Abstract
Ethylmalonic encephalopathy (EE) is an invariably fatal disease, characterized by the accumulation of hydrogen sulfide (H2S), a highly toxic compound. ETHE1, encoding sulfur dioxygenase (SDO), which takes part in the mitochondrial pathway that converts sulfide into harmless sulfate, is mutated in EE. The main source of H2S is the anaerobic bacterial flora of the colon, although in trace amount it is also produced by tissues, where it acts as a ‘gasotransmitter’. Here, we show that AAV2/8-mediated, ETHE1-gene transfer to the liver of a genetically, metabolically and clinically faithful EE mouse model resulted in full restoration of SDO activity, correction of plasma thiosulfate, a biomarker reflecting the accumulation of H2S, and spectacular clinical improvement. Most of treated animals were alive and well >6–8 months after birth, whereas untreated individuals live 26 ± 7 days. Our results provide proof of concept on the efficacy and safety of AAV2/8-mediated livergene therapy for EE, and alike conditions caused by the accumulation of harmful compounds in body fluids and tissues, which can directly be transferred to the clinic.
Keywords: adeno-associated virus, ethylmalonic encephalopathy, gene therapy, hydrogen sulfide, mitochondrial disease
INTRODUCTION
Ethylmalonic encephalopathy (EE; OMIM #602473) is a fatal, early onset, autosomal recessive mitochondrial disease caused by mutations in ETHE1 (Burlina et al, 1991; Mineri et al, 2008). ETHE1 encodes a ubiquitous mitochondrial sulfur dioxygenase (SDO) (Tiranti et al, 2009) involved in the detoxification of H2S (Hildebrandt & Grieshaber, 2008), which is produced in tissues by the catabolism of sulfurated amino acids (Kabil & Banerjee, 2010) and, in the large intestine, by anaerobic bacteria (Flannigan et al, 2011) (Supporting Information Fig S1A). In trace amounts, H2S, an elusive, highly volatile gas, is involved in the regulation of the vessel tone and, possibly, in neurotransmission (Gadalla & Snyder, 2010). However, at higher concentrations it acts as a pleiotropic, powerful poison of several enzymes, such as cytochrome c oxidase (COX) (Di Meo et al, 2011) and short chain acyl-CoA dehydrogenase (SCAD) (Tiranti et al, 2009), and directly damages the vascular endothelium (Giordano et al, 2012). These deleterious effects explain the main signs and symptoms of human EE, namely, rapidly progressive neurological failure due to the accumulation of multiple necrotic and haemorrhagic brain lesions (Yang et al, 2004), chronic haemorrhagic diarrhoea, vascular petechial purpura and orthostatic acrocyanosis. Biochemically, EE is characterized by high plasmatic and urinary levels of thiosulfate, a stable, easily measurable compound that directly reflects accumulation of H2S (Furne et al, 2001), and ethylmalonic acid (EMA), the carboxylated derivative of butyrate, which reflects the block of oxidative catabolism of butyryl-CoA (Corydon et al, 1996), by H2S-mediated inhibition of SCAD. In addition, generalized, H2S-mediated COX deficiency occurs in muscle, brain and colonic mucosa (Di Meo et al, 2011). These clinical and biochemical features are faithfully recapitulated in a recombinant Ethe1−/− mouse model (Tiranti et al, 2009).
Effective therapy for EE must aim at either reducing H2S production, increasing its clearance and detoxification, or both. This rationale underpinned a partially successful, however palliative, treatment, applied to both EE patients and Ethe1−/− mice, based on administration of either N-acetylcysteine (NAC), a precursor of H2S-buffering glutathione, or metronidazole, a bactericidal agent specific against anaerobic bacteria, or both (Viscomi et al, 2010). Here, we show the results of a strategy based on liver-restricted adeno-associated virus (AAV)-mediated gene-replacement.
