Abstract
Objective
This randomized controlled trial tested the efficacy of parent-based behavioral counseling for reducing secondhand smoke exposure (SHSe) among children with cancer. It also examined predictors of smoking and SHSe outcomes.
Methods
Participants were 135 parents or guardians of non-smoking children with cancer, <18 yrs, at least 30 days post-diagnosis, living with at least one adult smoker. Parents were randomized to either a standard care control group or an intervention consisting of six counseling sessions delivered over three months. Parent-reported smoking and child SHSe levels were obtained at baseline, 3, 6, 9, and 12 months. Children provided urine samples for cotinine analyses.
Results
Reductions in parent-reported smoking and exposure were observed in both the intervention and control conditions. There was a significantly greater reduction in parent-reported smoking and child SHSe at 3 months for the intervention group compared to the control group. Child SHSe was significantly lower at 12 months relative to baseline in both groups. Children’s cotinine levels did not show significant change over time in either group. Exposure outcomes were influenced by the number of smokers at home, smoking status of the parent participating in the trial, and the child’s environment (home vs. hospital) the day before the assessment.
Conclusions
Children’s SHSe can be reduced by advising parents to protect their child from SHSe, combined with routine reporting of their child’s exposure and cotinine testing, when delivered in the context of the pediatric cancer setting. More intensive interventions may be required to achieve greater reductions in SHSe.
Keywords: cancer, oncology, secondhand smoke exposure, intervention, cotinine
INTRODUCTION
Secondhand smoke exposure (SHSe) is carcinogenic, linked to respiratory and cardiovascular diseases, and represents a leading preventable cause of child morbidity and mortality [1]. The adverse health effects of SHSe among children include increased risk for respiratory illness, ear infections, bronchitis, pneumonia, asthma, and reduced pulmonary function [2–6], and the risk of complication increases with higher levels of exposure [7]. Children with cancer may be especially vulnerable to these health risks secondary to disease and treatment-related toxicities that may affect their respiratory, pulmonary, and cardiovascular functioning [8–10]. Newly diagnosed children with cancer who are exposed to smoke in their homes are more likely to present with a history of respiratory and pulmonary symptoms [11] and are potentially at risk for acute respiratory complications, particularly if immunocompromised and exposed during treatment. In addition to disease outcomes, youngsters who are exposed to SHS are also more likely to initiate smoking than those who are not exposed [12]. Adoption of smoking habits can be particularly detrimental to survivors of childhood cancer, who are already at risk for smoking-related diseases secondary to their disease and treatment [8–10].
Despite recent tobacco control efforts and community policies aimed at reducing SHSe in public places [13], children continue to be exposed to tobacco toxins in their own homes and cars. In the U.S., more than one-third of children and adolescents live in homes where residents and visitors smoke regularly [14–17]. Youth exposure to SHS while travelling in the car is also frequent [18,19] and may be 23 times more toxic than SHSe in the home due to the enclosed space [20]. Despite their compromised health status, children with cancer are at risk for being regularly exposed to tobacco smoke throughout their treatment, from multiple sources and in numerous settings. It has been reported that between 40–46% of newly diagnosed children with cancer live in smoking households that typically include at least one parent smoker [21]. At home, over half of these youngsters are directly exposed to someone’s cigarettes smoked in their presence with an even greater percentage of them frequently exposed in the family vehicle.
Interventions that reduce children’s SHSe have yielded mixed success [22]. The most successful trials have tested intensive, individualized, parent-based counseling approaches [22]. Reduction in children’s exposure to cigarettes [23–25], decreased cotinine levels [26], and decreased air nicotine [27] have been reported in children with asthma and respiratory problems as well as in healthy children. This study was the first to test the efficacy of a parent-based behavioral counseling intervention to reduce SHSe among children undergoing treatment for cancer. Child and parent sociodemographic characteristics and clinical variables were examined as predictors of parent-reported cigarette consumption and child SHSe as well as child biological cotinine outcomes.
