Abstract
Calcium is the major regulator of keratinocyte differentiation in vivo and in vitro. A calcium gradient within the epidermis promotes the sequential differentiation of keratinocytes as they traverse the different layers of the epidermis to form the permeability barrier of the stratum corneum. Calcium promotes differentiation by both outside–in and inside–out signaling. A number of signaling pathways involved with differentiation are regulated by calcium, including the formation of desmosomes, adherens junctions and tight junctions, which maintain cell–cell adhesion and play an important intracellular signaling role through their activation of various kinases and phospholipases that produce second messengers that regulate intracellular free calcium and PKC activity, critical for the differentiation process. The calcium receptor plays a central role by initiating the intracellular signaling events that drive differentiation in response to extracellular calcium. This review will discuss these mechanisms.
Keywords: cadherin, calcium, calcium receptor, catenin, keratinocyte, phospholipase, protein kinase C, Src kinase
Microanatomy of the epidermis
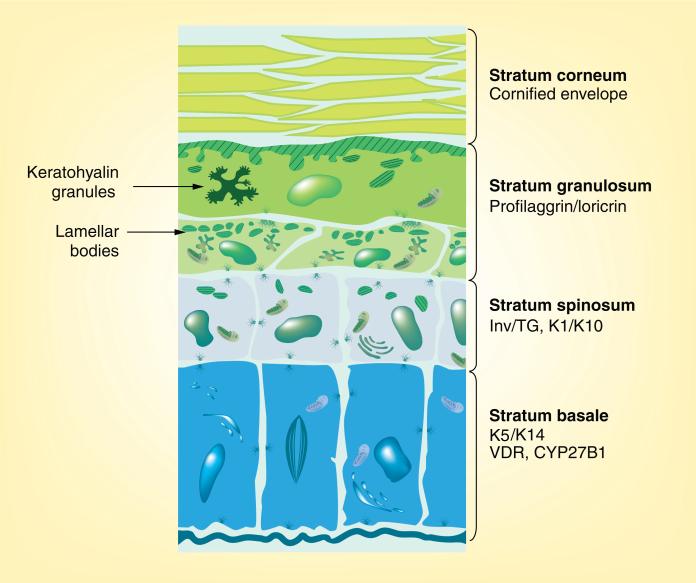
The epidermis is composed of four functionally different layers of keratinocytes at different stages of differentiation (Figure 1). The thickness of these layers varies in different sites, different species and under different conditions. The basal layer (stratum basale) rests on the basal lamina separating the dermis and epidermis. This layer contains the epidermal stem cells. These cells proliferate, providing the cells for the upper differentiating layers. They are large, columnar cells forming intercellular attachments with adjacent cells through desmosomes and adherens junctions. Desmogleins (Dsg) 2 and 3 and desmocollins (Dsc) 2 and 3 are the dominant cadherins in the desmosomes of the basal layers [1], whereas both P- and E-cadherins participate in the adherens junctions of these cells. However, as the keratinocytes move out of the basal layer and begin to differentiate, Dsg 1 and Dsc 1 become the dominant desmosomal cadherins, whereas P-cadherin is no longer produced as E-cadherin becomes the dominant cadherin in adherens junctions [1]. Furthermore, the expression of proteins (claudins and occludins) that form the tight junctions is initiated [2]. An asymmetric distribution of integrins on their lateral and basal surface enables their attachment to the basal lamina and adjacent cells [3–5] and helps to regulate their proliferation and subsequent differentiation [6]. Keratinocytes in the stratum basale express the keratins K5 and K14 [7]. As the cells leave the stratum basale, they switch from producing K5/K14 to producing keratins K1 and K10 in the stratum spinosum [8]. Cells of this layer also begin to produce involucrin [9], a component of the cornified envelope, and the enzyme, transglutaminase-K, which is responsible for the ε-(γ-glutamyl)lysine crosslinking of involucrin and other substrates into the insoluble cornified envelope [10]. The stratum granulosum, which is above the stratum spinosum, is so named because of the presence of electron-dense keratohyalin granules [11]. It is the uppermost nucleated layer. The larger of the two granule types contains profilaggrin, precursor of filaggrin, first thought to function as a bundling protein for the keratin filaments, and more recently thought to play a role in maintaining the water content of the epidermis, as it is degraded into smaller peptides with osmotic properties [12]. The smaller granules contain loricrin, a major component of the cornified envelope [13]. The stratum granulosum also contains lamellar bodies and lipid-filled vesicles responsible for secreting their lipid content (and lipid-processing enzymes) into the junction of the stratum granulosum and the stratum corneum [14]. The stratum corneum is the outermost layer of the epidermis. In this layer, the cornified envelope fully matures, surrounding the bundled keratin filaments and attached to the lipid envelope contributed by the processing of the lipids secreted by the lamellar bodies. The net result is a resilient impermeable structure protecting the viable layers underneath [15].
Figure 1. Microanatomy of the epidermis.
The epidermis is composed of four functionally different layers that may vary in thickness depending on location and species. The stratum basale rests on the basal lamina and contains the stem cells. This layer is distinguished by its production of keratins 5 and 14. The VDR and the 25OHD-1α CYP27B1 responsible for producing the active metabolite of vitamin D (1,25[OH]2D) are found in the highest concentration in this layer. Differentiation is initiated as the cells move from the stratum basale to the stratum spinosum, where involucrin, transglutaminase-I and the keratins K1 and K10 are expressed. In the next layer, the stratum granulosum, profilaggrin and loricrin are produced and packaged in keratohyalin granules. This layer is also where lipids for the waterproofing of the permeability barrier are produced and packaged into lamellar bodies. The stratum corneum is the enucleated layer critical for barrier function containing the cornified envelope within and the lipid matrix without the cells.
VDR: Vitamin D receptor.
Adapted with permission from [133].
Calcium forms a steep gradient within the epidermis, with the highest concentration in the stratum granulosum [16]. Although initial studies with ion-capture cytochemistry and proton-induced x-ray emission indicated that the calcium levels were lowest in the stratum basale, more recent studies with phasor representation of fluorescent lifetime imaging microscopy demonstrated that the calcium content of cells in the stratum basale was variable, with a number of the keratinocytes having substantial amounts of calcium relative to the keratinocytes of the stratum spinosum. Furthermore, these recent studies demonstrated that the calcium was essentially all intracellular [17]. Therefore, in the subsequent discussion of the calcium switch in vitro, it is important to bear in mind that the authors do not have precise measurements of the extracellular calcium concentration in the intact epidermis. It would not, for example, be appropriate to assume that the extracellular calcium concentration in the epidermis is the same as that in the bloodstream. The higher levels of calcium in some of the cells of the stratum basale versus the stratum spinosum may contribute to initiating the differentiation process, as keratinocytes from patients with Hailey–Hailey disease (mutations in the Golgi calcium pump, ATP2C1) or from patients with Darier's disease (mutations in the endoplasmic reticulum (ER) calcium pump, ATP2A2) have lower calcium content in their stratum basale with decreased cell adhesion and disruption of the transition from the basal expression of K14 to that of K10 [18,19]. When the barrier is disrupted, the calcium gradient is lost [20], with an increase in lamellar body secretion and decreased expression of the differentiation markers loricrin, profilaggrin and involucrin [21]. Exposure of the skin to a high extracellular calcium concentration (Cao) following barrier disruption restores the expression of these marker genes, whereas exposure to a low Cao maintains the lowered expression of these differentiation markers [21]. As will be clear in the section that follows, this in vivo study is consistent with the more extensive studies of the response of keratinocytes to Cao in vitro.