RESULTS
Using an AAV2/8 vector expressing GFP under the liver-specific thyroxine-binding globulin (TBG) promoter, we first established that the most effective route was intra-cardiac (i.c.) injections in avertin-anesthetized 21 day-old mice. Tail-vein injection was unfeasible due to the small size of the animals, and the intra-peritoneal route was ineffective, likely because of the imperviousness of the peritoneum to viral particles.
Next, we generated an AAV2/8 vector expressing human HA-tagged wild-type (wt) ETHE1 (h.ETHE1HA) cDNA (Tessitore et al, 2008) (Supporting Information Fig S1B). We then i.c. injected 4 × 1012 AAV2/8-TBG-h.ETHE1HA viral genomes (vg)/kg of body weight in four, 21-day old (P21) Ethe1−/− mice that had been given 1% NAC in drinking water since P18 to prevent rapid catastrophic downhill development of the clinical conditions occurring in naïve untreated Ethe1−/− individuals (Viscomi et al, 2010). Mice were euthanized at P35. The h.ETHE1HA cDNA and protein, as well as SDO activity, were detected exclusively in the liver (Supporting Information Fig S2A–B). Both protein amount and enzymatic activity reached approximately 60% of those in wt littermates. However, this partial biochemical correction was ineffective in significantly prolonging the lifespan of a second group of four (AAV + NAC)-treated Ethe1−/− mice, compared to that of Ethe1−/− littermates exposed only to NAC (Supporting Information Fig S2C). Accordingly, plasma thiosulfate remained very high in both groups (Supporting Information Fig S2D).
In a second pilot experiment, we administered a ten-fold higher titre (4 × 1013 vg/kg) of AAV2/8-TBG-h.ETHE1HA to three NAC-treated P21 Ethe1−/− mice. Again, h.ETHE1HA cDNA and protein was present only in, and evenly distributed throughout, the liver (Fig 1A–C), but expression, amount and activity were now comparable to those of wt littermates (Fig 1E–D). We then expanded the 4 × 1013 vg/kg treatment to ten additional, NAC-treated Ethe1−/− mice at P21. This dosage resulted in marked amelioration of the clinical conditions, reflected by highly significant prolongation of the lifespan. Five animals survived up to 4–6 months and were sacrificed for autoptic examination when still alive and well. The remaining five died during the observation time at an age ranging between 3 and 4 months. The median survival of ‘NAC-only’ treated Ethe1−/− mice was less than 2 months (log-rank test p < 0.0001) (Supporting Information Fig S3A).
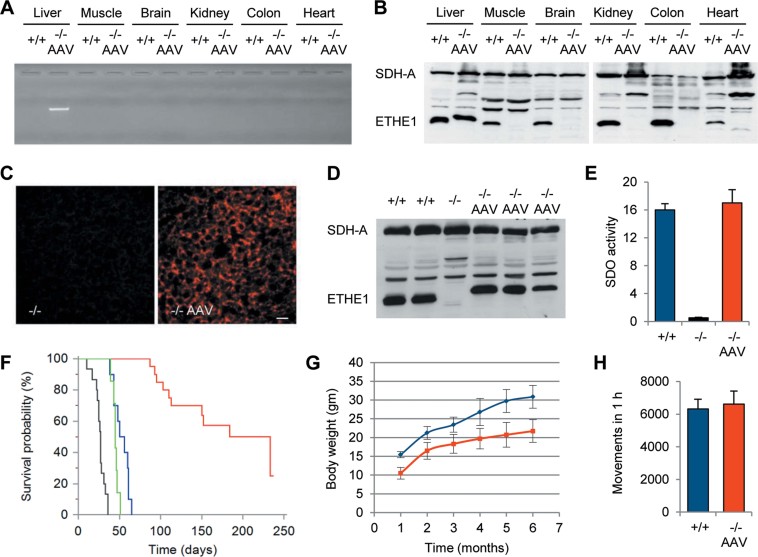
Figure 1. Molecular and clinical characterization of AAV2/8-TBG-h.Ethe1HA-treated mice.
A single i.c. injection of 4 × 1013 vg/kg was performed in each P21 animal.
- PCR analysis of AAV2/8 tissue distribution.