METHODS
Participants
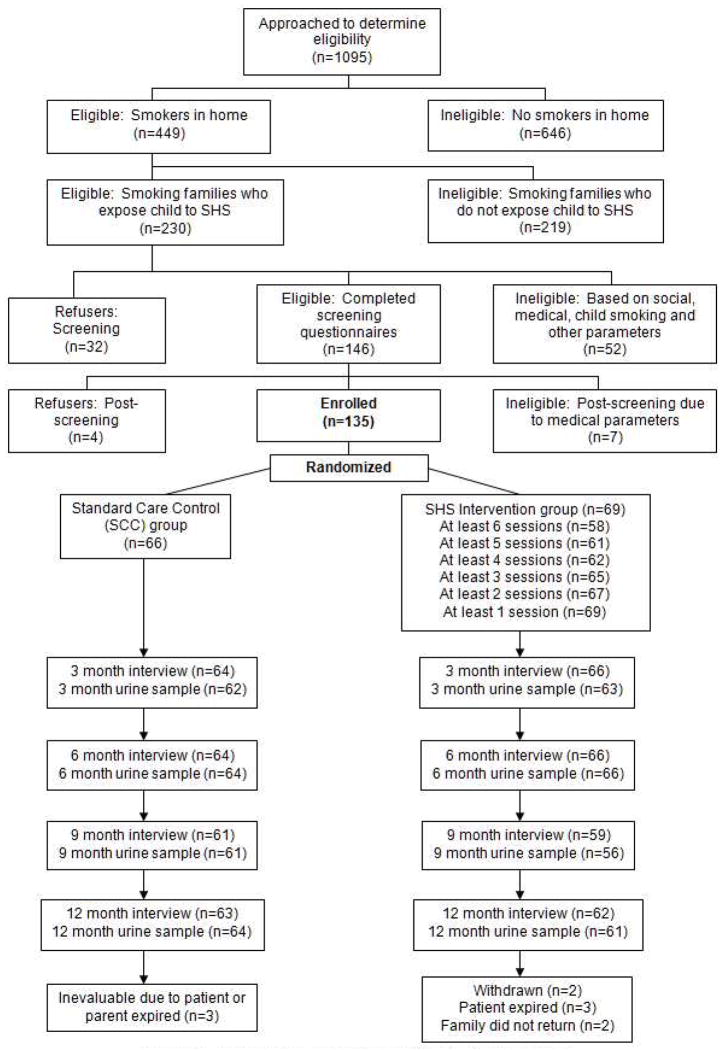
Participants were 135 parents or guardians of children receiving treatment for cancer who lived with at least one adult smoker and were exposed to SHS in the home or car setting, per parent report at the time of recruitment. Non-smoking patients (<18 yrs), at least 30 days post-diagnosis, were recruited along with their families. Patients/families were excluded if they had a high risk prognosis or had a medical or family social crisis precluding participation. Eligible families were initially identified from medical records and further screened in person to confirm their eligibility. Figure 1 outlines the number of families enrolled through completion of 12-month measures.
Figure 1.
CONSORT: Flow of Participants through Trial
Procedure
Design
Families were randomized (Figure 1) to either an intervention or a standard control group using a stratified, blocked randomization scheme with strata being child’s age (≤5, 6–12, 13–17 years), race (white, non-white), and smoking status of the participating parent (smoker, non-smoker). Parents/guardians were eligible for participation regardless of their smoking status. Families were followed longitudinally and parent-reported and child biological measures were obtained 5 times over 12 months (baseline, 3, 6, 9, and 12 months). Parents provided information about cigarettes they or others smoked and their child’s SHSe by completing structured interviews. The parent who accompanied the child to the hospital for clinical visits and participated in the study was designated as the “target” parent. Parents were compensated for completion of study questionnaires and participation in counseling sessions. Children received gift vouchers for each urine sample provided. Study procedures were reviewed and approved by the Institutional Review Board. All parents signed informed consent agreements and children (aged ≥7 years) provided assent.
Intervention Procedures
Parents in the intervention group received a multi-component behavioral program delivered by trained counselors over three months. Counselors were fully informed about each patient’s diagnosis, medical status, and treatment-related complications to enable them to deliver the counseling in the context of the child’s ongoing cancer treatment. Counseling consisted of three individual, face-to-face, bi-weekly 1-hour sessions followed by three 25-minute telephone sessions for a total of 6 individual contacts with their counselor. Parents also received letters from their child’s physician at the start and end of the counseling phase to acknowledge their participation and progress.
The intervention was based on previous behavioral trials [23–25, 28, 29] and included behavioral contracting for reducing children’s exposure, self-monitoring, problem-solving, and social reinforcement for successes. Sessions were designed to gradually shape participants’ behavior to remove children from sources of exposure (e.g. their own smoking, and smoking by family members and/or friends). Goal achievement resulted in prompting to do more. Parents were provided with literature about SHS-related health risks in children and for stress management. The study did not involve formal cessation counseling. Counselors invited and encouraged all family members to participate in the counseling sessions.
Standard Care Control Group (SCC)
Parents in the SCC group were asked about their smoking behaviors in the presence of their child and advised about the adverse health problems for children exposed to SHS. Parents were briefly advised to remove their child from sources of exposure and to protect their child from SHSe. This group received all study measures but did not receive SHSe counseling from the study counselors.
Measures
Parent-Reported Smoking
Parents reported the number of cigarettes smoked by all persons in the home and car over the past 7 days. Responses were used to calculate the all-source, 7-day total smoking as validated in prior studies [30–32].
Parent-Reported Child SHSe
Parents were asked to report on the number of cigarettes to which the child was exposed by all smoking persons in the home and car for the previous 7 days. Exposure was defined as the number of cigarettes smoked (even one puff) in the same room as the child or in the car when the child was present. Responses were used to calculate the all-source, 7-day total parent-reported child SHSe. Acceptable test-retest reliability and validity of parent reports of exposure in children with cancer in relation to cotinine assays are reported elsewhere [33].
Urine Cotinine Assays
Cotinine is a metabolite of nicotine and a reliable biomarker of recent SHS exposure [30]. All urine samples were obtained from patients in the hospital clinic setting and frozen. Batched samples were packed in dry ice and shipped to the mass spectrometry laboratories at San Diego State University for analysis of cotinine levels. All samples were analyzed using methods that are sensitive to low levels of SHSe [30, 31]. Level of detection was less than .05 ng/ml.
