Abstract
Most research on the association between restless legs syndrome (RLS) and depression has involved cross-sectional data. The objective of the present study was to evaluate this issue prospectively among Nurses' Health Study participants. A total of 56,399 women (mean age = 68 years) who were free of depression symptoms at baseline (2002) were followed until 2008. Physician-diagnosed RLS was self-reported. During 300,155 person-years of follow-up, the authors identified 1,268 incident cases of clinical depression (regular use of antidepressant medication and physician-diagnosed depression). Women with RLS at baseline were more likely to develop clinical depression (multivariate-adjusted relative risk (RR) = 1.5, 95% confidence interval (CI): 1.1, 2.1; P = 0.02) than those without RLS. The presence of RLS at baseline was also associated with higher scores on the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10) and the 15-item Geriatric Depression Scale (GDS-15) thereafter. Multivariable-adjusted mean differences were 1.00 (standard error, 0.12) for CESD-10 score and 0.47 (standard error, 0.07) for GDS-15 score between women with RLS and those without RLS (P < 0.0001). In conclusion, women with physician-diagnosed RLS had an increased risk of developing clinical depression and clinically relevant depression symptoms. Further prospective studies using refined approaches to ascertainment of RLS and depression are warranted.
Keywords: depression, meta-analysis, prospective studies, restless legs syndrome
Restless legs syndrome (RLS), also known as Ekbom syndrome, is a common, bothersome sensorimotor disorder that is characterized by an almost irresistible urge to move the legs, accompanied by an unpleasant sensation in the legs and a worsening of the symptoms in the evening or during the night (1). These symptoms can result in severe sleep disturbance and may be associated with the emergence of depression symptoms (2–4). To date, 17 population-based cross-sectional studies (5–21) and 5 clinic-based case-control studies (22–26) of RLS and depression have been conducted, and most of them have found a high co-occurrence of RLS and depression. However, to our knowledge, the association between RLS and depression has not been examined in a prospective study to date.
Interestingly, the association between RLS and depression appears to be inconsistent among women in these published studies. In 2000, Rothdach et al. (5) reported that men, but not women, with RLS had a higher likelihood of having depression, as compared with controls. This similar difference between men and women was observed in a subsequent Japanese study based on 1,008 men and 1,015 women (8). However, in a women-only Swedish study (15), women with RLS had approximately double the likelihood of self-reported depressed mood. Further, in a clinic-based German study including 519 women with RLS (27), 52.7% of them had abnormal anxiety/depression scores as evaluated by means of a 5-item quality-of-life scale called the EQ-5D (EuroQol Group, Rotterdam, the Netherlands), relative to approximately 4.9% in the general population (28). Given the inconsistent results on the association between RLS and depression in women, larger, better-controlled studies among women are needed.
Our purpose in the present study was to examine prospectively whether women with RLS had an elevated risk of developing depression during 6 years of follow-up, using data from the Nurses' Health Study. In a secondary analysis, we also examined the association between RLS and risk of clinically relevant depression symptoms.
MATERIALS AND METHODS
The Nurses' Health Study cohort was established in 1976, when 121,700 female registered nurses aged 30–55 years residing in 11 US states responded to a mailed questionnaire regarding their medical history and health practices. The cohort has been followed every 2 years with mailed questionnaires that update exposure information and inquire about newly diagnosed medical illnesses (29).
On the 2002 questionnaire, we asked 82,160 participants who were still alive and actively participating in the Nurses' Health Study whether they had ever been diagnosed with RLS by a physician. We therefore used 2002 as the baseline date in the present analysis and excluded women who reported having physician-diagnosed depression during or prior to 2002 (n = 9,054) or reported it after 2002 with an unknown start date (n = 89), those who reported regular use of antidepressant medication during or prior to 2002 (n = 6,059), and those who had a Mental Health Index Inventory score of 52 or less (i.e., depressive symptoms) at any of the study contacts made in 1992, 1996, and 2000 (n = 3,405) (see Web Figure 1, which appears on the Journal's website (http://aje.oxfordjournals.org/)). The Mental Health Inventory is a 5-item subscale of the Short Form 36 health status survey (30, 31), and a lower score (≤52) has been found to have high sensitivity and specificity for major depressive disorder (32). After further excluding women who did not have complete information on physician-diagnosed depression or regular use of antidepressant medication (n = 7,154), 56,399 women were included in the present analysis. Participants who were included had a similar age (mean age = 68.1 years vs. 67.7 years) and body mass index (26.5 vs. 27.3) as those who were excluded from the present analysis. The study protocol was approved by the institutional review boards of the Brigham and Women's Hospital and Harvard School of Public Health.