Calcium switch
Calcium is the best-studied prodifferentiating agent for keratinocytes. As noted above, the calcium gradient in the epidermis plays a role in epidermal differentiation. However, much of the understanding of the role of calcium in keratinocyte differentiation comes from in vitro studies. Some care must be taken in extrapolating the in vitro results to the in vivo situation, since the dermis clearly affects keratinocyte differentiation as evidenced by the fact that hair follicles will not develop in the absence of certain dermal structures (dermal papilla). Nevertheless, much has been learned about calcium-regulated differentiation from these in vitro studies, that is applicable to the in vivo situation. Keratinocytes in low calcium concentrations (e.g., 0.03 mM) proliferate but fail to differentiate into a stratified layer. When switched to calcium concentrations above 0.1 mM (the calcium switch), the differentiation process is initiated, involving both genomic and nongenomic pathways. The cells rapidly undergo morphologic changes with the development of cell–cell contacts that are critical for the differentiation process [22]. This is mediated by the redistribution to the membrane of desmoplakin to form desmosomes, occludins and claudins to form tight junctions, and E-cadherin with its associated catenins and kinases to form adherens junctions. As will be discussed subsequently, these membrane complexes provide not only adhesion between cells but also a signaling complex that participates in changes in actin distribution and sustained increases in intracellular calcium (Cai) [23–25]. These translocations to the membrane are dependent on the actin network in that cytochalasin blocks these events [24–27], but are rapid and not dependent on new protein synthesis. However, with the sustained increase in Cai, the cells begin to express in sequential fashion K1 and K10, involucrin and transglutaminase-I and loricrin and filaggrin, in that order [28–32]. A number of these genes (e.g., involucrin and K1) have known response elements, such as activator protein 1 (AP-1) sites for calcium and phorbol esters, acting at least in part by PKC activation. This regulation requires hours and is genomic [30–36]. Members of the Fos/Jun families bind to these sites following the calcium switch, but details of the actual mechanisms by which calcium controls the transcription of these genes remain elusive. A change in integrin expression accompanies the differentiation process and may be essential for keratinocytes to leave the stratum basale for the upper layers [37]. The response of keratinocytes to the calcium switch is multiphasic. The initial spike in Cai after an increase in Cao represents release from intracellular stores and is mediated by the calcium receptor (CaR), which will be discussed at length subsequently. However, it is the sustained increase in calcium that is critical for differentiation [27,30,32–34,38–41]. As the keratinocytes differentiate and the Cai increases, they become less sensitive to changes in Cao. The increase in Cai following the calcium switch is blocked by lanthanum, suggesting a requirement for calcium entry to sustain the Cai [42,43]. A number of different channels have been found in keratinocytes that may function as calcium channels (L-type calcium channels do not appear to be present) [44–48], with recent attention to being paid transient receptor potential vanilloid channel V6 (TRPV6) and the transient receptor potential cation (TRPC)1, 3 and 4 channels that may function as store-operated channels [49–51]. As will be discussed subsequently, several of these channels are regulated by phospholipase C (PLC)-γ1 and the CaR. Agents such as ATP that stimulate only the initial increase of Cai from intracellular stores do not promote differentiation [52–55].
A number of pathways are critical for the calcium response, and these will be reviewed prior to discussing the role of the CaR.
Phospholipase C
The calcium switch stimulates phosphoinositide metabolism, providing critical second messengers for the differentiation process [56–59]. The main enzymes involved are PLC β and γ, which hydrolyze phosphatidylinositol bisphosphate (PIP2) to inositol trisphosphate (IP3) and diacylglycerol (DAG). Both calcium and the active metabolite of vitamin D, 1,25(OH)2D, induce these enzymes [60,61]. Following the calcium switch, the rise in Cai and in IP3 and DAG are both immediate and prolonged. As noted above, other agents such as ATP raise Cai and IP3 levels as effectively as calcium, at least acutely, and yet do not stimulate differentiation [55], presumably owing to the transient nature of the increase in Cai. The prolonged increase in IP3 and Cai is due to calcium activation of PLC-γ1, as it is blocked by an antisense PLC-γ1 construct [61], whereas the initial increase in IP3 and Cai after the calcium switch appears to be mediated by PLC-β, as it is not blocked by the antisense PLC-γ1 construct but is blocked by the general PLC inhibitor U73122 and knockdown (by siRNA) of PLC-β1 [Xie Z, Bikle DD, Unpublished Data]. The critical role for PLC-γ1 in mediating calcium-induced differentiation is demonstrated by the ability of the PLC-γ1 antisense construct to prevent the calcium induction of differentiation markers such as involucrin and transglutaminase [61]. Furthermore, the PLC-γ1 antisense construct, as well as U73122, block the influx of calcium into the cells through putative store-operated channels TRPC1 and 4 following depletion of the intracellular stores with thapsigargin [50]. The mechanism leading to PLC-γ1 activation by calcium will be covered in the section describing the E-cadherin/catenin complex.
PLC-γ1 contains several important domains that are critical for its regulation [62–65]. The Src homology domain 2 (SH2) domains are responsible for binding PLC-γ1 to phosphotyrosines such as those found in a number of growth factor receptor kinases, for example the EGF receptor. The EGF receptor phosphorylates PLC-γ1 at three tyrosines (771, 783 and 1254 [66]), at least two of which (Y783 and Y1254) are required for maximal activation. A pleckstrin homology (PH) domain is found in the N-terminal portion of the molecule, with two half PH domains on either side of the SH2 and SH3 domains. These PH domains and the SH2 domains enable PLC-γ1 binding to phosphatidylinositols in the membrane [63–65], in particular phosphatidylinositol trisphosphate (PIP3). Calcium activates PLC-γ1 via PIP3 in the absence of tyrosine phosphorylation of PLC-γ1 [67], an important function of the E-cadherin/catenin complex, as will be discussed in a subsequent section. The means by which PLC-γ1 is activated dictates whether PLC-γ1 mediates growth factor-stimulated proliferation [68] or calcium-induced differentiation [67].
Protein kinase C
The rise in DAG and Cai following the calcium switch results in PKC activation. Studies of the role of PKC in keratinocyte differentiation utilize phorbol esters [54,56,69–73]. These compounds are capable of stimulating differentiation of keratinocytes at least in vitro even in low Cao conditions, although their effects can be potentiated by Cao [69–73]. Furthermore, PKC inhibitors block a number of effects of phorbol esters and Cao on keratinocyte differentiation [74,75]. However, phorbol esters and calcium differ in at least some aspects of their impact on differentiation. Phorbol esters, for example, do not stimulate K1 and K10 expression [76,77], unlike their effects on later differentiation markers, such as involucrin, loricrin and filaggrin. Cao and phorbol esters also differ in their patterns of protein phosphorylation [58,78,79], and, importantly, phorbol esters do not activate the PLC pathway [56,80]; rather, the PLC pathway activated by Cao results in PKC activation via generation of DAG [56,79,80]. Moreover, phorbol esters, at least in other cells, can interfere with PLC activation [81,82]. Nevertheless, PKC activation plays an important role in the mechanism by which calcium promotes keratinocyte differentiation.
There are a large number of PKC isozymes in the epidermis, generally products of different genes under different modes of regulation and distribution within the epidermal layers [83–87]. Of the classic PKC enzymes, only PKC-α is found in the keratinocyte. Classic PKC enzymes are characterized by their activation by calcium, phorbol esters and DAG. Three novel PKC enzymes, PKC-δ, ε and η, characterized by their responsiveness to phorbol esters and DAG but not calcium, are present in keratinocytes. The keratinocyte also expresses PKC-ζ, an atypical PKC that does not respond to calcium or phorbol esters. Different agents promoting differentiation may utilize different PKC isozymes. Several studies, including this one, showed that blocking the expression of PKC-α with antisense oligonucleotides prevented Cao induction of a number of differentiation markers [87,88]. However, not all studies have reached this conclusion. In particular, PKC-δ has been shown in some studies to be the most critical PKC for keratinocyte differentiation, whereas PKC-α overexpression was found to block calcium-induced differentiation [36]. These disparities remain unresolved, but may result from differences in species or between experimental approaches using overexpression versus reduction of the protein of interest.
As alluded to previously, activation of PKC leads to activation of transcription factors in the Fos/Jun families that probably mediate the effects of calcium, phorbol esters and DAG on keratinocyte differentiation [53,54,89–93]. These transcription factors bind to AP-1 sites in the regulatory regions of the genes that they regulate [94]. In addition to c-Fos and c-Jun, Fra-1, Fra-2, Jun B and Jun D are found in keratinocytes, and their distribution in the epidermis is both cell- and species-specific [95]. The best-studied gene in this regard is involucrin, in which the distal AP-1 site (critical for both phorbol ester and calcium regulation) binds Fra-1, Jun B and Jun D following PKC activation [96]. Surprisingly, a dominant negative mutant of c-Jun that blocks c-Jun/Fos-regulated prolactin expression [97] actually promotes transcription of involucrin [77], indicating that these Fos/Jun factors may have both stimulatory and inhibitory actions on the genes that they regulate.