Parent Satisfaction Survey
Parents in the intervention group were asked to complete a satisfaction survey that asked about their experience with counseling and requested their feedback about the intervention sessions.
Statistical Analyses
Analyses were based on the intention to treat rule with 135 families analyzed as randomized (n=69 in the intervention group and n=66 in the SCC group). Descriptive statistics, including means, standard deviations, percentages, and frequencies were reported for selected variables. Given that the distribution of the study outcome measures was highly skewed, geometric means and corresponding 95% confidence intervals were estimated for the intervention and control groups at each time point. T-tests were used to examine group and time-point comparisons. Cotinine levels were analyzed after logarithmic transformation.
Linear Mixed-effect Models (LMM)/Generalized Linear Mixed-effect Models (GLMM) were employed using the SAS Procedure PROC Mixed/Nlmixed (SAS, Cary, NC) to address intra-patient correlations with respect to repeated measurements over time. To analyze the intervention effect, the LMM/GLMM model examined group differences for smoking and exposure outcomes at baseline and across the 12 month study period. In this model, the variables of time (baseline, 3, 6, 9, and 12 months), group (intervention vs. control), and the interaction of time by group were included as covariates. Because the greatest reduction for parent-reported smoking and child exposure variables was observed in both groups at 3 months, a spline (discontinuity of slope) was also introduced into the model at the 3-month time point [34]. Outcomes were examined before and after the 3-month time point in order to test for treatment effects and effects through follow-up. As no such pattern was observed for cotinine outcomes, time was included as a continuous variable without a spline. Akaike’s information criterion (AIC) was used for covariance structure selection with compound symmetry assumption. For each outcome measure, covariates were selected from the following list: sociodemographic (parent and child age, race, gender; parent marital status and family socioeconomic status), child clinical (diagnosis and time from diagnosis), smoking-related covariates (number of smokers in the home and smoking status of the target parent), the child’s location/setting on the day prior to urine collection (hospital campus vs. home or other residence), number of counseling sessions received (coded as 0 sessions for the control group), and all two-way interactions between these variables. All factors significant at level alpha=0.15 in the univariate analyses were investigated in the multivariate model. The final model presented included the factors that were significant at level alpha=0.05.
RESULTS
Participant Characteristics
Table 1 presents the sociodemographic and child medical characteristics of the 135 families by intervention and control groups and overall. There were no significant group differences on demographic and child medical variables at baseline except for child gender. Male patients were more likely to be included in the intervention group.
Table 1.
Demographic and Smoking-Related Characteristics for Study Sample by Group
| Parent or Child Variable | All (N=135) | Intervention (n=69) | Control (n=66) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | n | % | n | % | |
| Child Gender
| ||||||
| Male | 71 | 52.6 | 44 | 63.8 | 27 | 40.9 |
| Female | 64 | 47.4 | 25 | 36.2 | 39 | 59.1 |
|
| ||||||
| Child Racea
| ||||||
| White | 102 | 75.6 | 52 | 75.4 | 50 | 75.8 |
| Non-white | 33 | 24.4 | 17 | 24.6 | 16 | 24.2 |
|
| ||||||
| Child Diagnosis
| ||||||
| CNS | 10 | 7.4 | 4 | 5.8 | 6 | 9.1 |
| Leukemia/Lymphoma | 88 | 65.2 | 43 | 62.3 | 45 | 68.2 |
| Solid tumor | 37 | 27.4 | 22 | 31.9 | 15 | 22.7 |
|
| ||||||
| Parent Gender
| ||||||
| Male | 23 | 17.0 | 10 | 14.5 | 13 | 19.7 |
| Female | 112 | 83.0 | 59 | 85.5 | 53 | 80.3 |
|
| ||||||
| Target Parent Racea
| ||||||
| White | 108 | 80.0 | 55 | 79.7 | 53 | 80.3 |
| Non-white | 27 | 20.0 | 14 | 20.3 | 13 | 19.7 |
|
| ||||||
| Parent SESb
| ||||||
| Low | 67 | 49.6 | 34 | 49.3 | 33 | 50.0 |
| Middle | 33 | 24.4 | 21 | 30.4 | 12 | 18.2 |
| High | 35 | 26.0 | 14 | 20.3 | 21 | 31.8 |
|
| ||||||
| Parent Marital Status
| ||||||
| Married | 78 | 57.8 | 36 | 52.2 | 42 | 63.6 |
| Not married | 57 | 42.2 | 33 | 47.8 | 24 | 36.4 |
|
| ||||||
| Target Parent Smoking Status
| ||||||
| Smoker | 95 | 70.4 | 49 | 71.0 | 46 | 69.7 |
| Non-smoker | 40 | 29.6 | 20 | 29.0 | 20 | 30.3 |
|
| ||||||
| Smokers in Homec
| ||||||
| 0 or 1 | 70 | 51.9 | 38 | 55.1 | 32 | 48.5 |
| ≥ 2 | 65 | 48.1 | 31 | 44.9 | 34 | 51.5 |
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||
| Child Age (yrs) | 8.6 | 5.2 | 8.9 | 5.2 | 8.4 | 5.1 |
| Parent Age (yrs) | 34.7 | 8.8 | 34.5 | 8.4 | 35.0 | 9.2 |
| Time Since Diagnosis (years) | .58 | .83 | .58 | .83 | .59 | .83 |
Abbreviations: CNS, central nervous system; SES, socioeconomic status
100 white and not of Hispanic origin and 2 whites of Hispanic origin. Remaining patients were 27 black except for 1 Asian and 3 of more than one race.
