Abstract
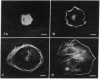
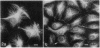
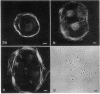
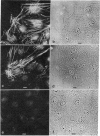
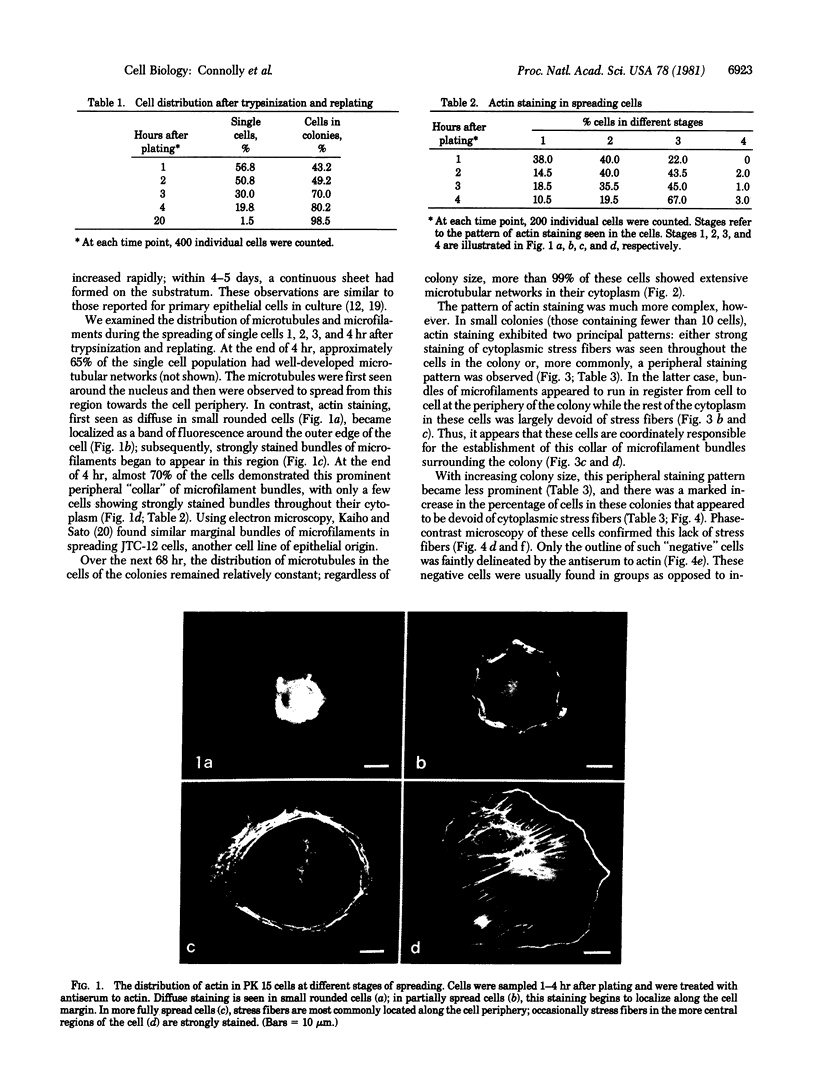
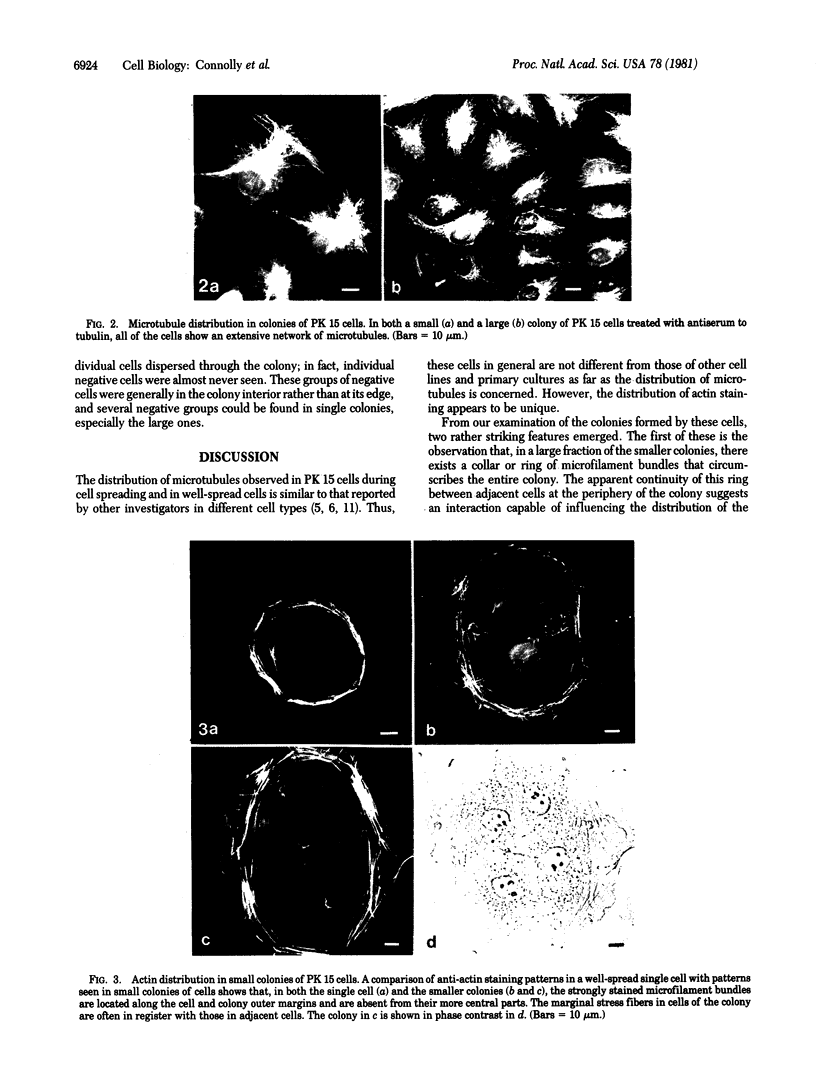
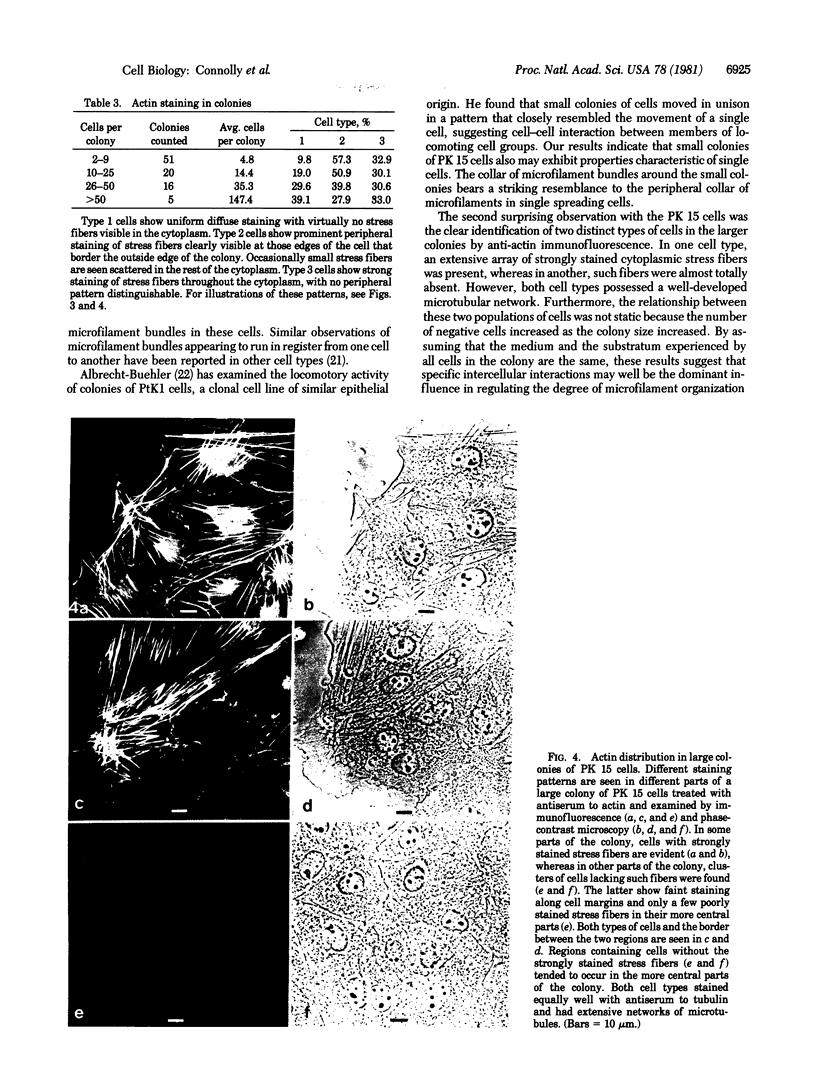
We have studied the distribution of microtubules and microfilaments during the cell spreading and subsequent colony formation in PK 15 pig kidney epithelial cells using indirect immunofluorescence. During the cell spreading on a solid substratum, microtubules grew out from the region around the nucleus, and a collar of microfilament bundles formed around the cell periphery. Although virtually all well-spread cells showed a complex microtubular network, distinctly different patterns of stress fibers were observed. In small colonies, the most commonly observed pattern was a ring of microfilament bundles that appeared to be in register between adjacent cells and encircled the entire colony in a fashion similar to that seen in single cells. In large colonies (more than 50 cells), approximately 60% of the cells displayed clearly stained microfilament bundles, either at the cell periphery or throughout their cytoplasm, whereas in the remaining 40%, no microfilament bundles were observed and only the outline of the cells was delineated by interaction with anti-actin. Such "negative" cells were seen in groups alongside "positive" cells (i.e., cells possessing extensive stress fiber networks) within the same colony. Independent of their stress fiber phenotype, all cells maintained a flattened shape and an extensive network of microtubules. We suggest that dense microfilament bundles are not a uniform feature of well-spread PI 15 cells in culture and that a loss of microfilament bundle occurs in some cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. Group locomotion of PtK1 cells. Exp Cell Res. 1979 Sep;122(2):402–407. doi: 10.1016/0014-4827(79)90319-7. [DOI] [PubMed] [Google Scholar]
