Abstract
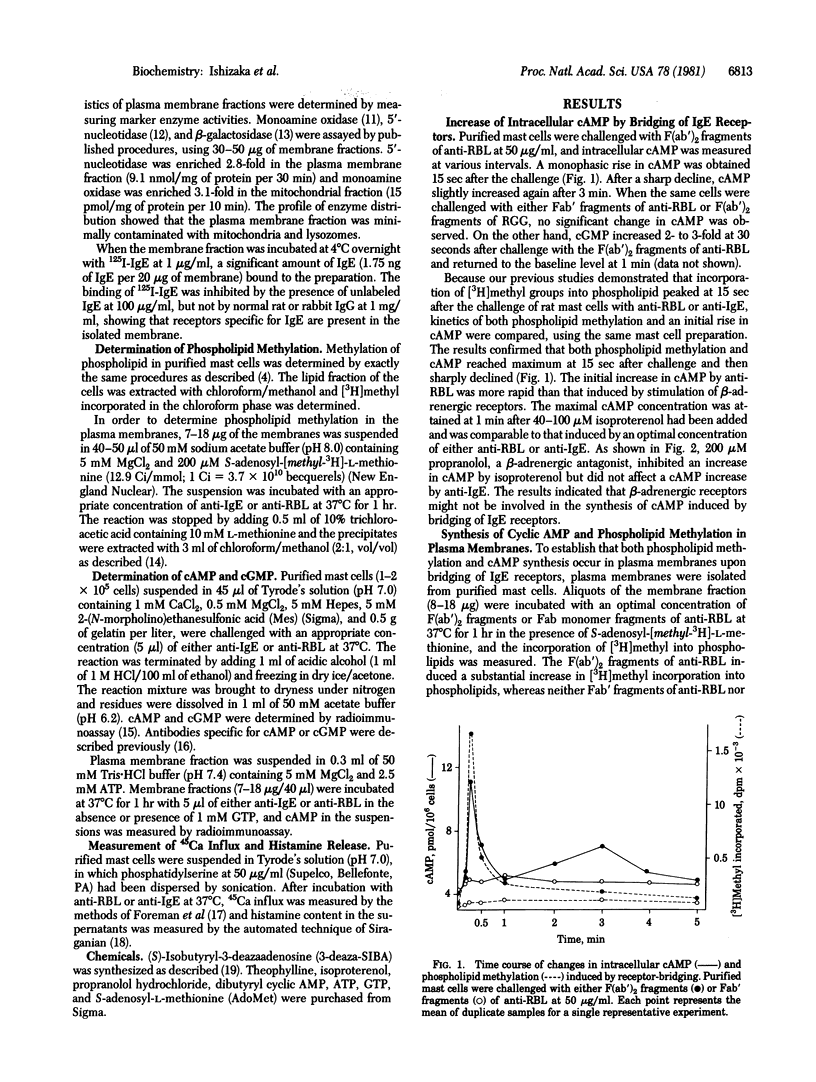
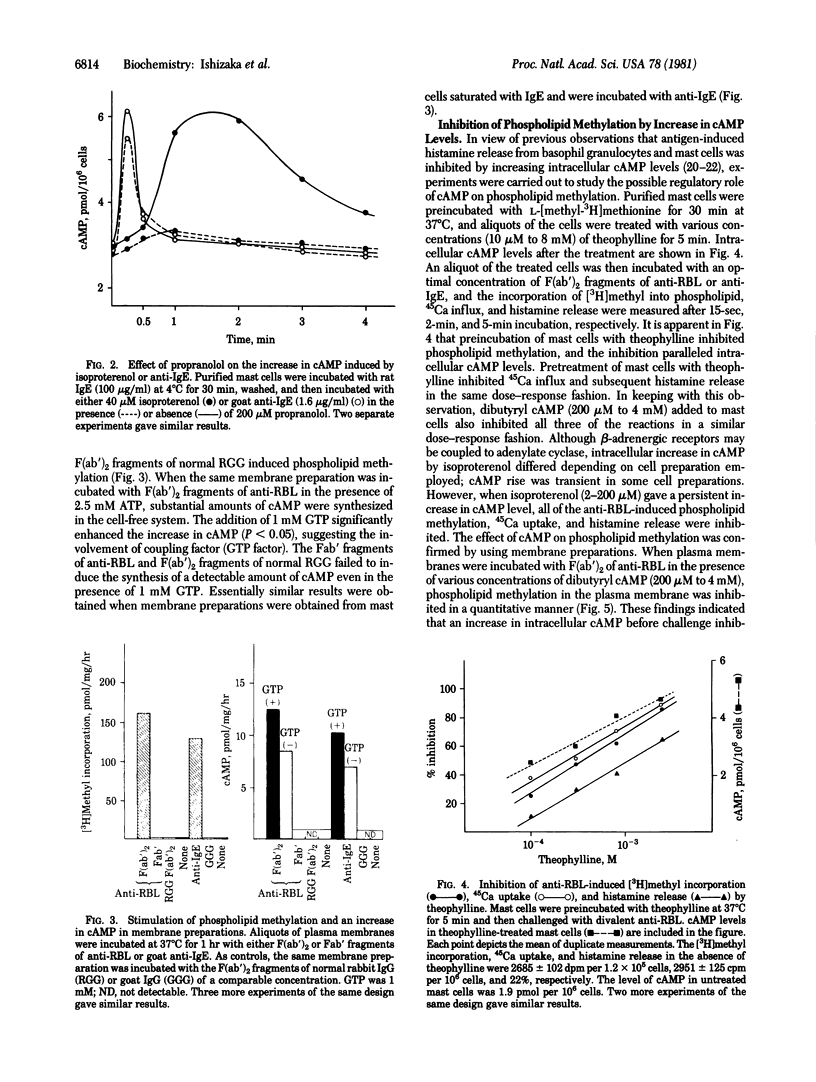
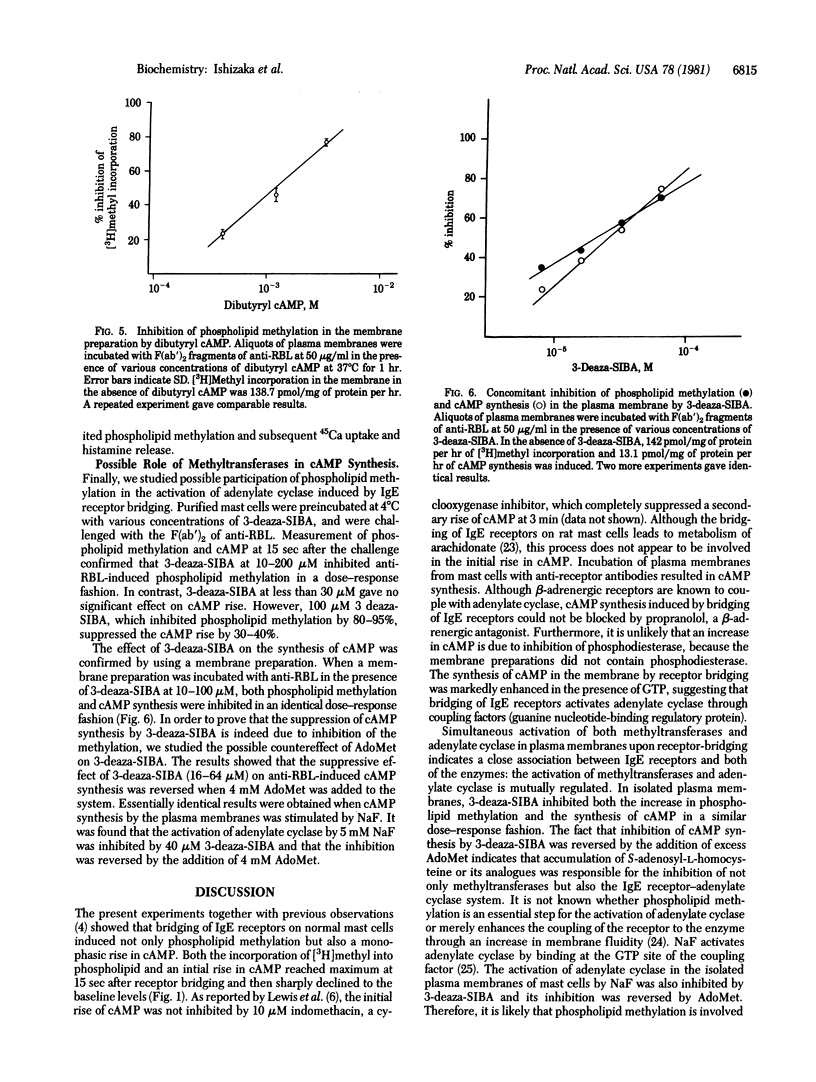
Bridging of IgE receptors on normal rat mast cells by divalent anti-receptor antibodies induced phospholipid methylation and an increase in intracellular cyclic AMP within 15 sec after the receptor bridging. These biochemical events were followed by Ca2+ influx and histamine release. When IgE receptors on isolated plasma membranes were bridged by the antibody, both the increase in the incorporation of [3H]methyl into lipid fraction and the synthesis of cyclic AMP were demonstrated. The synthesis of cyclic AMP in this system was enhanced in the presence of GTP. The results indicated that the bridged IgE receptors are linked to both methyltransferases and adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] in the plasma membrane. An increase in cyclic AMP prior to receptor bridging suppressed phospholipid methylation in the plasma membrane, Ca2+ uptake, and subsequent histamine release. On the other hand, inhibition of phospholipid methylation by (S)-isobutyryl-3-deazaadenosine resulted in the suppression of cyclic AMP synthesis in the plasma membrane. These findings suggest that the activation of phospholipid methylation and the activation of adenylate cyclase are e, and subsequent histamine release. On the other hand, inhibition of phospholipid methylation by (S)-isobutyryl-3-deazadenosine resulted in the suppression of cyclic AMP synthesis in the plasma membrane. These findings suggest that the activation of phospholipid methylation and the activation of adenylate cyclase are e, and subsequent histamine release. On the other hand, inhibition of phospholipid methylation by (S)-isobutyryl-3-deazadenosine resulted in the suppression of cyclic AMP synthesis in the plasma membrane. These findings suggest that the activation of phospholipid methylation and the activation of adenylate cyclase are mutually