SES measured using Hollingshead index.42
Only 1 family had 4 smokers. 5 children lived in nonsmoking primary residences but were in homes where there was regular smoke exposure.
Intervention Effects
Parent-Reported Smoking and Child SHSe
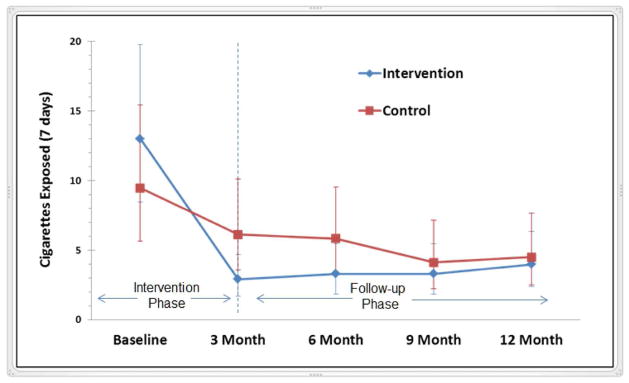
Geometric means and corresponding 95% CI values for parent-reported cigarettes smoked and child SHSe by all sources in the child’s home and car over a 7-day period for the intervention and control groups at each assessment point are presented in Table 2. There were no significant group differences for cigarettes smoked and child SHSe at baseline. After adjusting for sociodemographic and child medical covariates, results of GLMM modeling indicated a significant reduction in reported smoking and child SHSe from baseline to 3 months in both the control and intervention groups (Table 3). A significantly steeper decline (greater change in slope) was observed for the intervention group (p<.05). A 65.8% reduction from baseline to 3 months in parent-reported child SHSe levels was observed for the intervention group compared to a 32.8% reduction for the control group (p<.05). From 3 to 12 months, the slope (rate of change) for cigarettes smoked and child SHSe slightly increased for the intervention group (p<.05). For the control group, a slightly decreased slope was observed after 3 months (p<.05). The group difference for average cigarettes smoked and child SHSe was not significantly different at the 12 month follow-up (p>.05). However, child SHSe, the target of our intervention, was significantly lower at 12 months relative to baseline in both the intervention and control groups (p<.05). Figure 2 demonstrates these results for reported child SHSe.
Table 2.
Parent-Reported Smoking and Child SHSe Outcomes at Baseline and Follow-up
| Variable | Baseline | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|
| Geometric Mean* (95% C.I.) | |||||
| Reported Smoking (cigarettes per week) | |||||
| Intervention | 30.6 (20.0, 46.4) | 8.5 (4.7, 14.7) | 7.6 (4.3, 13.0) | 7.2 (3.9, 12.8) | 11.5 (6.7, 19.3) |
| Control | 25.3 (15.1, 41.9) | 15.2 (8.6, 26.4) | 15.6 (8.5, 27.8) | 12.0 (6.6, 21.4) | 13.2 (7.3, 23.3) |
| Reported Exposure (cigarettes per week) | |||||
| Intervention | 13.0 (8.4, 19.8) | 2.9 (1.7, 4.7) | 3.3 (1.8, 5.5) | 3.3 (1.8, 5.5) | 4.0 (2.4, 6.4) |
| Control | 9.5 (5.7, 15.4) | 6.1 (3.6, 10.1) | 5.8 (3.4, 9.5) | 4.1 (2.2, 7.1) | 4.5 (2.5, 7.7) |
| Child Cotinine Levels (ng/ml) | |||||
| Intervention | 3.9 (2.9, 5.3) | 3.9 (2.8, 5.5) | 3.8 (2.5, 5.7) | 5.3 (3.7, 7.5) | 4.0 (2.9, 5.6) |
| Control | 3.7 (2.4, 5.5) | 3.5 (2.3, 5.3) | 3.1 (2.1, 4.6) | 3.6 (2.4, 5.4) | 3.9 (2.6, 6.0) |
Prior to and after calculating geometric mean, 1 was added and then subtracted to the cigarettes smoked or exposed since there were 0 exposures. This method43 has been confirmed by simulation.
Table 3.