E-cadherin–catenin complex
As noted in the discussion of the response of the keratinocyte to the calcium switch, cell–cell contacts are established. These consist of adherens junctions, tight junctions and desmosomes [6,24]. These contacts serve not only as mechanisms for cellular adhesion but also as important signaling complexes. Cao plays a critical role in enabling the intercellular contacts, although the precise role played by Cao in the binding of the extra-cellular domains of the proteins remains unclear [1,98]. What does seem clear is that the strength of these intercellular bonds is regulated by the intracellular portions of these complexes. The E-cadherin–catenin complex, the major component of adherens junctions, is the best studied with respect to its signaling function, and plays a key role in the mechanism by which Cao stimulates differentiation [99]. Within minutes of the calcium switch, E-cadherin is translocated to the plasma membrane in a complex with α, β, γ and p120 catenins, RhoA, Src family kinases, PI3K and phosphatidylinositol 4 phosphate 5 kinase 1α (PIP5K1α) [100–108]. E-cadherin is expressed throughout the epidermis, unlike P-cadherin, which is expressed only in the basal layer [109]. Deletion of E-cadherin prevents the formation of adherens junctions and impairs differentiation of the keratinocytes, although cell–cell adhesion persists, presumably owing to the continued expression of P-cadherin and proteins involved with desmosome and tight junction formation [102,105,110]. The results in vivo with E-cadherin deletion are somewhat discrepant, with one report demonstrating the maintenance of adherens junctions, although the epidermis was hyperplastic and lacked full expression of terminal differentiation markers [111], whereas a second report demonstrated a high rate of perinatal mortality not found in the earlier report, with breakdown of the permeability barrier and loss of the catenins [112].
The chain of events leading to the formation of the E-cadherin–catenin complex following the calcium switch is discussed later. When activated by calcium, the CaR, in turn activates RhoA [113]. This leads to activation of Src family kinases, Fyn and Src, in particular, responsible for the tyrosine phosphorylation of the catenins and their binding to E-cadherin [67,100,101,105,108,114]. Surprisingly, this does not require an increase in Cai, in that the Cai chelator BAPTA fails to block calcium-induced E-cadherin–catenin complex formation, although, as noted earlier, the increase in Cai is required for subsequent differentiation [115]. p120 catenin binds to the juxtamembrane region of E-cadherin and stabilizes the complex [67,116]. Knockdown of p120 catenin leads to loss of E-cadherin and β- and α-catenin, with epidermal hyperplasia and chronic inflammation [117] similar to that seen in the E-cadherin null mouse reported by Tinkle et al. [111]. β- and γ- (phakoglobin) catenins bind separately to the catenin-binding domain of the E-cadherin tail. Through their N-termini, they bind α-catenin, which in turn links the complex to the underlying actin cytoskeleton [118]. In mouse keratinocytes, PI3K binds to p120 and γ-catenin [102], but in human keratinocytes and most other cells, p120 and β-catenin serve as the anchors for PI3K binding [105]. In human keratinocytes, γ-catenin plays little role in the formation or maintenance of the E-cadherin–complex, and its deletion has little impact on Cao-induced differentiation, unlike deletion of the other catenins and E-cadherin itself [105].
There are a number of PI3Ks with different regulatory and catalytic subunits and distinct modes of regulation [119]. Class 1A enzymes are composed of one of four regulatory subunits (p85-α and -β, p55 and p50), and one of three catalytic subunits (p110-α, -β and -δ). The regulatory subunits bind to activated receptor tyrosine kinases or other phosphotyrosine-containing proteins via their SH2/SH3 domains. Within the E-cadherin complex, this binding is to the catenins that enable phosphorylation of the regulatory subunit, releasing and thus activating the catalytic subunit. Class 1B PI3K has one known catalytic subunit, p110γ, and one known regulatory subunit, p101. This isoform is activated by the β/γ subunits of G proteins, such that agonists of G protein-coupled receptors, including CaR, may also activate PI3K by this mechanism. Inhibition of PI3K activity blocks the ability of calcium to activate PLC-γ1 and induce differentiation [67,105].
PIP5K1α is also bound to the E-cadherin–catenin complex following the calcium switch, and this binding is also dependent on β-catenin [106]. Deletion of PIP5K1α in vitro blocks both PI3K and PLC-γ1 activity following the calcium switch. Furthermore, deletion of PIP5K1α blocks the acute rise in Cai following Cao, ATP or ionomycin, indicating a reduction in Cai stores. As with the inhibition of PI3K, this results in an inhibition of Cao-induced differentiation.
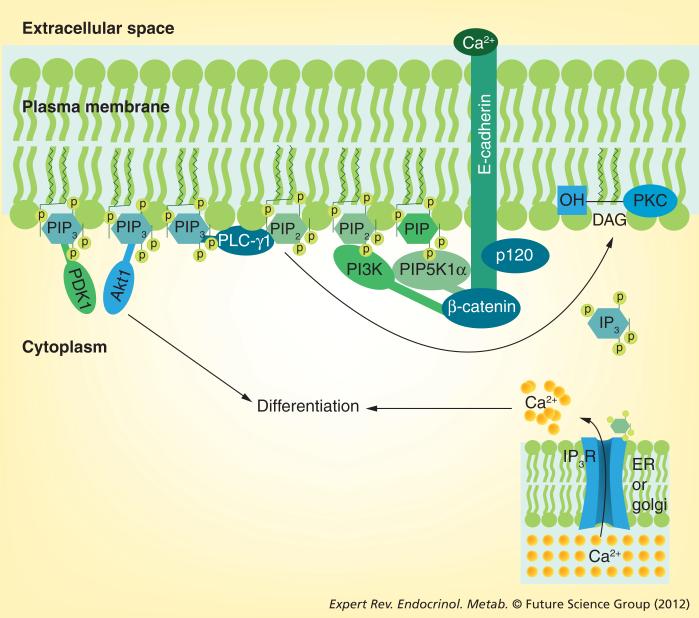
The model the authors propose that links the E-cadherin–catenin complex to calcium-induced differentiation is shown in Figure 2. p120 and β catenin when bound to E-cadherin recruit PI3K and PIP5K1α to the membrane. PIP5K1α phosphorylates phosphatidylinositol phosphate to PIP2, and PI3K phosphorylates PIP2 to PIP3. PIP3 activates PLC-γ1, which in turn hydrolyzes PIP2 to IP3 and DAG. These second messengers release calcium from intracellular stores and activate PKC. The rise in Cai and activation of PKC contribute to the induction of genes that are responsible for the differentiation process. Deletion of PIP5K1α results in a reduction of PIP2 production, thus limiting the ability of any of the PLCs to produce IP3 and DAG, and limiting the amount of PIP3 that can be produced by PI3K. Deletion or inactivation of PI3K prevents PIP3 formation, thus blocking calcium-induced activation of either PLC-γ1 or Akt (also known as protein kinase B, which is dependent on PIP3 for its recruitment to the membrane and subsequent phosphorylation by PI3K). The net result is that formation of the E-cadherin complex following the calcium switch is critical for the pathways, in particular the PKC- and PLC-mediated pathways, required for differentiation. CaR plays a key role in initiating these events in response to Cao.
Figure 2. Signaling by the E-cadherin–catenin complex.
Following the calcium switch, the E-cadherin–catenin complex forms in the plasma membrane. Extracellular calcium serves to link the extracellular domains of E-cadherin together, forming the adherens junctions (outside–in signaling). Within the cell, p120- and β-catenins bind to the juxtamembrane and cytoplasmic tail, respectively, of E-cadherin. γ-catenin (not shown) competes with β-catenin for this site, but in human keratinocytes it does not appear to have a major role in calcium-induced keratinocyte differentiation. α-catenin (not shown) attaches to β-catenin and links the complex to the cytoskeleton. β-catenin also serves as the binding site for PIP5K1α and PI3K, enzymes that phosphorylate PIP to PIP2 and PIP2 to PIP3, respectively. PIP3 activates PLC-γ1, which hydrolyzes PIP2 to IP3 and DAG, leading to the release of calcium from intracellular stores and activation of PKC, respectively. PIP3 also serves as a binding site for Akt and PDK1, which also contribute to the differentiation process.
DAG: Diacylglycerol; ER: Endoplasmic reticulum; IP3: Inositol trisphosphate; IP3R: Inositol trisphosphate receptor; P: Phosphorylation; PIP: Phosphatidylinositol phosphate; PIP2: Phosphatidylinositol bisphosphate; PIP3: Phosphatidylinositol trisphosphate; PIP5K1α: Phosphatidylinositol 4 phosphate 5 kinase 1α.