- Western-blot analysis of tissue homogenates using an anti-(α)-ETHE1 antibody: the h.ETHE1HA protein is detected only in the liver of AAV-treated animals as a band slightly slower than that corresponding to the endogenous mouse Ethe1 protein present in all wt (+/+) tissues. SDH-A, the 70 kDa subunit of succinate dehydrogenase is used as a protein-loading standard.
- Immunofluorescence of liver using an α-HA antibody. The h.ETHE1HA protein is diffusely distributed in the liver of AAV-treated animals (−/− AAV, right panel), whereas it is absent in naïve −/− animals (left panel). Scale bar: 50 µm.
- Western-blot analysis of h.ETHE1HA protein in liver: the amount detected in three AAV-treated mice (−/− AAV) is comparable to that of wt littermates (+/+), whereas the protein is absent in a sample from a naïve −/− animal.
- SDO activity in liver expressed in nmol O2/min/mg: the activity of AAV-treated Ethe1−/− samples (−/− AAV; red bar; n = 3) is comparable to that of wt samples (+/+; blue bar; n = 2). Note that SDO activity is virtually absent in untreated Ethe1−/− samples (−/−; black bar; n = 3).
- Kaplan–Meier survival probability graph. Significance was assessed by log-rank test. Grey: untreated Ethe1−/− mice (n = 15); green: Ethe1−/− mice treated with NAC for 10 days (n = 7); blue: Ethe1−/− treated with NAC ad libitum (n = 10); red: AAV-treated Ethe1−/− mice (n = 20).
- Variation of body weights over time: for each point the difference between AAV-treated Ethe1−/− mice (red) versus wt littermates (blue) was significant (p < 0.001).
- Activity cage test: no significant difference in spontaneous locomotor activity was measured between −/− AAV-treated mice (n = 11) versus wt (+/+) littermates at 3–5 months of age.
In a second trial, ten Ethe1−/− mice were treated with the same protocol and dosage, but NAC administration was suspended at P28. Survival was monitored up to 8 months. Again, of the 6 individuals that offset 7 months of age, five were alive and well at the time of sacrifice, whereas one died at >7 months; one individual died at 6 months; two at 5 months; one at 3. The median survival of naive untreated Ethe1−/− mice (with no NAC) was <1 month (log-rank test p < 0.0001) (Supporting Information Fig S3B).
The survival probability between the two AAV-treated groups did not differ significantly; a cumulative Kaplan–Meier distribution of survival probability is shown in Fig 1F. Lifespan prolongation was accompanied by increased body-weight over time, which was similar to, albeit less than, wt littermates (Fig 1G). In sharp contrast with untreated Ethe1−/− mice, 3–5 month-old AAV-treated animals (n = 11) showed a striking increase of spontaneous motor activity, comparable to that of wt littermates (n = 9) (Fig 1H).
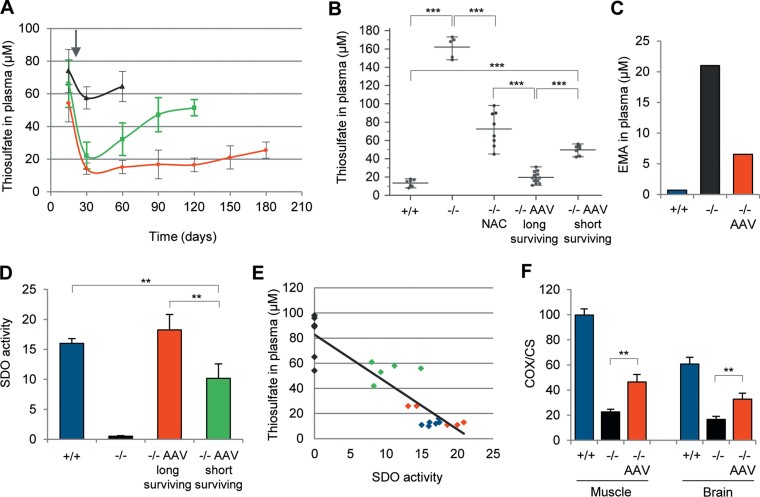