regulated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang P. K., Cantoni G. L., Bader J. P., Shannon W. M., Thomas H. J., Montgomery J. A. Adenosylhomocysteine hydrolase inhibitors: synthesis of 5'-deoxy-5'-(isobutylthio)-3-deazaadenosine and its effect on Rous sarcoma virus and Gross murine leukemia virus. Biochem Biophys Res Commun. 1978 May 30;82(2):417–423. doi: 10.1016/0006-291x(78)90892-6. [DOI] [PubMed] [Google Scholar]
- Conrad D. H., Froese A., Ishizaka T., Ishizaka K. Evidence for antibody activity against the receptor for IgE in a rabbit antiserum prepared against IgE-receptor complexes. J Immunol. 1978 Feb;120(2):507–512. [PubMed] [Google Scholar]
- Emmelot P., Bos C. J., van Hoeven R. P., van Blitterswijk W. J. Isolation of plasma membranes from rat and mouse livers and hepatomas. Methods Enzymol. 1974;31:75–90. doi: 10.1016/0076-6879(74)31008-7. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic methylation of phosphatidylethanolamine increases erythrocyte membrane fluidity. Nature. 1978 Sep 21;275(5677):219–220. doi: 10.1038/275219a0. [DOI] [PubMed] [Google Scholar]
- Hirata F., Strittmatter W. J., Axelrod J. beta-Adrenergic receptor agonists increase phospholipid methylation, membrane fluidity, and beta-adrenergic receptor-adenylate cyclase coupling. Proc Natl Acad Sci U S A. 1979 Jan;76(1):368–372. doi: 10.1073/pnas.76.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Lewis R. A., Austen K. F. Role of adenylate cyclase in immunologic release of mediators from rat mast cells: agonist and antagonist effects of purine- and ribose-modified adenosine analogs. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6800–6804. doi: 10.1073/pnas.77.11.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Foreman J. C., Sterk A. R., Ishizaka K. Induction of calcium flux across the rat mast cell membrane by bridging IgE receptors. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5858–5862. doi: 10.1073/pnas.76.11.