Calcium receptor
The acute response of the keratinocyte to calcium resembles that of the parathyroid cell [120], which senses Cao via a seven-transmembrane domain, the GTP-binding protein-coupled CaR [121,122]. This receptor was originally discovered in the parathyroid gland, but is found in a number of other tissues, including the keratinocyte [41,123–126]. The human CaR is composed of 1078 amino acids with a number of glycosylation sites that appear to be important for function. Moreover, the keratinocyte and a number of other tissues (bone, cartilage and kidney) produce an alternatively spliced variant of the CaR (CaRalt) as they differentiate; this variant lacks exon 5 and so would be missing residues 461–537 in the extracellular domain [123,124]. At least in the skin, CaRalt appears to have little function and may even act as a dominant negative. The original global knockout of CaR was produced using a neomycin cassette inserted in exon 5, so that this mouse continued to express (and actually increased the expression of) CaRalt. Nevertheless, these mice died within a few weeks of severe hyperparathyroidism and hypercalcemia. The authors have recently developed a mouse model in which exon 7, which codes the transmembrane domain and cytoplasmic tail of CaR, is floxed, allowing it to be deleted in the tissue of our choice [127]. By deleting CaR only in keratinocytes, the authors have avoided the systemic problems of hyperparathyroidism and hypercalcemia in the global CaR null model. These mice demonstrated a reduction in differentiation markers and permeability barrier function in the epidermis, defects more pronounced in mice ingesting a low calcium diet [128]. Turksen and Troy, however, overexpressed the CaR in keratinocytes and demonstrated accelerated development of the barrier during embryologic development [129].
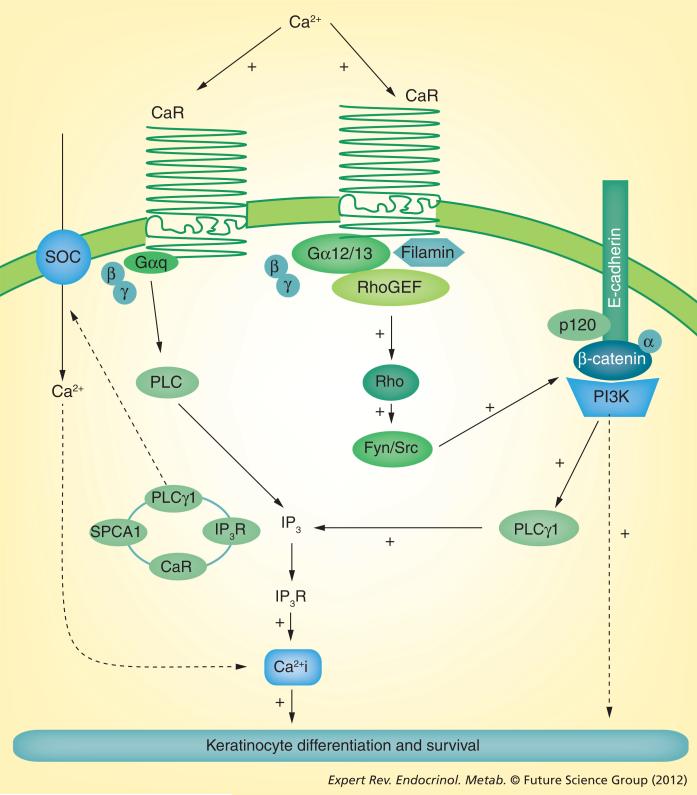
Blocking the expression of CaR with an antisense construct in keratinocytes decreases the ability of calcium to raise Cai and induce the involucrin and transglutaminase-I genes [115,125]. There are several mechanisms by which CaR mediates calcium-induced increases in Cai, thereby promoting differentiation. CaR in the plasma membrane responds to Cao with activation of PLC, which in turn hydrolyzes PIP2 to IP3 and DAG as discussed earlier. IP3 then releases calcium from intracellular stores, but probably also stimulates calcium influx. The activation of PLC occurs by at least two mechanisms (Figure 3). CaR is a G-protein-coupled receptor that directly activates PLCs, most likely through Gq. This mechanism would activate both PLC-β and -γ. As noted earlier, this is the mechanism favored for PLC-β activation and is not blocked by inhibition of PLC-γ1 activity or expression. This mechanism has received little study in keratinocytes, and its role in Cao-induced differentiation is unknown. However, the failure of ATP, which increases Cai transiently presumably by this mechanism, does not stimulate differentiation. PLC-γ1 activation following the calcium switch requires the E-cadherin–catenin complex as described previously. Blocking CaR expression prevents the calcium-induced formation and stabilization of the E-cadherin–catenin complex and the subsequent activation of PLC-γ1 [115]. The mechanism is as follows: Cao induces the formation of a complex containing CaR, filamin A and RhoA with the E-cadherin complex [113]. RhoA is activated in the process, in turn activating the Src family kinases Fyn and Src, leading to tyrosine phosphorylation of the catenins and their binding to E-cadherin. This enables recruitment of PIP5K1α and PI3K, and activation of PLC-γ1 via PIP3 as described earlier. Filamin A is a scaffolding protein that links CaR with RhoA. Interference with the binding of CaR to filamin A blocks calcium-stimulated RhoA activation and formation of the E-cadherin–catenin complex, with subsequent inhibition of keratinocyte differentiation [113]. Blocking RhoA activity did not prevent the initial rise in Cai following the calcium switch, unlike deletion of CaR, although it did prevent the prolonged increase in Cai. These results are consistent with the hypothesis that the mechanism for the sustained increase in Cai necessary for differentiation requires PLC-γ1 activation via the E-cadherin–catenin complex.
Figure 3. The role of the calcium receptor in calcium-induced differentiation.
Calcium stimulates intracellular signaling (inside–out signaling) through its binding to CaR. In one pathway, CaR activates the G protein Gαq, leading to PLC activation and generation of IP3 with the release of calcium from intracellular stores. This is the predominant mechanism for the initial spike in Ca2+i concentration following the calcium switch. CaR also activates Rho that, in turn, activates the src kinases Src and Fyn to tyrosine phosphorylate the catenins, enabling their binding to the E-cadherin–catenin complex. As noted in the legend of Figure 2, this results in PLC-γ1 activation, leading to a sustained increase in Ca2+ i concentration both by release of calcium from intracellular stores and by stimulating the influx of calcium through store-operated channels. The latter role may be played by PLC-γ1 as part of the complex in Golgi with the Golgi calcium pump SPCA1, CaR and the IP3 receptor.
Ca2+i: Intracellular calcium; CaR: Calcium receptor; IP3: Inositol trisphosphate; IP3R: Inositol trisphosphate receptor; PLC: Phospholipase C; SOC: Store-operated channel.
However, most of the CaR is located intracellularly in the ER and trans-Golgi, the major sources of Cai [130]. In the ER, the CaR is primarily unglycosylated, unlike the forms in the trans-Golgi and plasma membrane [130]. In the trans-Golgi, the CaR forms a complex with PLC-γ1, the IP3 receptor and the Golgi calcium pump (SPCA1, the gene product of ATP2C1) [130]. Both the ER and Golgi fractions are capable of ATP-stimulated calcium uptake and IP3-stimulated calcium release [130]. Blocking CaR expression, through stimulating the production of both the endoplasmic reticulum (ER) and Golgi calcium pumps (SERCA2 and SPCA1, respectively), reduces the intracellular stores and limits the amount of releasable calcium following the addition of Cao, ionomycin or thapsigargin [130]. The importance of Cai stores in regulating the differentiation process is illustrated by diseases such as Dariers disease and Hailey–Hailey disease that likewise have reduced levels of calcium in their ER and Golgi, respectively, and impaired calcium-stimulated differentiation due to mutations in their respective calcium pumps SERCA2 and SPCA1 [131,132]. One explanation for the reduction in Cai stores is that PLC-γ1 is required for the activation of store-operated channels (in particular TRPC-1 and -4) in keratinocytes as previously noted [50]. TRPC1 forms a complex with PLC-γ1 and the IP3 receptor [50], and its knockdown impairs Cao-induced increases in Cai and the expression of differentiation markers [50,51]. The CaR is required for calcium activation of PLC-γ1 and, as such, would be expected to be required for calcium influx through the TRPC channels, in addition to its presumed role in causing IP3-mediated release of calcium from intracellular stores.
Summary
Cao regulation of keratinocyte differentiation is well established in vivo and in vitro. Calcium signals by both outside–in and inside–out mechanisms. Central to both mechanisms is the CaR. Although Cao is necessary to facilitate the intercellular adhesion mediated by the extracellular domains of the proteins forming the desmosomes, adherens junctions and tight junctions, it is the intracellular signaling that results in stability of these complexes and promotes the differentiation process. CaR, through RhoA and Src family kinases, enables the formation of a stable E-cadherin–catenin complex in the membrane that recruits two important enzymes, PI3K and PIP5K1α, to the membrane where they maintain levels of PIP3 and PIP2 that respectively activate and serve as substrates for PLC-γ1. When cleaved by PLC-γ1, PIP2 produces two important signaling molecules, IP3 and DAG. These in turn stimulate the release of calcium from intracellular stores and activate PKC. PLC-γ1 is also required for the activation of store-operated channels by which Cai stores are replenished. The prolonged rise in Cai coupled with PKC-regulated transcription factors in the Fos/Jun families regulate transcription of the genes required for keratinocyte differentiation. In addition, the CaR forms a complex with the IP3 receptor and the Golgi calcium pump, possibly to regulate the uptake and release of calcium from these intracellular stores. With the development of the keratinocyte-specific CaR-null mouse, one can expect the rapid progress in understanding calcium-regulated keratinocyte differentiation to continue.