- Ash J. F., Vogt P. K., Singer S. J. Reversion from transformed to normal phenotype by inhibition of protein synthesis in rat kidney cells infected with a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3603–3607. doi: 10.1073/pnas.73.10.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Cytoplasmic fibrils in living cultured cells. A light and electron microscope study. Protoplasma. 1967;64(4):349–380. doi: 10.1007/BF01666538. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Cleveland D. W., Kirschner M. W. Intracellular localization of the high molecular weight microtubule accessory protein by indirect immunofluorescence. J Cell Biol. 1978 Mar;76(3):781–786. doi: 10.1083/jcb.76.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I. Visualization of centrioles and basal bodies by fluorescent staining with nonimmune rabbit sera. J Cell Biol. 1978 Nov;79(2 Pt 1):526–532. doi: 10.1083/jcb.79.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipasquale A. Locomotion of epithelial cells. Factors involved in extension of the leading edge. Exp Cell Res. 1975 Oct 15;95(2):425–439. doi: 10.1016/0014-4827(75)90568-6. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D. R., Steinberg M. S. Cell locomotion within a contact-inhibited monolayer of chick embryonic liver parenchyma cells. J Cell Sci. 1975 Aug;18(3):405–425. doi: 10.1242/jcs.18.3.405. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., Subrahmanyan L., Turnbull C., Kalnins V. I. Localization of the neurofilament protein in neuroblastoma cells by immunofluorescent staining. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3192–3196. doi: 10.1073/pnas.73.9.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiho M., Misu Y. The role of microfilaments in the spreading process of JTC-12 cells. Exp Cell Res. 1980 Jun;127(2):434–438. doi: 10.1016/0014-4827(80)90448-6. [DOI] [PubMed] [Google Scholar]
- Kaiho M., Sato A. Circular distribution of microfilaments in cells spreading in vitro. Exp Cell Res. 1978 Apr;113(1):222–227. doi: 10.1016/0014-4827(78)90106-4. [DOI] [PubMed] [Google Scholar]
- Kopelovich L., Conlon S., Pollack R. Defective organization of actin in cultured skin fibroblasts from patients with inherited adenocarcinoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3019–3022. doi: 10.1073/pnas.74.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Hubbard B. D. