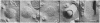Abstract
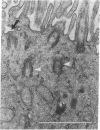
Observations of freeze-fracture preparations of human nasal epithelium have provided a unique perspective of the spatial distribution of epithelial cell cilia unattainable by more conventional ultrastructural techniques. The initial stages of ciliogenesis were characterized ultrastructurally in these preparations by differentiation of the lumenal aspect of the epithelial cell membrane prior to the emergence and maturation of new cilia. Morphometric analyses of the resultant electron micrographs indicate that the development of an optimal ciliary population during differentiation of ciliated cells may be integral to the adequate functioning of respiratory mucociliary mechanisms. The frequency with which such ciliogenic structures are observed indicates that ciliogenesis is a common feature of the nasal epithelium and suggests that epithelial cell turnover in the nasal cavities is relatively rapid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS G. D., BAUER H., SPRINZ H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963 Mar;12:355–364. [PubMed] [Google Scholar]
- Breeze R. G., Wheeldon E. B. The cells of the pulmonary airways. Am Rev Respir Dis. 1977 Oct;116(4):705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Clyde W. A., Jr Ciliary membrane alterations occurring in experimental Mycoplasma pneumoniae infection. Science. 1979 Oct 19;206(4416):349–351. doi: 10.1126/science.113877. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Tyler W. S., Dungworth D. L. Lesions in respiratory bronchioles and conducting airways of monkeys exposed to ambient levels of ozone. Exp Mol Pathol. 1977 Jun;26(3):384–400. doi: 10.1016/0014-4800(77)90041-7. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974 Dec;141(4):461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- Cordier A. C., Haumont S. Origin of necklace particles in thymic ciliating cells. Am J Anat. 1979 Sep;156(1):91–97. doi: 10.1002/aja.1001560109. [DOI] [PubMed] [Google Scholar]
- Fabrikant J. I. The kinetics of cellular proliferation in human tissues. Determination of duration of DNA synthesis using double labeling autoradiography. Br J Cancer. 1970 Mar;24(1):122–127. doi: 10.1038/bjc.1970.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula N. B., Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972 May;53(2):494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins V. I., Chung C. K., Turnbull C. Procentrioles in ciliating and ciliated cells of chick trachea. Z Zellforsch Mikrosk Anat. 1972;135(4):461–471. doi: 10.1007/BF00583430. [DOI] [PubMed] [Google Scholar]
- Kanda T., Hilding D. Development of respiratory tract cilia in fetal rabbits. Electron microscopic investigation. Acta Otolaryngol. 1968 Jun;65(6):611–624. doi: 10.3109/00016486809119295. [DOI] [PubMed] [Google Scholar]
- Mellick P. W., Dungworth D. L., Schwartz L. W., Tyler W. S. Short term morphologic effects of high ambient levels of ozone on lungs of rhesus monkeys. Lab Invest. 1977 Jan;36(1):82–90. [PubMed] [Google Scholar]
- Muse K. E., Collier A. M., Baseman J. B. Scanning electron microscopic study of hamster tracheal organ cultures infected with Bordetella pertussis. J Infect Dis. 1977 Dec;136(6):768–777. doi: 10.1093/infdis/136.6.768. [DOI] [PubMed] [Google Scholar]
- Sorokin S. P. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968 Jun;3(2):207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]