Expert commentary
Calcium is a major regulator of keratinocyte differentiation. The CaR is central to the mechanism by which calcium affects this role. However, most of the information comes from in vitro studies. Although a calcium gradient exists in the epidermis, its role in vivo in the differentiation process is less clear. The development of the conditional CaR-null mouse that enables specific deletion of the CaR in keratinocytes will help answer the degree to which the effects of calcium in vitro can be translated to the in vivo situation. In addition, very few studies are available on the role of calcium in hair follicle cycling. Whether calcium affects this process to the degree and by the same mechanisms that it regulates epidermal differentiation remains to be determined.
Five-year view
Separating the inside–out and outside–in mechanisms by which calcium regulates keratinocyte differentiation should play an increasingly important role in future research in this area. The role of the desmosomes, adherens junctions and tight junctions not only as the means of attaching cells and/or regulating paracellular transport of fluids and ions but also as intracellular signaling complexes will become better appreciated and studied. Currently, the E-cadherin–catenin complex has received the most study in this regard, but it is likely that the desmosomes and tight junctions play similar roles. In addition, the mechanisms by which the CaR regulates not only events at the plasma membrane but also the handling of calcium by the ER and Golgi have received little attention and remain wide open for future investigation.
Key issues.
Keratinocytes cultured in low calcium concentrations remain proliferative; when switched to high calcium concentrations (the calcium switch), the cells begin to differentiate and form intercellular contacts important for the differentiation process.
Calcium initiates the differentiation by outside–in and inside–out mechanisms. Specifically, calcium enables the formation of desmosomes, adherens junctions and tight junctions that bind cells together in the presence of calcium, and stimulates the calcium receptor (CaR) to initiate intracellular mechanisms required for differentiation.
The E-cadherin–catenin complex, the major component of adherens junctions, exemplifies the role that the intercellular contacts play, as it helps bind cells together while also serving as a scaffold for intracellular processes required for differentiation.
The sustained increase in intracellular calcium (Cai) following the calcium switch is required for differentiation; transient increases in Cai do not suffice.
When activated by calcium, the CaR increases Cai by stimulating phospholipase C (PLC) activity leading to hydrolysis of phosphatidylinositol bisphosphate to form two second messengers, inositol trisphosphate (IP3) and diacylglycerol, important for keratinocyte differentiation. IP3 stimulates the release of calcium from intracellular stores, and diacylglycerol stimulates PKC.
Two PLCs play different roles in the response of the keratinocyte to calcium. PLC-β is primarily responsible for the initial spike in Cai, whereas PLC-γ1 is primarily responsible for the sustained increase in Cai both by increasing IP3 levels to release Cai and by increasing calcium influx through store-operated channels.
PLC-β is activated by Gq as a direct result of CaR binding to calcium, whereas PLC-γ1 is activated via the CaR-mediated formation of the E-cadherin–catenin complex that brings together the enzymes required for phosphatidylinositol triphosphate production, the direct activator of PLC-γ1 following the calcium switch.
Acknowledgements
The authors acknowledge the administrative support of Teresa Tong and the efforts of a number of previous postdoctoral fellows in the laboratory who contributed to this work, including Kumar Pillai, Maria-Laura Mancianti, Mei-Ji Su, Anita Ratnam, David Gibson, Dean Ng, J-K Cho and L-C Yang, and key collaborators including Peter Elias, Kenneth Feingold, Theodora Mauro, Wenhan Chang, Lazlo Komuves and Yuko Oda.
DD Bikle is supported by grants VA Merit Review, NIH RO1 AR050023 and DOD CA110338.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J. Invest. Dermatol. 2007;127(11):2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- 2.Yuki T, Haratake A, Koishikawa H, Morita K, Miyachi Y, Inoue S. Tight junction proteins in keratinocytes: localization and contribution to barrier function. Exp. Dermatol. 2007;16(4):324–330. doi: 10.1111/j.1600-0625.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 3.Marchisio PC, Bondanza S, Cremona O, Cancedda R, De Luca M. Polarized expression of integrin receptors (α 6 β 4, α 2 β 1, α 3 β 1, and α v β 5) and their relationship with the cytoskeleton and basement membrane matrix in cultured human keratinocytes. J. Cell Biol. 1991;112(4):761–773. doi: 10.1083/jcb.112.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peltonen J, Larjava H, Jaakkola S, et al. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J. Clin. Invest. 1989;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo M, Kim LT, Akiyama SK, Gralnick HR, Yamada KM, Grinnell F. Altered processing of integrin receptors during keratinocyte activation. Exp. Cell Res. 1991;195(2):315–322. doi: 10.1016/0014-4827(91)90379-9. [DOI] [PubMed] [Google Scholar]
- 6.Müller EJ, Williamson L, Kolly C, Suter MM. Outside–in signaling through integrins and cadherins: a central mechanism to control epidermal growth and differentiation? J. Invest. Dermatol. 2008;128(3):501–516. doi: 10.1038/sj.jid.5701248. [DOI] [PubMed] [Google Scholar]
- 7.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 8.Eichner R, Sun TT, Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J. Cell Biol. 1986;102(5):1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warhol MJ, Roth J, Lucocq JM, Pinkus GS, Rice RH. Immuno-ultrastructural localization of involucrin in squamous epithelium and cultured keratinocytes. J. Histochem. Cytochem. 1985;33(2):141–149. doi: 10.1177/33.2.2578499. [DOI] [PubMed] [Google Scholar]
- 10.Thacher SM, Rice RH. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985;40(3):685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- 11.Steven AC, Bisher ME, Roop DR, Steinert PM. Biosynthetic pathways of filaggrin and loricrin - two major proteins expressed by terminally differentiated epidermal keratinocytes. J. Struct. Biol. 1990;104(1–3):150–162. doi: 10.1016/1047-8477(90)90071-j. [DOI] [PubMed] [Google Scholar]
- 12.Dale BA, Resing KA, Lonsdale-Eccles JD. Filaggrin: a keratin filament associated protein. Ann. NY Acad. Sci. 1985;455:330–342. doi: 10.1111/j.1749-6632.1985.tb50420.x. [DOI] [PubMed] [Google Scholar]
- 13.Mehrel T, Hohl D, Rothnagel JA, et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61(6):1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 14.Elias PM, Menon GK, Grayson S, Brown BE. Membrane structural alterations in murine stratum corneum: relationship to the localization of polar lipids and phospholipases. J. Invest. Dermatol. 1988;91(1):3–10. doi: 10.1111/1523-1747.ep12463279. [DOI] [PubMed] [Google Scholar]
- 15.Hohl D. Cornified cell envelope. Dermatologica. 1990;180(4):201–211. doi: 10.1159/000248031. [DOI] [PubMed] [Google Scholar]
- 16.Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J. Invest. Dermatol. 1985;84(6):508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- 17.Celli A, Sanchez S, Behne M, Hazlett T, Gratton E, Mauro T. The epidermal Ca(2+) gradient: measurement using the phasor representation of fluorescent lifetime imaging. Biophys. J. 2010;98(5):911–921. doi: 10.1016/j.bpj.2009.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leinonen PT, Hägg PM, Peltonen S, et al. Reevaluation of the normal epidermal calcium gradient, and analysis of calcium levels and ATP receptors in Hailey–Hailey and Darier epidermis. J. Invest. Dermatol. 2009;129(6):1379–1387. doi: 10.1038/jid.2008.381. [DOI] [PubMed] [Google Scholar]
- 19.Dhitavat J, Cobbold C, Leslie N, Burge S, Hovnanian A. Impaired trafficking of the desmoplakins in cultured Darier's disease keratinocytes. J. Invest. Dermatol. 2003;121(6):1349–1355. doi: 10.1046/j.1523-1747.2003.12557.x. [DOI] [PubMed] [Google Scholar]