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Biology of immunoglobulin E. Molecular basis of reaginic hypersensitivity. Prog Allergy. 1975;19:60–121. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Triggering of histamine release from rat mast cells by divalent antibodies against IgE-receptors. J Immunol. 1978 Mar;120(3):800–805. [PubMed] [Google Scholar]
- Ishizaka T., König W., Kurata M., Mauser L., Ishizaka K. Immunologic properties of mast cells from rats infected with Nippostrongylus brasiliensis. J Immunol. 1975 Oct;115(4):1078–1083. [PubMed] [Google Scholar]
- Ishizaka T. The Robert A. Cooke memorial lecture. Analysis of triggering events in mast cells for immunoglobulin E-mediated histamine release. J Allergy Clin Immunol. 1981 Feb;67(2):90–96. doi: 10.1016/0091-6749(81)90002-6. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Margolis S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968 Aug 30;161(3844):902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Eisen S. A., Parker C. W. Modulation of cyclic AMP in purified rat mast cells. II. Studies on the relationship between intracellular cyclic AMP concentrations and histamine release. J Immunol. 1975 May;114(5):1480–1485. [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Kulczycki A., Jr, Parker C. W. Modulation of cyclic AMP in purified rat mast cells. III. Studies on the effects of concanavalin A and anti-IgE on cyclic AMP concentrations during histamine release. J Immunol. 1976 Sep;117(3):713–716. [PubMed] [Google Scholar]
- Sun A. S., Poole B. Fractionation of rat fibroblasts in a zonal rotor by means of a viscosity barrier. Anal Biochem. 1975 Sep;68(1):260–273. doi: 10.1016/0003-2697(75)90704-6. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Jr, Brady R. O., Suzuki K. Enzymic activities associated with membranous cytoplasmic bodies and isolated brain lysosomes. J Neurochem. 1971 Sep;18(9):1775–1777. doi: 10.1111/j.1471-4159.1971.tb03754.x. [DOI] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]