Model Estimates: Parent-Reported Smoking, Child SHSe, and Cotinine
| Variable | Estimate | Std Error | P-Value |
|---|---|---|---|
| Cigarettes Smoked | |||
| Intercept | 4.133 | 0.635 | <.001 |
| Intervention and Time Effect | |||
| Time | −0.048 | 0.006 | <.001 |
| Splinea | 0.017 | 0.007 | 0.025 |
| Interventionb | 0.022 | 0.297 | 0.940 |
| Time*Interventionc | −0.151 | 0.009 | <.001 |
| Spline*Interventionc | 0.210 | 0.012 | <.001 |
| Predictive Covariates | |||
| Two or More Smokers in Homeb | 0.424 | 0.022 | <.001 |
| Race (White) b | 0.688 | 0.347 | 0.050 |
| Parent Age | −0.033 | 0.017 | 0.051 |
| Child SHSe | |||
| Intercept | 2.079 | 0.264 | <.001 |
| Intervention and Time Effect | |||
| Time | −0.154 | 0.009 | <.001 |
| Splinea | 0.131 | 0.012 | <.001 |
| Interventionb | 0.332 | 0.337 | 0.326 |
| Time*Interventionc | −0.138 | 0.015 | <.001 |
| Spline*Interventionc | 0.181 | 0.019 | <.001 |
| Predictive Covariates | |||
| Two or More Smokers in Homeb | 0.228 | 0.034 | <.001 |
| Race (White) b | 0.919 | 0.398 | 0.022 |
| Log Cotinine | |||
| Intercept | 1.829 | 0.215 | <.001 |
| Intervention and Time Effect | |||
| Time | 0.014 | 0.008 | 0.076 |
| Interventionb | 0.126 | 0.197 | 0.523 |
| Predictive Covariates | |||
| Two or More Smokers in Homeb | 0.310 | 0.125 | 0.017 |
| Smoking Status of Target Parent (Smoker) b | 0.732 | 0.223 | 0.001 |
| Location (On Campus)d | −0.237 | 0.056 | <.001 |
Abbreviations: SHSe, secondhand smoke exposure; Std Error, standard error
In the employed LMM/GLMM models, a Spline was used to indicate the slope change before and after the 3 month time points. The spline variable was constructed in the following way: if time ≤ 3 months, then spline = 0; if time > 3 months, then spline = time − 3.34
For the variables “Intervention,” “Two or More Smokers in Home,” “Race (White),” and “Smoking Status of Target Parent (Smoker),” the reference groups are “SCC (standard care control),” “0 or 1 smoker,” “Non-white,” and “Non-smoker,” respectively.
The terms “Time*Intervention” and “Spline*Intervention” were used to indicate the interaction between time and/or spline and the intervention, respectively.
”Location (On Campus)” indicates the setting where the child spent the majority of time on the day preceding the urine collection (on the hospital campus vs. home or other residence).
Figure 2.
Geometric Mean of Parent-Reported Child SHSe at Baseline and Follow-up by Group
Cotinine
The geometric means and 95% CI for cotinine outcomes for the intervention and control groups at baseline and follow-up time points are presented in Table 2. There were no significant group differences in cotinine levels at baseline. Children’s cotinine levels did not show a significant change over time in either the intervention or control group. No significant group differences or time by group interactions were observed.
Predictors of Parent-Reported Smoking and Child SHSe
In the model to predict parent-reported smoking from all sources in the past 7 days (Table 3), more than one smoker in the household was significantly associated with a greater number of cigarettes smoked. Parents of white children smoked a marginally greater number of cigarettes while older parents smoked less cigarettes. Higher levels of child SHSe were reported among white families and were significantly associated with the presence of more than one household smoker. More than one smoker in the home and a target parent who smoked were significant predictors of higher average child cotinine levels (Table 3). Children who spent the majority of time on the hospital campus (vs. other settings) on the day prior to urine sample collection had significantly lower cotinine levels.
Intervention Adherence and Satisfaction
Eighty-four percent of parents randomly assigned to the intervention group successfully completed all six counseling sessions. Almost 90% completed at least 4 of the sessions. Feedback from 88.4% (61/69) parents who participated in our intervention and completed a satisfaction survey was generally positive in terms of the number and content of sessions and the sensitivity of the counseling approach. Almost 92% of parents reported they acquired information regarding the health effects of SHSe and learned specific strategies to reduce their child’s SHSe. Approximately 93% of parents reported that the number of session contacts was “just right.” No concerns were raised regarding the timing of our intervention delivery; almost 97% reported the information was provided at the appropriate time during the child’s treatment while two parents wanted earlier access to this information.
DISCUSSION
This was the first study to test an intervention to reduce SHSe among children undergoing treatment for cancer. In this trial, parent-reported measures indicated significantly greater reduction in children’s exposure to cigarette smoke in the intervention group compared to the control group at 3 months (after completion of the intervention phase). Differential patterns of reduction in SHSe for the two groups indicated that the control families showed a slower and more gradual decline in SHSe over the study period while the intervention group showed a significant initial counseling effect with slight increases in exposure after the first 3 months (during the follow-up phase). Although reported child SHSe levels for families who received the intervention were significantly lower at 12 months relative to baseline, results suggests that maintenance of low levels of exposure and/or greater reduction of SHSe will likely require counseling of greater duration or booster sessions that occur during the follow-up phase.