- 20.Mauro T, Bench G, Sidderas-Haddad E, Feingold K, Elias P, Cullander C. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. J. Invest. Dermatol. 1998;111(6):1198–1201. doi: 10.1046/j.1523-1747.1998.00421.x. [DOI] [PubMed] [Google Scholar]
- 21.Elias PM, Ahn SK, Denda M, et al. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J. Invest. Dermatol. 2002;119(5):1128–1136. doi: 10.1046/j.1523-1747.2002.19512.x. [DOI] [PubMed] [Google Scholar]
- 22.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 23.Hennings H, Holbrook KA. Calcium regulation of cell–cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp. Cell Res. 1983;143(1):127–142. doi: 10.1016/0014-4827(83)90115-5. [DOI] [PubMed] [Google Scholar]
- 24.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J. Invest. Dermatol. 2007;127(11):2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 25.Zamansky GB, Nguyen U, Chou IN. An immunofluorescence study of the calcium-induced coordinated reorganization of microfilaments, keratin intermediate filaments, and microtubules in cultured human epidermal keratinocytes. J. Invest. Dermatol. 1991;97(6):985–994. doi: 10.1111/1523-1747.ep12491899. [DOI] [PubMed] [Google Scholar]
- 26.Inohara S, Tatsumi Y, Cho H, Tanaka Y, Sagami S. Actin filament and desmosome formation in cultured human keratinocytes. Arch. Dermatol. Res. 1990;282(3):210–212. doi: 10.1007/BF00372627. [DOI] [PubMed] [Google Scholar]
- 27.Yoneda K, Fujimoto T, Imanura S, et al. Fodrin is localized in the cytoplasm of keratinocytes cultured in low calcium medium: immunoelectron microscopic study. Acta Histochem. Cytochem. 1990;23:139–148. [Google Scholar]
- 28.Pillai S, Bikle DD, Mancianti ML, Cline P, Hincenbergs M. Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J. Cell. Physiol. 1990;143(2):294–302. doi: 10.1002/jcp.1041430213. [DOI] [PubMed] [Google Scholar]
- 29.Rubin AL, Parenteau NL, Rice RH. Coordination of keratinocyte programming in human SCC-13 squamous carcinoma and normal epidermal cells. J. Cell. Physiol. 1989;138(1):208–214. doi: 10.1002/jcp.1041380128. [DOI] [PubMed] [Google Scholar]
- 30.Su MJ, Bikle DD, Mancianti ML, Pillai S. 1,25-dihydroxyvitamin D3 potentiates the keratinocyte response to calcium. J. Biol. Chem. 1994;269(20):14723–14729. [PubMed] [Google Scholar]
- 31.Hohl D, Lichti U, Breitkreutz D, Steinert PM, Roop DR. Transcription of the human loricrin gene in vitro is induced by calcium and cell density and suppressed by retinoic acid. J. Invest. Dermatol. 1991;96(4):414–418. doi: 10.1111/1523-1747.ep12469779. [DOI] [PubMed] [Google Scholar]
- 32.Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J. Cell Biol. 1989;109(3):1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng DC, Su MJ, Kim R, Bikle DD. Regulation of involucrin gene expression by calcium in normal human keratinocytes. Front. Biosci. 1996;1:a16–a24. doi: 10.2741/a101. [DOI] [PubMed] [Google Scholar]
- 34.Huff CA, Yuspa SH, Rosenthal D. Identification of control elements 3′ to the human keratin 1 gene that regulate cell type and differentiation-specific expression. J. Biol. Chem. 1993;268(1):377–384. [PubMed] [Google Scholar]
- 35.Denning MF, Dlugosz AA, Williams EK, Szallasi Z, Blumberg PM, Yuspa SH. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995;6(2):149–157. [PubMed] [Google Scholar]
- 36.Deucher A, Efimova T, Eckert RL. Calcium-dependent involucrin expression is inversely regulated by protein kinase C (PKC)α and PKCdelta. J. Biol. Chem. 2002;277(19):17032–17040. doi: 10.1074/jbc.M109076200. [DOI] [PubMed] [Google Scholar]
- 37.Ryynänen J, Jaakkola S, Engvall E, Peltonen J, Uitto J. Expression of β 4 integrins in human skin: comparison of epidermal distribution with β 1-integrin epitopes, and modulation by calcium and vitamin D3 in cultured keratinocytes. J. Invest. Dermatol. 1991;97(3):562–567. doi: 10.1111/1523-1747.ep12481896. [DOI] [PubMed] [Google Scholar]
- 38.Pillai S, Bikle DD. Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J. Cell. Physiol. 1991;146(1):94–100. doi: 10.1002/jcp.1041460113. [DOI] [PubMed] [Google Scholar]
- 39.Hennings H, Kruszewski FH, Yuspa SH, Tucker RW. Intracellular calcium alterations in response to increased external calcium in normal and neoplastic keratinocytes. Carcinogenesis. 1989;10(4):777–780. doi: 10.1093/carcin/10.4.777. [DOI] [PubMed] [Google Scholar]
- 40.Kruszewski FH, Hennings H, Tucker RW, Yuspa SH. Differences in the regulation of intracellular calcium in normal and neoplastic keratinocytes are not caused by RAS gene mutations. Cancer Res. 1991;51(16):4206–4212. [PubMed] [Google Scholar]
- 41.Bikle DD, Ratnam A, Mauro T, Harris J, Pillai S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of the calcium receptor. J. Clin. Invest. 1996;97(4):1085–1093. doi: 10.1172/JCI118501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillai S, Bikle DD. Lanthanum influx into cultured human keratinocytes: effect on calcium flux and terminal differentiation. J. Cell. Physiol. 1992;151(3):623–629. doi: 10.1002/jcp.1041510323. [DOI] [PubMed] [Google Scholar]
- 43.Reiss M, Lipsey LR, Zhou ZL. Extracellular calcium-dependent regulation of transmembrane calcium fluxes in murine keratinocytes. J. Cell. Physiol. 1991;147(2):281–291. doi: 10.1002/jcp.1041470213. [DOI] [PubMed] [Google Scholar]
- 44.Galietta LJ, Barone V, De Luca M, Romeo G. Characterization of chloride and cation channels in cultured human keratinocytes. Pflugers Arch. 1991;418(1–2):18–25. doi: 10.1007/BF00370447. [DOI] [PubMed] [Google Scholar]
- 45.Mauro TM, Pappone PA, Isseroff RR. Extracellular calcium affects the membrane currents of cultured human keratinocytes. J. Cell. Physiol. 1990;143(1):13–20. doi: 10.1002/jcp.1041430103. [DOI] [PubMed] [Google Scholar]
- 46.Mauro TM, Isseroff RR, Lasarow R, Pappone PA. Ion channels are linked to differentiation in keratinocytes. J. Membr. Biol. 1993;132(3):201–209. doi: 10.1007/BF00235738. [DOI] [PubMed] [Google Scholar]
- 47.Grando SA, Horton RM, Mauro TM, Kist DA, Lee TX, Dahl MV. Activation of keratinocyte nicotinic cholinergic receptors stimulates calcium influx and enhances cell differentiation. J. Invest. Dermatol. 1996;107(3):412–418. doi: 10.1111/1523-1747.ep12363399. [DOI] [PubMed] [Google Scholar]
- 48.Oda Y, Timpe LC, McKenzie RC, Sauder DN, Largman C, Mauro T. Alternatively spliced forms of the cGMP-gated channel in human keratinocytes. FEBS Lett. 1997;414(1):140–145. doi: 10.1016/s0014-5793(97)00927-7. [DOI] [PubMed] [Google Scholar]
- 49.Müller M, Essin K, Hill K, et al. Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation. J. Biol. Chem. 2008;283(49):33942–33954. doi: 10.1074/jbc.M801844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tu CL, Chang W, Bikle DD. Phospholipase Cγ1 is required for activation of store-operated channels in human keratinocytes. J. Invest. Dermatol. 2005;124(1):187–197. doi: 10.1111/j.0022-202X.2004.23544.x. [DOI] [PubMed] [Google Scholar]
- 51.Cai S, Fatherazi S, Presland RB, et al. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflugers Arch. 2006;452(1):43–52. doi: 10.1007/s00424-005-0001-1. [DOI] [PubMed] [Google Scholar]
- 52.Pillai S, Bikle DD, Mancianti ML, Hincenbergs M. Uncoupling of the calcium-sensing mechanism and differentiation in squamous carcinoma cell lines. Exp. Cell Res. 1991;192(2):567–573. doi: 10.1016/0014-4827(91)90077-8. [DOI] [PubMed] [Google Scholar]
- 53.Holladay K, Fujiki H, Bowden GT. Okadaic acid induces the expression of both early and secondary response genes in mouse keratinocytes. Mol. Carcinog. 1992;5(1):16–24. doi: 10.1002/mc.2940050106. [DOI] [PubMed] [Google Scholar]
- 54.Bollag WB, Xiong Y, Ducote J, Harmon CS. Regulation of FOS-LACZ fusion gene expression in primary mouse epidermal keratinocytes isolated from transgenic mice. Biochem. J. 1994;300(Pt 1):263–270. doi: 10.1042/bj3000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pillai S, Bikle DD. Adenosine triphosphate stimulates phosphoinositide metabolism, mobilizes intracellular calcium, and inhibits terminal differentiation of human epidermal keratinocytes. J. Clin. Invest. 1992;90(1):42–51. doi: 10.1172/JCI115854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaken S, Yuspa SH. Early signals for keratinocyte differentiation: role of Ca 2+-mediated inositol lipid metabolism in normal and neoplastic epidermal cells. Carcinogenesis. 1988;9(6):1033–1038. doi: 10.1093/carcin/9.6.1033. [DOI] [PubMed] [Google Scholar]