The levels of plasma thiosulfate reflected the lifespan, and in fact predicted the outcome, of AAV-treated individuals (Fig 2A), since those that displayed consistent correction to nearly normal levels (19.0 ± 6.0 µM; wt control mean: 14.0 ± 4.0 µM; NS) lived much longer than those showing a progressive increase (51.0 ± 4.6 µM; p < 0.0001) (Fig 2B). Plasma EMA in the AAV-treated group was much lower than that in the untreated group (Fig 2C). SDO activity in liver mitochondria of AAV-treated mice was virtually normal in longer-surviving (18.2 ± 2.6 nmol O2/min/mg; normal values: 16.0 ± 0.8; NS), but lower-than-normal in shorter-surviving, individuals (10.2 ± 2.4; p = 0.001) (Fig 2D). Thiosulfate levels in plasma correlated with liver SDO activity (R2 = 0.82, p < 0.0001) (Fig 2E), and AAV DNA copy number in hepatocytes was significantly higher in longer- than in shorter-surviving groups (24.6 ± 2.5 AAV DNA copies/diploid genome vs. 10.4 ± 2.0; p = 0.007). The amount of thiosulfate was much lower in tissues than in plasma, and for some tissues, such as brain, it was below the detectability threshold (Tiranti et al, 2009). Nevertheless, we found a significant reduction of thiosulfate levels in muscle homogenates from AAV-treated Ethe1−/− animals compared to untreated Ethe1−/− individuals (Supporting Information Fig S4), which suggests that the clearance of circulating H2S is effective in reducing its concentration in critical, extrahepatic tissues.
Figure 2. Biochemical analysis.
- Plasma thiosulfate measured at different time-points in shorter- (green) and longer- (red) surviving Ethe1−/− individuals treated with 4 × 1013 vg/kg AAV2/8-TBG-h.ETHE1HA, and in NAC-only treated Ethe1−/− mice (gray). The arrow indicates the time of the AAV injection (P21).
- Thiosulfate concentration at the last time-point before death. In longer-surviving AAV-treated Ethe1−/− animals, the thiosulfate concentration is comparable to that of the wt ones, whereas in shorter-surviving AAV-treated animals it is comparable to that of the ‘NAC-only’ treated ones. ***: unpaired Student's t-test p < 0.0001.
- Plasma EMA in pooled samples.
- SDO activities expressed as nmol O2/min/mg in wt controls (+/+; blue bar; n = 3); untreated Ethe1−/− animals (black bar; n = 3); longer-surviving AAV-treated animals (red bar; n = 5) and shorter-surviving AAV-treated animals (green bar; n = 5). **: Student's t-test p = 0.008.
- Correlation analysis of plasma thiosulfate versus liver SDO activity. Black diamonds: ‘NAC-only’ treated Ethe1−/−; green: AAV-treated, shorter-surviving Ethe1−/−; red: AAV-treated, longer-surviving Ethe1−/−; blue: Ethe1+/+. χ2-test correlation R2 = 0.82 (p = 0.001).
- COX/CS activity in muscle and brain of wt controls (+/+; blue bar; n = 3); untreated Ethe1−/− animals (black bar; n = 3); AAV-treated animals (red bar; n = 5) **: Student's t-test p = 0.004.
Biochemical assays for COX activity normalized to that of citrate synthase (CS) showed significant recovery in both skeletal muscle and brain of treated versus untreated animals (Fig 2F and Supporting Information Table S1).