In this study, reductions in parent-reported exposure were observed in both the intervention and control conditions. The decrease in parent-reported exposure for the intervention and control groups may be partially accounted for by reactivity of standard measurement procedures recognized in prior exposure studies [23, 26]. Asking parents to report on their child’s exposure, while also sampling their child’s urine, may have contributed to the observed reductions in exposure that may not be specifically attributed to behavioral counseling alone. Participants in the control group also received a minimal intervention consisting of brief advice about protecting the child from sources of SHSe. This advice, combined with routine reporting procedures and cotinine testing, may account for observed reductions in exposure observed for the control group. This approach was employed as an ethical minimum for our control participants, which also served to enhance recruitment and cohort retention. Because the trial was conducted in the clinical setting, it is also likely that the setting took on discriminative properties that served to remind parents, in both conditions, of the dangers of SHSe and prompt greater attention to their smoking behaviors around their child. This follows behavioral ecological theory [35], where the child’s diagnosis of disease, medical treatment, and clinical setting serve as motivating operations that promote change in parental smoking behaviors in their child’s presence.
It is important to note that a variety of patterns in cotinine concentrations have been reported across SHSe studies [23, 26, 28, 36, 37]. We did not demonstrate significant differences in cotinine levels between the intervention and control groups over time. Our findings are similar to those of Greenberg and colleagues [37] who demonstrated decreased exposure levels based on parent reports but no significant reductions in urine cotinine levels. This finding should not diminish the significance of the study. While cotinine is an endpoint of interest for evaluating health risk, the more proximal targeted behavior (i.e. fewer cigarettes smoked in the child’s presence) that is relevant for testing the efficacy of our behavioral intervention, was significantly changed. Reliance on cotinine, by itself, for evaluating the effects of the intervention may result in faulty conclusions.
The finding that cotinine levels did not change over the course of the trial in the context of reduced parent-reported exposure may have several possible explanations. First, a reporting bias on part of the parent respondents should be considered. It is possible that the hospital setting and delivery of the intervention during the child’s treatment could influence the degree of accurate disclosure by parents about their smoking and child’s SHSe. Another potential reporting bias was that the smoking status of the target parent may have influenced the accuracy of the reported exposure. Smoking parents tend to provide more accurate estimates of child SHSe than nonsmoking parents [32, 33], since they are often the sources of the child’s exposure. Additionally, parents may not be able to accurately report on exposure that is not directly observed. Lastly, the definition of reported “exposure” for our trial, and many others, required that the child be present in the room or car when a cigarette was smoked. Yet children may be in close proximity to a room where smoking occurs or they may enter a room soon after cigarettes are smoked, thus exposing them to SHS contaminants. Therefore, our parent-reported measures were not as inclusive in measuring all sources of SHSe, as was cotinine.
On a related note, it is likely that residual exposure could account for the failure to obtain significant reductions in children’s cotinine levels, even when parents reported reduced smoking in the home or other environments or smoked when the child was not at home. It is now well known that children are at particular risk to thirdhand smoke exposure (THSe) through contamination of home surfaces by volatile SHSe components that can be off-gassed into the air and affect cotinine levels [38–40]. It is likely that the homes of families in our study may have been contaminated for some time, suggesting that children may have been exposed to THSe, which was not separately measured in this study. The fact that families in our study were not required to completely ban smoking in their homes and cars and were permitted to implement less restrictive options to protect their children from SHSe (e.g. restricting smoking to certain rooms of the home, smoking in the home when the child is not present) was not sufficient to offset the risk from THS. Complete elimination of smoking in the home and/or car for an extended time period would be necessary to result in substantive reductions in both SHS and THS exposures that are measured by the child’s cotinine levels. Our study and the counseling interventions conducted to date have not sufficiently focused on this goal.
The considerable variability in cotinine levels for children in our sample, as noted in prior studies [41], may reflect differences in opportunity for exposure and account for the lack of a differential reduction in cotinine levels between the intervention and control groups. Due to treatment-related schedules and the distance some families travel to receive treatment, patients were not consistently in their primary residence or in the same location/setting in the 24 hours prior to urine sample collection. Therefore, children spending more time on the smoke-free hospital campus setting prior to urine sample collection likely had less opportunity for exposure, as reflected in lower cotinine levels.
The collection of urine samples and parent reports in this study was intended to capture exposure during the same one week period prior to assessment. Urine samples were obtained at the time the parent completed the exposure reports for the past 7 days. However, only a single urine sample was obtained at each assessment point, providing accurate estimates of only recent exposure (2–3 days) due to the short half-life of cotinine [30]. With a single urine sample, it is also possible that our estimates of exposure were artificially biased if the timing of the urine samples reflected episodic high or low level exposure events. More frequent and targeted cotinine measurements, preferably in the child’s home environment, may be necessary to obtain more representative levels of exposure for this mobile patient population [39]. Travel, time away from home, and treatment schedules may present methodological challenges for cotinine measurement among other pediatric populations, in addition to cancer, who often cannot be treated locally.
