Abstract
Digital processing techniques can be used to greatly enhance the available information in an optical image. Although this technology has been routinely used in many fields for a number of years, little application of digital image-processing techniques have been made toward analysis and enhancement of the types of images seen most often by the research biologist. We describe here a computer-based video microscope system that is capable of performing extensive manipulation and enhancement of microscope images in real time. The types of manipulations possible with these techniques greatly surpass the enhancement capabilities of photographic or video techniques alone. The speed and flexibility of this system enables experimental manipulation of the microscopic specimen based on its live processed image. These features greatly extend the power and versatility of the light microscope.
Full text
PDF
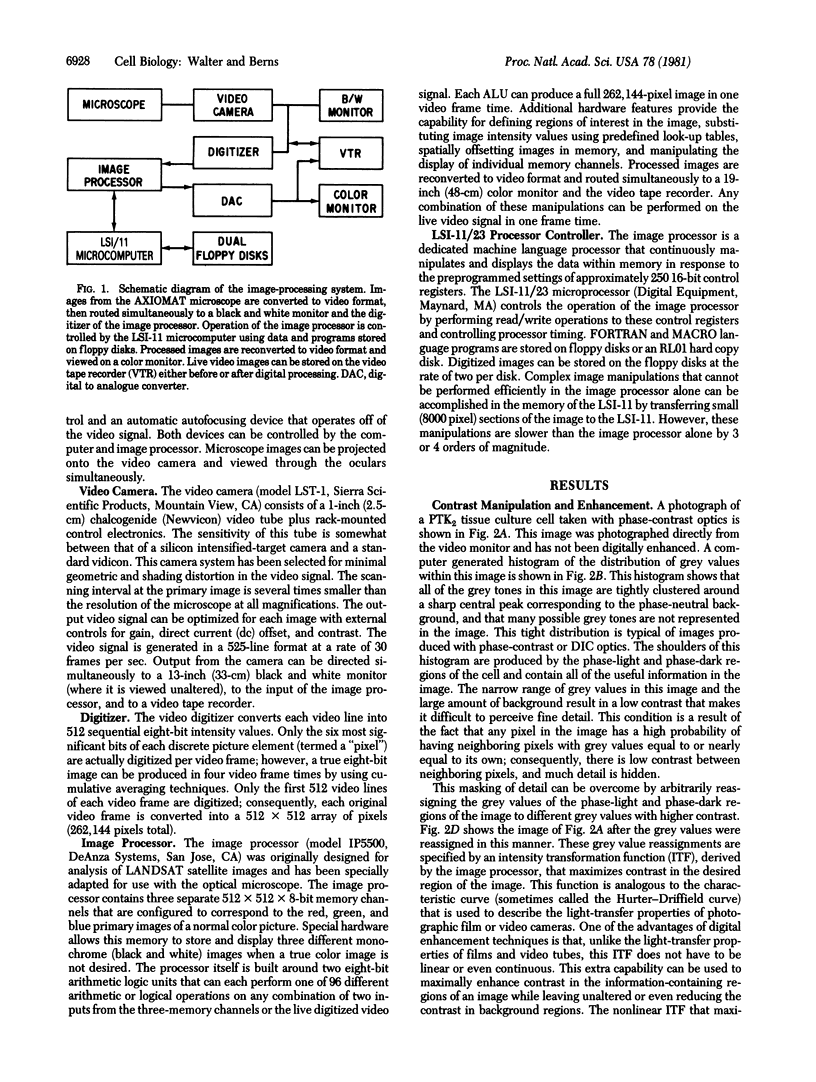
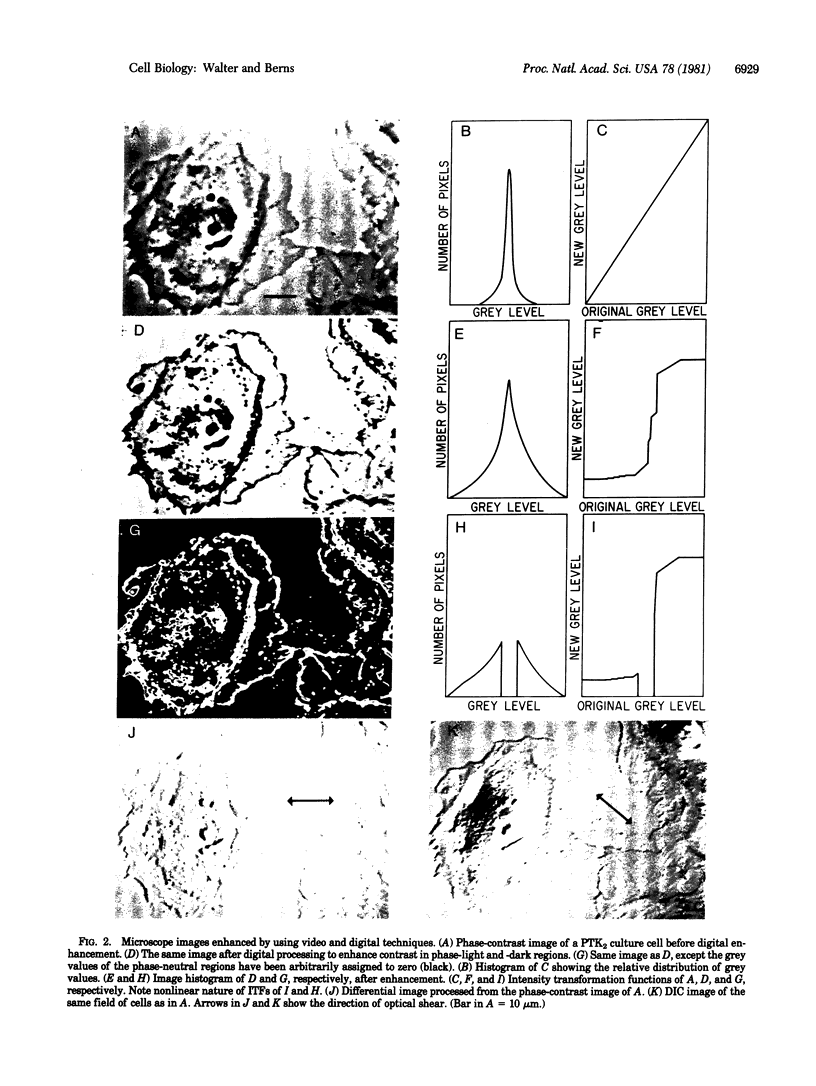


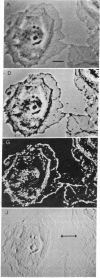
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Allen N. S., Travis J. L. Video-enhanced contrast, differential interference contrast (AVEC-DIC) microscopy: a new method capable of analyzing microtubule-related motility in the reticulopodial network of Allogromia laticollaris. Cell Motil. 1981;1(3):291–302. doi: 10.1002/cm.970010303. [DOI] [PubMed] [Google Scholar]
- Allen R. D., David G. B., Nomarski G. The zeiss-Nomarski differential interference equipment for transmitted-light microscopy. Z Wiss Mikrosk. 1969 Nov;69(4):193–221. [PubMed] [Google Scholar]
- Allen R. D., Travis J. L., Allen N. S., Yilmaz H. Video-enhanced contrast polarization (AVEC-POL) microscopy: a new method applied to the detection of birefringence in the motile reticulopodial network of Allogromia laticollaris. Cell Motil. 1981;1(3):275–289. doi: 10.1002/cm.970010302. [DOI] [PubMed] [Google Scholar]