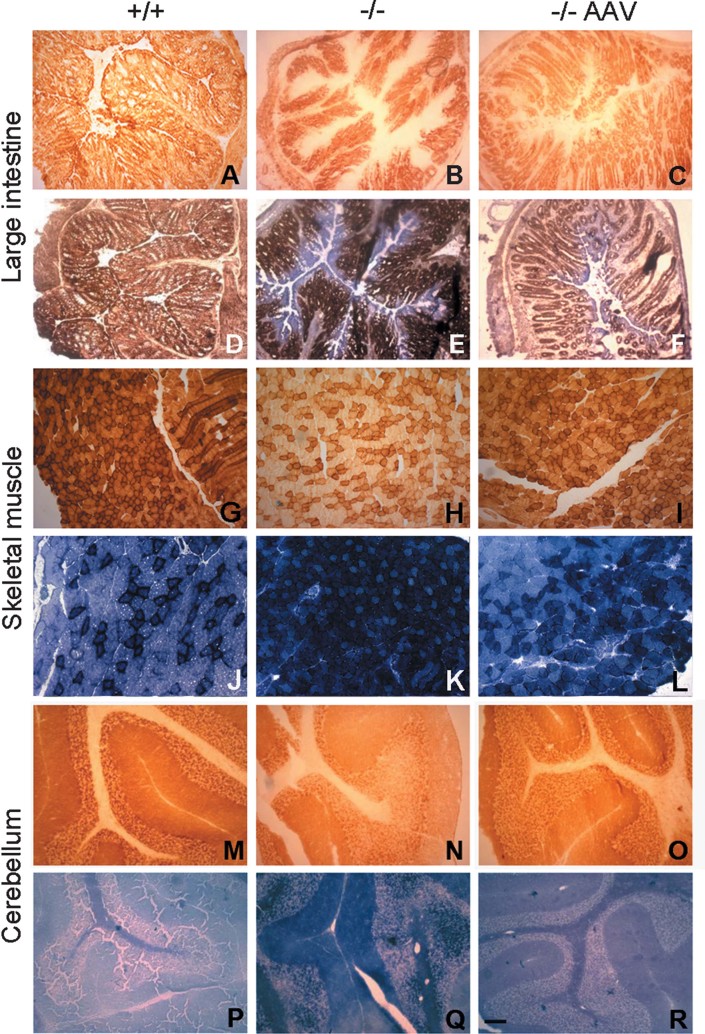
Histochemical reaction to COX and SDH was performed in skeletal muscle, brain (cerebellum), and large intestine of Ethe1+/+, Ethe1−/− and AAV-treated Ethe1−/− mice (n = 3 for each group). Whilst naive Ethe1−/− mice displayed marked COX deficiency due to H2S poisoning (Di Meo et al, 2011) in the luminal surface of the colonic mucosa, muscle fibres and brain cells, the AAV treatment was associated with clearly visible, albeit partial, correction to nearly normal levels (Fig 3). Likewise, markedly hyperintense ‘SDH-like’ blue staining that was evident in naive Ethe1−/− mice returned to nearly normal levels in tissues from AAV-treated Ethe1−/− mice (Fig 3). We have previously shown that hyper intense blue staining in Ethe1−/− tissues is a ‘spurious’ reaction caused by direct H2S-mediated reduction of colourless tetrazolium, the histochemical SDH substrate, into blue formazan (Di Meo et al, 2011). Thus, the lesser intense blue reaction in AAV-treated tissues reflects lower H2S concentration in situ.
Figure 3. Histochemical analysis.
Frozen sections from a 6 month-old Ethe1+/+ (+/+), a 1 month-old Ethe1−/− (−/−), and a 6 month-old AAV-treated Ethe1−/− (−/− AAV) mouse.
- A–C. COX reaction.
- D–F. Combined COX + SDH reactions in the large intestine.
- G–I. COX reaction.
- J–L. SDH reaction based on the conversion of tetrazolium into blue formazan.
- M–O. COX reaction.
- P–R. SDH reaction based on the conversion of tetrazolium into blue formazan. Scale bar: 50 mm. See text for details.
The paper explained
PROBLEM:
Ethylmalonic encephalopathy (EE) is an invariably fatal disease characterized by the accumulation of hydrogen sulfite (H2S), a highly toxic compound. The main source of H2S is the anaerobic bacterial flora of the colon, although it is also produced by tissues in trace amounts, where it acts as a “gasotransmitter”. EE is caused by mutations in ETHE1, a gene encoding a mitochondrial sulfur dioxygenase (SDO), which takes part in the pathway that converts sulfite into harmless sulfate. We have previously proposed a pharmacological therapy based on the off-label use of approved drugs (Metronidazole and NAC) to lower the production and promote the intra-cellular detoxification of H2S. Although beneficial, this palliative therapy is far from being curative.