An important observation from this trial was that only 16% of families assigned to the intervention group did not complete all 6 counseling sessions and the intervention was delivered without high rates of withdrawal at follow-up. These findings, combined with the positive feedback from parents, support their willingness to participate in multi-session tobacco-based counseling during their child’s cancer treatment. However, families of children with cancer may require even more powerful clinical interventions, which also include decontamination procedures to reduce THSe [42,43], to ensure larger reductions in SHSe as measured by both parent report and cotinine levels. A logical next step would be an intervention trial that requires complete home and car smoking bans with longer follow-up intervals to capture reductions in home contamination that contribute to child cotinine measurements. Our finding that more smokers in the home was associated with higher SHSe and child cotinine levels highlights the need to engage multiple family members, particularly parent smokers, in the counseling process. Repeated advice by health care providers to prohibit smoking in all environments inhabited by children is critical. An ongoing dialogue about smoking and exposure between the clinical team and families at each clinical contact may be the most ecological way to reduce SHSe for children with cancer.
Acknowledgments
This study was supported in part by grants CA 085406 and CA 21765 from the National Cancer Institute and the American Lebanese Associated Charities. The authors thank the patients and their families participating in this study.
Footnotes
CONFLICT OF INTERESTS STATEMENT
The authors declare that they have no conflict of interest.
Clinicaltrials.gov ID: NCT00766766
References
- 1.United States Department of Health and Human Services (USDHHS) The health consequences of involuntary exposure to tobacco smoke: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2006. [Google Scholar]
- 2.Cook DG, Strachan DP. Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etzel RA. Environmental Tobacco Smoke. Immunol Allergy Clin North Am. 1994;14:621–633. [Google Scholar]
- 4.National Research Council. Environmental tobacco smoke: Measuring exposures and assessing health effects. Washington, DC: National Academy Press; 1986. pp. 209–211. [PubMed] [Google Scholar]
- 5.Office of Health and Environmental Assessment, Office of Research and Development, Environmental Protection Agency. Respiratory health effects of passive smoking: Lung cancer and other disorders. Washington, D.C: 1993. [Google Scholar]
- 6.USDHHS (Department of Health and Human Services) The health consequences of involuntary smoking: A report by the US Surgeon General. Rockville, MD: United States Department of Health and Human Services; 1986. [Google Scholar]
- 7.DiFranza JR, Lew RA. Morbidity and mortality in children associated with the use of tobacco products by other people. Pediatrics. 1996;97:560. [PubMed] [Google Scholar]
- 8.Benoist MR, Lemerle J, Jean R, et al. Effects of pulmonary function of whole lung irradiation for Wilm’s tumour in children. Thorax. 1982;37:175–180. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 10.O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med. 1990;323:378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- 11.Tyc VL, Throckmorton-Belzer L, Klosky JL, et al. Smoking among parents of pediatric cancer patients and children’s exposure to environmental tobacco smoke. J Child Health Care. 2004;8:288–300. doi: 10.1177/1367493504047319. [DOI] [PubMed] [Google Scholar]
- 12.Bettcher DW, Peruga A, Fishburn B, et al. Exposure to secondhand smoke among students aged 13–15 years -- Worldwide, 2000–2007. MMWR: Morbidity & Mortality Weekly Report. 2007;56:497–500. [PubMed] [Google Scholar]
- 13.Norman GJ, Ribisl KM, Howard-Pitney B, et al. The relationship between home smoking bans and exposure to state tobacco control efforts and smoking behaviors. Am J Health Promot. 2000;15:81–88. doi: 10.4278/0890-1171-15.2.81. [DOI] [PubMed] [Google Scholar]
- 14.American Legacy Foundation. Secondhand smoke: Youth exposure and adult attitudes-results from three national surveys. Washington, DC: American Legacy Foundation; 2005. [Google Scholar]
- 15.Centers for Disease Control and Prevention. Vital signs: Nonsmokers’ exposure to secondhand smoke - United States, 1999–2008. MMWR: Morbidity & Mortality Weekly Report. 2010;59:1141–1146. [PubMed] [Google Scholar]
- 16.Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003–2006. Pediatrics. 2009;124:1299–1305. doi: 10.1542/peds.2009-0880. [DOI] [PubMed] [Google Scholar]
- 17.Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Arch Pediatr Adolesc Med. 2002;156:1094–1100. doi: 10.1001/archpedi.156.11.1094. [DOI] [PubMed] [Google Scholar]
- 18.Norman GJ, Ribisl KM, Howard-Pitney B, Howard KA. Smoking bans in the home and car: Do those who really need them have them? Prev Med. 1999;29:581–589. doi: 10.1006/pmed.1999.0574. [DOI] [PubMed] [Google Scholar]
- 19.Pyle SA, Haddock CK, Hymowitz N, et al. Family rules about exposure to environmental tobacco smoke. Fam syst health. 2005;23:3–16. [Google Scholar]
- 20.Ontario Medical Association. Exposure to secondhand smoke: Are we protecting our kids? Ontario, Toronto, Canada: 2005. [Google Scholar]