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Two general classes of cytoplasmic actin filaments in tissue culture cells: the role of tropomyosin. J Supramol Struct. 1976;5(4):531(383)–563(415). doi: 10.1002/jss.400050410. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton C. A. The control of epithelial cell locomotion in tissue culture. Ciba Found Symp. 1973;14:251–270. [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tubulin-specific antibody and the expression of microtubules in 3T3 cells after attachment to a substratum. Further evidence for the polar growth of cytoplasmic microtubules in vivo. Exp Cell Res. 1976 Dec;103(2):331–340. doi: 10.1016/0014-4827(76)90270-6. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Auger J., Barber B. H., Edwards A. J., Walsh F. S., Crumpton M. J. Actin may be present on the lymphocyte surface. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4484–4488. doi: 10.1073/pnas.75.9.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M., Pollack R. Tumor promoters induce changes in the chick embryo fibroblast cytoskeleton. Cell. 1979 Oct;18(2):361–368. doi: 10.1016/0092-8674(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Sanford K. K., Frankel R. Tubulin and actin in paired nonneoplastic and spontaneously transformed neoplastic cell lines in vitro: fluorescent antibody studies. Cell. 1978 Apr;13(4):629–642. doi: 10.1016/0092-8674(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Vollet J. J., Brugge J. S., Noonan C. A., Butel J. S. The role of SV40 gene A in the alteration of microfilaments in transformed cells. Exp Cell Res. 1977 Mar 1;105(1):119–126. doi: 10.1016/0014-4827(77)90157-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Lazarides E., Goldman R. D., Vogel A., Pollack R. Localization and distribution of actin fibers in normal transformed and revertant cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):363–369. doi: 10.1101/sqb.1974.039.01.047. [DOI] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Yamada K. M., Yamada S. S., Pouysségur J., Pastan I. Microfilament bundles and cell shape are related to adhesiveness to substratum and are dissociable from growth control in cultured fibroblasts. Cell. 1977 Mar;10(3):375–380. doi: 10.1016/0092-8674(77)90024-1. [DOI] [PubMed] [Google Scholar]