RESULTS:
In order to develop a more effective, etiological therapy for EE, we have used AAV-mediated gene targeting to express the human ETHE1 protein in vivo. AAV2/8-mediated ETHE1-gene transfer to the liver of a genetically, metabolically and clinically faithful EE mouse model resulted in full restoration of in vitro SDO activity, correction of plasma thiosulfate, a biomarker reflecting the accumulation of H2S, and efficient clinical improvement. Most of the treated animals were alive and well >6–8 months after birth, whereas untreated individuals lived 26 ± 7 days.
IMPACT:
Our results provide proof of concept on the efficacy and safety of AAV2/8-mediated liver gene therapy for EE and alike conditions caused by the accumulation of harmful compounds in body fluids and tissues, which can directly be transferred to the clinic.
DISCUSSION
The key-pathomechanism of EE is the accumulation of H2S in the bloodstream and tissues up to toxic levels, which eventually inhibits crucial enzymes such as COX and SCAD and directly damages the endothelial lining. We reasoned that the clearance of circulating H2S, by expressing the missing ETHE1 gene in a filtering organ such as the liver, could decrease the levels of H2S, thus acting as a detoxifying means for effective disease treatment. To test this hypothesis, we used an AAV2/8-TBG vector to express h.ETHE1HA in the liver of Ethe1−/− mice. After optimization of route and titer, we then treated a total of 20 animals that all showed marked amelioration of the phenotype and spectacular prolongation of the lifespan. This remarkable clinical result was associated with partial or complete correction of the main metabolic and biochemical indexes of disease, including EMA and thiosulfate levels in plasma as well as COX activity in tissues. Interestingly, the levels of thiosulfate, a stable biomarker mirroring the amount of labile H2S, were much higher in plasma of untreated Ethe1−/− animals than in muscle homogenates, suggesting that circulating H2S can have more prominent and widespread damaging effects than H2S produced endogenously in individual tissues. This is concordant with the observation that conditional brain- or muscle-specific Ethe1−/− mice display hardly any tissue damage or clinical effect (Di Meo et al, 2011). We conclude that liver-specific AAV-based treatment can correct, at least partially, H2S-mediated inhibition of SCAD and COX in brain, muscle and colonic mucosa, by just lowering H2S concentration in the bloodstream, as reflected by normalization (or marked decrease) of circulating thiosulfate. We propose that the restoration of H2S detoxifying competency by the hepatic filter can prevent the most ominous pathological feature in human EE, i.e. the direct damage of endothelia in critical organs (Giordano et al, 2012) caused by circulating H2S (Yang et al, 2004). Unfortunately, this hypothesis is difficult to test in Ethe1−/− mice, whose vascular lesions, possibly because of their very short lifespan, are much less prominent than those of EE patients (Giordano et al, 2012).
Long-term therapeutic levels of coagulation Factor IX have recently been achieved in hemophilia B patients treated with a single intravascular administration of the recombinant AAV2/8 vector (Nathwani et al, 2011). Likewise, our results open realistic perspectives to the treatment of human EE, providing proof-of-principle, evidence-based demonstration that AAV-driven ETHE1 gene therapy is an effective and feasible approach, directly translatable to clinical practice.
In a broader perspective, the same strategy can be applied to other conditions such as mitochondrial neuro-gastro-intestinal encephalomyopathy (MNGIE) (Nishino et al, 1999) caused by the accumulation of thymidine up to mutagenic levels for mtDNA.
MATERIALS AND METHODS
Construction of AAV2/8 vectors
AAV2/8-TBG-h.ETHE1HA and AAV2/8-TBG-eGFP vectors were produced by the AAV Vector Core of the Telethon Institute of Genetics and Medicine (TIGEM, Naples, Italy) by triple transfection of 293 cells and purified by CsCl gradients (Xiao et al, 1999). Physical titers of the viral preparations (genome copies/mL) were determined by real-time PCR (Gao et al, 2000) (Applied Biosystems, Foster City, CA, USA) and dot-blot analysis.