- 57.Tang W, Ziboh VA, Isseroff R, Martinez D. Turnover of inositol phospholipids in cultured murine keratinocytes: possible involvement of inositol triphosphate in cellular differentiation. J. Invest. Dermatol. 1988;90(1):37–43. doi: 10.1111/1523-1747.ep12462536. [DOI] [PubMed] [Google Scholar]
- 58.Moscat J, Fleming TP, Molloy CJ, Lopez-Barahona M, Aaronson SA. The calcium signal for Balb/MK keratinocyte terminal differentiation induces sustained alterations in phosphoinositide metabolism without detectable protein kinase C activation. J. Biol. Chem. 1989;264(19):11228–11235. [PubMed] [Google Scholar]
- 59.Lee E, Yuspa SH. Aluminum fluoride stimulates inositol phosphate metabolism and inhibits expression of differentiation markers in mouse keratinocytes. J. Cell. Physiol. 1991;148(1):106–115. doi: 10.1002/jcp.1041480113. [DOI] [PubMed] [Google Scholar]
- 60.Pillai S, Bikle DD, Su MJ, Ratnam A, Abe J. 1,25-dihydroxyvitamin D3 upregulates the phosphatidylinositol signaling pathway in human keratinocytes by increasing phospholipase C levels. J. Clin. Invest. 1995;96(1):602–609. doi: 10.1172/JCI118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Z, Bikle DD. Phospholipase C-γ1 is required for calcium-induced keratinocyte differentiation. J. Biol. Chem. 1999;274(29):20421–20424. doi: 10.1074/jbc.274.29.20421. [DOI] [PubMed] [Google Scholar]
- 62.Carpenter G, Ji Q. Phospholipase C-γ as a signal-transducing element. Exp. Cell Res. 1999;253(1):15–24. doi: 10.1006/excr.1999.4671. [DOI] [PubMed] [Google Scholar]
- 63.Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase C γ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17(2):414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phospholipase Cγ-mediated calcium signaling. J. Biol. Chem. 1998;273(37):23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- 65.Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273(8):4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 66.Kim HK, Kim JW, Zilberstein A, et al. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-γ 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65(3):435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 67.Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires Src- and Fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-γ1. Mol. Biol. Cell. 2005;16(7):3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie Z, Chen Y, Lias E-Y, Jian Y, Liu F-Y, Pennypacker SD. Phospholipase C-γ1 is required for the epidermal growth factor receptor-induced squamous cell carcinoma cell mitogenesis. Biochem. Biophy. Res. Comm. 2010;397:296–300. doi: 10.1016/j.bbrc.2010.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawley-Nelson P, Stanley JR, Schmidt J, Gullino M, Yuspa SH. The tumor promoter, 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp. Cell Res. 1982;137(1):155–167. doi: 10.1016/0014-4827(82)90017-9. [DOI] [PubMed] [Google Scholar]
- 70.Yuspa SH, Ben T, Hennings H, Lichti U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982;42(6):2344–2349. [PubMed] [Google Scholar]
- 71.Kitajima Y, Inoue S, Nagao S, Nagata K, Yaoita H, Nozawa Y. Biphasic effects of 12-O-tetradecanoylphorbol-13-acetate on the cell morphology of low calcium-grown human epidermal carcinoma cells: involvement of translocation and down regulation of protein kinase C. Cancer Res. 1988;48(4):964–970. [PubMed] [Google Scholar]
- 72.Rice RH, Rong XH, Chakravarty R. Suppression of keratinocyte differentiation in SSC-9 human squamous carcinoma cells by benzo[a]pyrene, 12-O-tetradecanoylphorbol-13-acetate and hydroxyurea. Carcinogenesis. 1988;9(10):1885–1890. doi: 10.1093/carcin/9.10.1885. [DOI] [PubMed] [Google Scholar]
- 73.Yuspa SH, Ben T, Hennings H. The induction of epidermal transglutaminase and terminal differentiation by tumor promoters in cultured epidermal cells. Carcinogenesis. 1983;4(11):1413–1418. doi: 10.1093/carcin/4.11.1413. [DOI] [PubMed] [Google Scholar]
- 74.Dlugosz AA, Yuspa SH. Protein kinase C regulates keratinocyte transglutaminase (TGK) gene expression in cultured primary mouse epidermal keratinocytes induced to terminally differentiate by calcium. J. Invest. Dermatol. 1994;102(4):409–414. doi: 10.1111/1523-1747.ep12372171. [DOI] [PubMed] [Google Scholar]
- 75.Matsui MS, Illarda I, Wang N, DeLeo VA. Protein kinase C agonist and antagonist effects in normal human epidermal keratinocytes. Exp. Dermatol. 1993;2(6):247–256. doi: 10.1111/j.1600-0625.1993.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 76.Dlugosz AA, Yuspa SH. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J. Cell Biol. 1993;120(1):217–225. doi: 10.1083/jcb.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ng DC, Shafaee S, Lee D, Bikle DD. Requirement of an AP-1 site in the calcium response region of the involucrin promoter. J. Biol. Chem. 2000;275(31):24080–24088. doi: 10.1074/jbc.M002508200. [DOI] [PubMed] [Google Scholar]
- 78.Filvaroff E, Stern DF, Dotto GP. Tyrosine phosphorylation is an early and specific event involved in primary keratinocyte differentiation. Mol. Cell. Biol. 1990;10(3):1164–1173. doi: 10.1128/mcb.10.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chakravarthy BR, Isaacs RJ, Morley P, Durkin JP, Whitfield JF. Stimulation of protein kinase C during Ca(2+)-induced keratinocyte differentiation. Selective blockade of MARCKS phosphorylation by calmodulin. J. Biol. Chem. 1995;270(3):1362–1368. doi: 10.1074/jbc.270.3.1362. [DOI] [PubMed] [Google Scholar]
- 80.Punnonen K, Denning M, Lee E, Li L, Rhee SG, Yuspa SH. Keratinocyte differentiation is associated with changes in the expression and regulation of phospholipase C isoenzymes. J. Invest. Dermatol. 1993;101(5):719–726. doi: 10.1111/1523-1747.ep12371682. [DOI] [PubMed] [Google Scholar]
- 81.Hepler JR, Earp HS, Harden TK. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation. Evidence for a role of protein kinase C in agonist-induced desensitization. J. Biol. Chem. 1988;263(16):7610–7619. [PubMed] [Google Scholar]
- 82.Kojima I, Shibata H, Ogata E. Phorbol ester inhibits angiotensin-induced activation of phospholipase C in adrenal glomerulosa cells. Its implication in the sustained action of angiotensin. Biochem. J. 1986;237(1):253–258. doi: 10.1042/bj2370253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dlugosz AA, Mischak H, Mushinski JF, Yuspa SH. Transcripts encoding protein kinase C-α, Δ, ε, ζ, and η are expressed in basal and differentiating mouse keratinocytes in vitro and exhibit quantitative changes in neoplastic cells Mol. Carcinog. 1992;5(4):286–292. doi: 10.1002/mc.2940050409. [DOI] [PubMed] [Google Scholar]
- 84.Denning MF, Dlugosz AA, Howett MK, Yuspa SH. Expression of an oncogenic RASHA gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C delta. J. Biol. Chem. 1993;268(35):26079–26081. [PubMed] [Google Scholar]
- 85.Reynolds NJ, Baldassare JJ, Henderson PA, et al. Translocation and downregulation of protein kinase C isoenzymes-α and -epsilon by phorbol ester and bryostatin-1 in human keratinocytes and fibroblasts. J. Invest. Dermatol. 1994;103(3):364–369. doi: 10.1111/1523-1747.ep12394957. [DOI] [PubMed] [Google Scholar]
- 86.Fisher GJ, Tavakkol A, Leach K, et al. Differential expression of protein kinase C isoenzymes in normal and psoriatic adult human skin: reduced expression of protein kinase C-β II in psoriasis. J. Invest. Dermatol. 1993;101(4):553–559. doi: 10.1111/1523-1747.ep12365967. [DOI] [PubMed] [Google Scholar]
- 87.Goodnight J, Mischak H, Mushinski JF. Selective involvement of protein kinase C isozymes in differentiation and neoplastic transformation. Adv. Cancer Res. 1994;64:159–209. doi: 10.1016/s0065-230x(08)60838-6. [DOI] [PubMed] [Google Scholar]
- 88.Yang LC, Ng DC, Bikle DD. Role of protein kinase C α in calcium induced keratinocyte differentiation: defective regulation in squamous cell carcinoma. J. Cell. Physiol. 2003;195(2):249–259. doi: 10.1002/jcp.10248. [DOI] [PubMed] [Google Scholar]
- 89.Downward J, Waterfield MD, Parker PJ. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J. Biol. Chem. 1985;260(27):14538–14546. [PubMed] [Google Scholar]