- 21.Tyc VL, Klosky J, Throckmorton-Belzer L, et al. Parent-reported environmental tobacco smoke exposure among preadolescents and adolescents treated for cancer. Psycho-Oncology. 2004;13:537–546. doi: 10.1002/pon.771. [DOI] [PubMed] [Google Scholar]
- 22.Priest N, Roseby R, Waters E, et al. Family and career smoking control programs for reducing children’s exposure to environmental tobacco smoke. John Wiley & Sons, Ltd; 2008. [DOI] [PubMed] [Google Scholar]
- 23.Hovell MF, Meltzer SB, Zakarian JM, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: A controlled trial. Chest. 1994;106:440–446. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- 24.Hovell MF, Zakarian JM, Matt GE, et al. Effect of counseling mothers on their children’s exposure to environmental tobacco smoke: Randomised controlled trial. BMJ. 2000;321:337–342. doi: 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlgren DR, Hovell MF, Meltzer SB, et al. Reduction of environmental tobacco smoke exposure in asthmatic children. A 2-year follow-up. Chest. 1997;111:81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- 26.Hovell MF, Meltzer SB, Wahlgren DR, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: A controlled trial. Pediatrics. 2002;110:946. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- 27.Emmons KM, Hammond SK, Fava JL, et al. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108:18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- 28.Hovell MF, Zakarian JM, Matt GE, et al. Counseling to reduce children’s secondhand smoke exposure and help parents quit smoking: A controlled trial. Nicotine Tob Res. 2009;11:1383–1394. doi: 10.1093/ntr/ntp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson SR, Yamada EG, Sudhakar R, et al. A Controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120:1709–1722. doi: 10.1378/chest.120.5.1709. [DOI] [PubMed] [Google Scholar]
- 30.Matt GE, Bernert JT, Hovell MF. Measuring secondhand smoke exposure in children: An ecological measurement approach. J Pediatr Psychol. 2008;33:156–175. doi: 10.1093/jpepsy/jsm123. [DOI] [PubMed] [Google Scholar]
- 31.Matt GE, Hovell MF, Zakarian JM, et al. Measuring secondhand smoke exposure in babies: The reliability and validity of mother reports in a sample of low-income families. Health Psychol. 2000;19:232–241. doi: 10.1037//0278-6133.19.3.232. [DOI] [PubMed] [Google Scholar]
- 32.Matt GE, Wahlgren DR, Hovell MF, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: Empirical findings and discussion. Tob Control. 1999;8:282–289. doi: 10.1136/tc.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyc VL, Lensing S, Vukadinovich CM, Hovell MF. Can parents of children with cancer accurately report their child’s passive smoking exposure? Nicotine Tob Res. 2009;11:1289–1295. doi: 10.1093/ntr/ntp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maudgal M, Raman J. Alternative methods of estimating piecewise linear and higher order regression models using SAS Software. Proceedings of SAS Users Group International. 1990;15:523–527. [Google Scholar]
- 35.Hovell MF, Hughes SC. The behavioral ecology of secondhand smoke exposure: A pathway to complete tobacco control. Nicotine Tob Res. 2009;11:1254–1264. doi: 10.1093/ntr/ntp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gehrman CA, Hovell MF. Protecting children from environmental tobacco smoke (ETS) exposure: A critical review. Nicotine Tob Res. 2003;5:289–301. doi: 10.1080/1462220031000094231. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg RA, Strecher VJ, Bauman KE, et al. Evaluation of a home-based intervention program to reduce infant passive smoking and lower respiratory illness. J Behav Med. 1994;17:273–290. doi: 10.1007/BF01857953. [DOI] [PubMed] [Google Scholar]
- 38.Singer BC, Hodgson AT, Geuvarra KS, et al. Gas-phase organics in environmental tobacco smoke: 1-effects of smoking rate, ventilation and furnishing level on emission factors. Environ Sci Technol. 2002;36:846–853. doi: 10.1021/es011058w. [DOI] [PubMed] [Google Scholar]
- 39.Singer BC, Hodgson AT, Nazaroff WW. Gas-phase organics in environmental tobacco smoke: 2---exposure relevant emission factors and indirect exposures from habitual smoking. Atmos Environ. 2003;37:5551–5561. [Google Scholar]
- 40.Sleiman M, Gundel LA, Pankow JF, et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proceedings of the National Academy of Sciences. 2010;107:6576–6581. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matt GE, Hovell MF, Quintana PJE, et al. The variability of urinary cotinine levels in young children: Implications for measuring ETS exposure. Nicotine Tob Res. 2007;9:83–92. doi: 10.1080/14622200601078335. [DOI] [PubMed] [Google Scholar]
- 42.Matt GE, Quintana PJE, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13:29–37. doi: 10.1136/tc.2003.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matt GE, Quintana PJE, Hovell MF, et al. Residual tobacco smoke pollution in used cars for sale: Air, dust, and surfaces. Nicotine Tob Res. 2008;10:1467–1475. doi: 10.1080/14622200802279898. [DOI] [PubMed] [Google Scholar]
- 44.Hollingshead AB. Unpublished manuscript. New Haven, CT: Yale University; Four factor index of social status. [Google Scholar]
- 45.Aitchison J. On the distribution of a positive random variable having a discrete probability mass at the origin. J Am Stat Assoc. 1955;50:901–908. [Google Scholar]