] [PubMed] [Google Scholar]
- 25.Jones NM, Bergeron M. Hypoxia-induced ischemic tolerance in neonatal rat brain involves enhanced ERK1/2 signaling. J Neurochem. 2004;89(1):157. doi: 10.1111/j.1471-4159.2004.02324.x. [DOI] [PubMed] [Google Scholar]
- 26.Lu GW, Yu S, Li RH, Cui XY, Gao CY. Hypoxic preconditioning: a novel intrinsic cytoprotective strategy. Mol Neurobiol. 2005;31(1–3):255. doi: 10.1385/MN:31:1-3:255. [DOI] [PubMed] [Google Scholar]
- 27.Wang CH, Chang A, Tsai MJ, Cheng H, Liao LP, Lin AM. Kainic acid-induced oxidative injury is attenuated by hypoxic preconditioning. Ann N Y Acad Sci. 2005;1042:314. doi: 10.1196/annals.1338.054. [DOI] [PubMed] [Google Scholar]
- 28.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327. doi: 10.1146/annurev.pharmtox.37.1.327. [DOI] [PubMed] [Google Scholar]
- 29.Ishii D, Schenk AD, Baba S, Fairchild RL. Role of TNFalpha in early chemokine production and leukocyte infiltration into heart allografts. Am J Transplant. 2010;10(1):59. doi: 10.1111/j.1600-6143.2009.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Birch SE, He R, et al. Remote ischemic preconditioning by hindlimb occlusion prevents liver ischemic/reperfusion injury: the role of High Mobility Group-Box 1. Ann Surg. 2010;251(2):292. doi: 10.1097/SLA.0b013e3181bfda8c. [DOI] [PubMed] [Google Scholar]
- 31.Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81(2):154. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- 32.Carini R, De Cesaris MG, Splendore R, et al. Signal pathway involved in the development of hypoxic preconditioning in rat hepatocytes. Hepatology. 2001;33(1):131. doi: 10.1053/jhep.2001.21050. [DOI] [PubMed] [Google Scholar]
- 33.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol. 2004;286(2):G285. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 34.Harada N, Okajima K, Murakami K, et al. Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. J Pharmacol Exp Ther. 2000;294(3):1034. [PubMed] [Google Scholar]
- 35.Poucher SM, Keddie JR, Singh P, et al. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br J Pharmacol. 1995;115(6):1096. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klotz KN. Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4–5):382. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- 37.Ji X, Kim YC, Ahern DG, Linden J, Jacobson KA. [3H]MRS 1754, a selective antagonist radioligand for A(2B) adenosine receptors. Biochem Pharmacol. 2001;61(6):657. doi: 10.1016/s0006-2952(01)00531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111(4):2024. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang D, Zhang Y, Nguyen HG, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116(7):1913. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullane K, Bullough D. Harnessing an endogenous cardioprotective mechanism: cellular sources and sites of action of adenosine. J Mol Cell Cardiol. 1995;27(4):1041. doi: 10.1016/0022-2828(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 41.Takano H, Bolli R, Black RG, Jr, et al. A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88(5):520. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 42.Joo JD, Kim M, Horst P, et al. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293(6):F1847. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- 43.Thiel M, Holzer K, Kreimeier U, Moritz S, Peter K, Messmer K. Effects of adenosine on the functions of circulating polymorphonuclear leukocytes during hyperdynamic endotoxemia. Infect Immun. 1997;65(6):2136. doi: 10.1128/iai.65.6.2136-2144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukashev DE, Smith PT, Caldwell CC, Ohta A, Apasov SG, Sitkovsky MV. Analysis of A2a receptor-deficient mice reveals no significant compensatory increases in the expression of A2b, A1, and A3 adenosine receptors in lymphoid organs. Biochem Pharmacol. 2003;65(12):2081. doi: 10.1016/s0006-2952(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 45.Martignoni A, Tschop J, Goetzman HS, et al. CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock. 2008;29(5):591. doi: 10.1097/SHK.0b013e318157f427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK. cAMP-adenosine pathway in the proximal tubule. J Pharmacol Exp Ther. 2006;317(3):1219. doi: 10.1124/jpet.106.101360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.