- 90.Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J. Cell Biol. 1986;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kolch W, Heidecker G, Kochs G, et al. Protein kinase C α activates RAF-1 by direct phosphorylation. Nature. 1993;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 92.Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3 β by protein kinase C isotypes. J. Biol. Chem. 1992;267(24):16878–16882. [PubMed] [Google Scholar]
- 93.Yaar M, Gilani A, DiBenedetto PJ, Harkness DD, Gilchrest BA. Gene modulation accompanying differentiation of normal versus malignant keratinocytes. Exp. Cell Res. 1993;206(2):235–243. doi: 10.1006/excr.1993.1143. [DOI] [PubMed] [Google Scholar]
- 94.Stanwell C, Denning MF, Rutberg SE, Cheng C, Yuspa SH, Dlugosz AA. Staurosporine induces a sequential program of mouse keratinocyte terminal differentiation through activation of PKC isozymes. J. Invest. Dermatol. 1996;106(3):482–489. doi: 10.1111/1523-1747.ep12343690. [DOI] [PubMed] [Google Scholar]
- 95.Welter JF, Eckert RL. Differential expression of the Fos and Jun family members c-Fos, FosB, Fra-1, Fra-2, c-Jun, JunB and JunD during human epidermal keratinocyte differentiation. Oncogene. 1995;11(12):2681–2687. [PubMed] [Google Scholar]
- 96.Welter JF, Crish JF, Agarwal C, Eckert RL. Fos-related antigen (Fra-1), JunB, and JunD activate human involucrin promoter transcription by binding to proximal and distal AP1 sites to mediate phorbol ester effects on promoter activity. J. Biol. Chem. 1995;270(21):12614–12622. doi: 10.1074/jbc.270.21.12614. [DOI] [PubMed] [Google Scholar]
- 97.Forman BM, Casanova J, Raaka BM, Ghysdael J, Samuels HH. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol. Endocrinol. 1992;6(3):429–442. doi: 10.1210/mend.6.3.1316541. [DOI] [PubMed] [Google Scholar]
- 98.Troyanovsky S. Cadherin dimers in cell–cell adhesion. Eur. J. Cell Biol. 2005;84(2–3):225–233. doi: 10.1016/j.ejcb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 99.van Roy F, Berx G. The cell–cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008;65(23):3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Paolo Dotto G. Tyrosine phosphorylation and Src family kinases control keratinocyte cell–cell adhesion. J. Cell Biol. 1998;141(6):1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calautti E, Grossi M, Mammucari C, et al. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell–cell adhesion. J. Cell Biol. 2002;156(1):137–148. doi: 10.1083/jcb.200105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J. Biol. Chem. 2005;280(38):32856–32865. doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- 103.Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell–cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J. Biol. Chem. 1999;274(27):19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- 104.Pang JH, Kraemer A, Stehbens SJ, Frame MC, Yap AS. Recruitment of phosphoinositide 3-kinase defines a positive contribution of tyrosine kinase signaling to E-cadherin function. J. Biol. Chem. 2005;280(4):3043–3050. doi: 10.1074/jbc.M412148200. [DOI] [PubMed] [Google Scholar]
- 105.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin–catenin complex at the plasma membrane is required for calcium-induced phospholipase C-γ1 activation and human keratinocyte differentiation. J. Biol. Chem. 2007;282(12):8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 106.Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD. Phosphatidylinositol-4-phosphate 5-kinase 1α mediates extracellular calcium-induced keratinocyte differentiation. Mol. Biol. Cell. 2009;20(6):1695–1704. doi: 10.1091/mbc.E08-07-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112(4):535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 108.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J. Cell Biol. 1997;137(6):1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Furukawa F, Fujii K, Horiguchi Y, et al. Roles of E- and P-cadherin in the human skin. Microsc. Res. Tech. 1997;38(4):343–352. doi: 10.1002/(SICI)1097-0029(19970815)38:4<343::AID-JEMT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 110.Young P, Boussadia O, Halfter H, et al. E-cadherin controls adherens junctions in the epidermis and the renewal of hair follicles. EMBO J. 2003;22(21):5723–5733. doi: 10.1093/emboj/cdg560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tinkle CL, Lechler T, Pasolli HA, Fuchs E. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc. Natl Acad. Sci. USA. 2004;101(2):552–557. doi: 10.1073/pnas.0307437100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tunggal JA, Helfrich I, Schmitz A, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24(6):1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tu CL, Chang W, Bikle DD. The calcium-sensing receptor-dependent regulation of cell–cell adhesion and keratinocyte differentiation requires Rho and filamin A. J. Invest. Dermatol. 2011;131(5):1119–1128. doi: 10.1038/jid.2010.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell–cell contacts. Annu. Rev. Cell Dev. Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 115.Tu CL, Chang W, Xie Z, Bikle DD. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell–cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J. Biol. Chem. 2008;283(6):3519–3528. doi: 10.1074/jbc.M708318200. [DOI] [PubMed] [Google Scholar]
- 116.Anastasiadis PZ. p120-ctn: a nexus for contextual signaling via Rho GTPases. Biochim. Biophys. Acta. 2007;1773(1):34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 117.Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124(3):631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 119.Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Res. 2001;3(5):304–312. doi: 10.1186/bcr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nemeth EF, Scarpa A. Rapid mobilization of cellular Ca 2+ in bovine parathyroid cells evoked by extracellular divalent cations. Evidence for a cell surface calcium receptor. J. Biol. Chem. 1987;262(11):5188–5196. [PubMed] [Google Scholar]
- 121.Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 122.Garrett JE, Capuano IV, Hammerland LG, et al. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J. Biol. Chem. 1995;270(21):12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- 123.Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J. Biol. Chem. 1998;273(36):23344–23352. doi: 10.1074/jbc.273.36.23344. [DOI] [PubMed] [Google Scholar]
- 124.Oda Y, Tu CL, Chang W, et al. The calcium sensing receptor and its alternatively spliced form in murine epidermal differentiation. J. Biol. Chem. 2000;275(2):1183–1190. doi: 10.1074/jbc.275.2.1183. [DOI] [PubMed] [Google Scholar]
- 125.Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J. Biol. Chem. 2001;276(44):41079–41085. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- 126.Ratnam AV, Cho JK, Bikle DD. 1,25-dihydroxyvitamin D3 enhances the calcium response of keratinocytes. J. Invest. Dermatol. 1996;106:910. doi: 10.1002/(SICI)1097-4652(199902)178:2<188::AID-JCP8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 127.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci. Signal. 2008;1(35):ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tu C-L, Crumrine D, Man M-Q, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J. Invest. Dermatol. 2012 doi: 10.1038/jid.2012.159. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Turksen K, Troy TC. Overexpression of the calcium sensing receptor accelerates epidermal differentiation and permeability barrier formation in vivo. Mech. Dev. 2003;120(6):733–744. doi: 10.1016/s0925-4773(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 130.Tu CL, Chang W, Bikle DD. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. J. Invest. Dermatol. 2007;127(5):1074–1083. doi: 10.1038/sj.jid.5700633. [DOI] [PubMed] [Google Scholar]
- 131.Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey–Hailey disease. Nat. Genet. 2000;24(1):61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 132.Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat. Genet. 1999;21(3):271–277. doi: 10.1038/6784. [DOI] [PubMed] [Google Scholar]
- 133.Bikle DD. Vitamin D: role in skin and hair. In: Feldman D, Pike W, Glorieux F, editors. Vitamin D. 2nd Edition Vol. 1. Elsevier Academic Press; CA, USA: 2005. pp. 609–630. [Google Scholar]