Genomic DNA extraction, PCR and quantitative PCR
Total DNA was extracted from frozen tissues (Viscomi et al, 2009). AAV-derived DNA was detected by standard PCR amplification using primer pairs specific to the h.ETHE1 gene. SYBR-GREEN based real-time quantitative PCR was carried out as previously described (Di Meo et al, 2011) using primers specific to the h.ETHE1 gene; the RNAseP gene was used as a reference. Oligonucleotide sequences are available on request.
Immunoblotting
Western-blot analysis was performed on mouse tissue homogenates with an α-ETHE1 (Tiranti et al, 2004) and an α-succinate dehydrogenase-A (α-SDHA; 0.1 µg/mL) using the ECL chemiluminescence kit (Amersham) (Di Meo et al, 2011).
Immunofluorescence, histochemical and biochemical analyses
Immunofluorescence analysis was carried out on cryostat sections fixed with methanol using an α-HA polyclonal primary antibody (Abcam). Standard histochemical reactions for COX and SDH were performed on cryostat sections (Sciacco & Bonilla, 1996). SDO activity in liver and thiosulfate in plasma and tissues were measured as described (Hildebrandt & Grieshaber, 2008). Plasma EMA was assessed by GC/MS (Wilcken et al, 2003). Spectrophotometric analysis was carried out as described (Di Meo et al, 2011).
Statistical analysis
For comparison between groups we used two-tailed, unpaired Student's t-test. Correlation significance was determined by χ2 test. Survival probability analysis was calculated using Kaplan–Meier estimate and log-rank test.
Experimental ethics policy
Animal studies were approved by the Ethics Committee of the Foundation ‘Carlo Besta’ Neurological Institute, in accordance with the guidelines of the Italian Ministry of Health. The use and care of animals followed the Italian Law D.L. 116/1992 and the EU directive 86/609/CEE. The mice were kept on a C57Bl6/129Sv mixed background, and wt littermates were used as controls. Standard food and water were given ad libitum.
Acknowledgments
This work was supported by the Pierfranco and Luisa Mariani Foundation Italy, Telethon-Italy GPP10005 and GGP11011, AFM 15927, Cariplo 2011-0526, E-RARE JTC2011, and the Italian Association of Mitochondrial Disease Patients (Mitocon).
Supporting Information is available at EMBO Molecular Medicine Online.
The authors declare that they have no conflict of interest.
Author contributions
IDM performed the in vivo experiments, AA shared expertise and provided the AAV vectors and viruses, CL performed histochemical analysis, AB performed tandem mass measurements in plasma, CV and MZ designed the experimental plan and wrote the manuscript.