- Bowie J. E., Young I. T. An analysis technique for biological shape-III. Acta Cytol. 1977 Nov-Dec;21(6):739–746. [PubMed] [Google Scholar]
- Bradbury S. Microscopical image analysis: problems and approaches. J Microsc. 1979 Mar;115(2):137–150. doi: 10.1111/j.1365-2818.1979.tb00163.x. [DOI] [PubMed] [Google Scholar]
- Castleman K. R., Melnyk J., Frieden H. J., Persinger G. W., Wall R. J. Computer-assisted karyotyping. J Reprod Med. 1976 Jul;17(1):53–57. [PubMed] [Google Scholar]
- Dunn R. F., O'Leary D. P., Kumley W. E. Quantitative analysis of micrographs by computer graphics. J Microsc. 1975 Nov;105(2):205–213. doi: 10.1111/j.1365-2818.1975.tb04051.x. [DOI] [PubMed] [Google Scholar]
- Inoué S. Video image processing greatly enhances contrast, quality, and speed in polarization-based microscopy. J Cell Biol. 1981 May;89(2):346–356. doi: 10.1083/jcb.89.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin L. E., Watt W. C., Kirsch R. A. The analysis, synthesis, and description of biological images. Ann N Y Acad Sci. 1966 Jan 31;128(3):984–1012. doi: 10.1111/j.1749-6632.1965.tb11712.x. [DOI] [PubMed] [Google Scholar]
- Olson A. C., Larson N. M., Heckman C. A. Classification of cultured mammalian cells by shape analysis and pattern recognition. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1516–1520. doi: 10.1073/pnas.77.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prewitt J. M., Mendelsohn M. L. The analysis of cell images. Ann N Y Acad Sci. 1966 Jan 31;128(3):1035–1053. doi: 10.1111/j.1749-6632.1965.tb11715.x. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Calcium ion distribution in cytoplasm visualised by aequorin: diffusion in cytosol restricted by energized sequestering. Science. 1975 Dec 19;190(4220):1204–1206. doi: 10.1126/science.1198106. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The visualization of fluorescent proteins in living cells by video intensification microscopy (VIM). Cell. 1978 Mar;13(3):501–507. doi: 10.1016/0092-8674(78)90323-9. [DOI] [PubMed] [Google Scholar]