For more information
TELETHON-Italy Foundation official website: http://www.telethon.it
Association Francaise contre les Myopathies (AFM) official website: http://www.afm-telethon.fr
TIGEM (Telethon Institute of Genetic Medicine) official website: http://www.tigem.it
Pierfranco and Luisa Mariani Center for the Study of Paediatric Mitochondrial Disease: http://www.mitopedia.org
Mitocon-Italian Association of Mitochondrial Disease Patients: http://www.mitocon.it
OMIM Online Mendelian Inheritance in Man: http://www.ncbi.nlm.nih.gov/omim/
National Center for Biotechnology Information: http://www.ncbi.nlm.nih.gov
The Foundation Carlo Besta Institute of Neurology – IRCCS official website: http://www.istituto-besta.it
Clinical Trials on AAV-mediated gene replacement can be found at: http://www.clinicaltrials.gov
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Burlina A, Zacchello F, Dionisi-Vici C, Bertini E, Sabetta G, Bennet MJ, Hale DE, Schmidt-Sommerfeld E, Rinaldo P. New clinical phenotype of branched-chain acyl-CoA oxidation defect. Lancet. 1991;338:1522–1523. doi: 10.1016/0140-6736(91)92338-3. [DOI] [PubMed] [Google Scholar]
- Corydon MJ, Gregersen N, Lehnert W, Ribes A, Rinaldo P, Kmoch S, Christensen E, Kristensen TJ, Andresen BS, Bross P, et al. Ethylmalonic aciduria is associated with an amino acid variant of short chain acyl-coenzyme A dehydrogenase. Pediatr Res. 1996;39:1059–1066. doi: 10.1203/00006450-199606000-00021. [DOI] [PubMed] [Google Scholar]
- Di Meo I, Fagiolari G, Prelle A, Viscomi C, Zeviani M, Tiranti V. Chronic exposure to sulfide causes accelerated degradation of cytochrome c oxidase in ethylmalonic encephalopathy. Antioxid Redox Signal. 2011;15:353–362. doi: 10.1089/ars.2010.3520. [DOI] [PubMed] [Google Scholar]
- Flannigan KL, McCoy KD, Wallace JL. Eukaryotic and prokaryotic contributions to colonic hydrogen sulfide synthesis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G188–G193. doi: 10.1152/ajpgi.00105.2011. [DOI] [PubMed] [Google Scholar]
- Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol. 2001;62:255–259. doi: 10.1016/s0006-2952(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Qu G, Burnham MS, Huang J, Chirmule N, Joshi B, Yu QC, Marsh JA, Conceicao CM, Wilson JM. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- Giordano C, Viscomi C, Orlandi M, Papoff P, Spalice A, Burlina A, Di Meo I, Tiranti V, Leuzzi V, d'Amati G, et al. Morphologic evidence of diffuse vascular damage in human and in the experimental model of ethylmalonic encephalopathy. J Inherit Metab Dis. 2012;35:451–458. doi: 10.1007/s10545-011-9408-3. [DOI] [PubMed] [Google Scholar]
- Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–-21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineri R, Rimoldi M, Burlina AB, Koskull S, Perletti C, Heese B, von Döbeln U, Mereghetti P, Di Meo I, Invernizzi F, et al. Identification of new mutations in the ETHE1 gene in a cohort of 14 patients presenting with ethylmalonic encephalopathy. J Med Genet. 2008;45:473–478. doi: 10.1136/jmg.2008.058271. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–692. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- Sciacco M, Bonilla E. Cytochemistry and immunocytochemistry of mitochondria in tissue sections. Methods Enzymol. 1996;264:509–521. doi: 10.1016/s0076-6879(96)64045-2. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Faella A, O'Malley T, Cotugno G, Doria M, Kunieda T, Matarese G, Haskins M, Auricchio A. Biochemical, pathological, and skeletal improvement of mucopolysaccharidosis VI after gene transfer to liver but not to muscle. Mol Ther. 2008;16:30–37. doi: 10.1038/sj.mt.6300325. [DOI] [PubMed] [Google Scholar]
- Tiranti V, D'Adamo P, Briem E, Ferrari G, Mineri R, Lamantea E, Mandel H, Balestri P, Garcia-Silva MT, Vollmer B, et al. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am J Hum Genet. 2004;74:239–252. doi: 10.1086/381653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, Tiveron C, Levitt MD, Prelle A, Fagiolari G, Rimoldi M, et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- Viscomi C, Burlina AB, Dweikat I, Savoiardo M, Lamperti C, Hildebrandt T, Tiranti V, Zeviani M. Combined treatment with oral metronidazole and N-acetylcysteine is effective in ethylmalonic encephalopathy. Nat Med. 2010;16:869–871. doi: 10.1038/nm.2188. [DOI] [PubMed] [Google Scholar]
- Viscomi C, Spinazzola A, Maggioni M, Fernandez-Vizarra E, Massa V, Pagano C, Vettor R, Mora M, Zeviani M. Early-onset liver mtDNA depletion and late-onset proteinuric nephropathy in Mpv17 knockout mice. Hum Mol Genet. 2009;18:12–26. doi: 10.1093/hmg/ddn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348:2304–2312. doi: 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.