Information on potential confounders, including age, body weight, menopausal status and estrogen hormone therapy, smoking status, physical activity, use of aspirin and multiple vitamin supplements, and history of major chronic conditions, including hypertension, elevated cholesterol concentration, diabetes, arthritis, heart diseases (myocardial infarction and/or angina), stroke, and cancer, was collected via biennial questionnaires throughout the follow-up period. Body mass index was calculated from self-reported weight and height as weight in kilograms divided by height in meters squared. Information on alcohol consumption was collected using validated food frequency questionnaires every 4 years. In 2002, we also collected information on sleep duration and snoring frequency.
Case ascertainment
A participant was considered clinically depressed if she reported both physician-diagnosed depression and regular use of antidepressant medication. The questions on regular antidepressant medication use and physician-diagnosed depression were asked in 2002, and data were updated biennially through 2008.
Depression symptoms were evaluated by means of 2 commonly used self-report measures: the 10-item version of the Center for Epidemiologic Studies Depression Scale (CESD-10) in 2004 and the 15-item version of the Geriatric Depression Scale (GDS-15) in 2008. The CESD-10 is a short-form scale with 10 items that is designed to identify depressive symptoms in the general population (33). The CESD-10 has shown good predictive accuracy when compared with the full-length 20-item version of the Center for Epidemiologic Studies Depression Scale, with a kappa index of 0.97 (33). Responses are recorded using a 4-point Likert scale ranging from rarely (scored 0) to all of the time (scored 3), and points are summed across the 10 items to provide a total CESD-10 score ranging from 0 to 30. Respondents who provided data on at least 9 of the CESD-10 items were included in the analyses, with mean imputation used to replace the missing items. The optimum cutoff point for the CESD-10 scores was 10 (33). The GDS-15 is a 15-item short version of the Geriatric Depression Scale. Giving 1 point for each matching answer and summing the points results in the GDS-15 score (34). Respondents who provided data on all 15 items of the GDS-15 were included in the analyses. The GDS-15 has excellent properties in screening for major depression (35) as assessed by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (36). The optimum cutoff point for the GDS-15 is 5, yielding a sensitivity of 92% and a specificity of 85% in predicting major depression (35).
Clinically relevant depressive symptoms were defined as either CESD-10 score ≥10 or GDS-15 score ≥5.
Statistical methods
Person-years for each participant were calculated from the date on which the 2002 questionnaire was returned to the diagnosed date of depression or death; June 1, 2008; or the date of return of the last questionnaire, whichever came first. Time-dependent Cox proportional hazards models were used to estimate age- and multivariate-adjusted relative risks (and 95% confidence intervals) of clinical depression and clinically relevant depression symptoms for women with RLS compared with women without RLS. The age-adjusted mean values for the CESD-10 score and GDS-15 score and multivariate-adjusted mean differences in scores between women with RLS and women without RLS were compared using a generalized linear model.
The basic Cox model adjusted for age (continuous, in years), ethnicity (Caucasian, African-American, or Asian/other), smoking status (never smoker, former smoker, or current smoker), alcohol drinking (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, or ≥15 g/day of alcohol), body mass index (<23, 23–24.9, 25–29.9, 30–34.9, or ≥35), physical activity (quintiles), marital status (married/in a partnership, widowed, or separated/divorced/single), living status (alone or with others; binary), regular involvement in a social or community group (yes or no; binary), menopausal status, use of estrogen hormone therapy (no use, past use, or current use), and the presence of hypertension, elevated cholesterol level, diabetes, arthritis, myocardial infarction/angina, stroke, Parkinson's disease, and cancer (yes/no). In the final Cox and generalized linear models, we also adjusted for use of iron-specific supplements (yes/no), sleep duration (≤5, 6, 7, 8, or ≥9 hours per 24-hour period), and snoring frequency (every night, most nights, a few nights per week, occasionally, or almost never), because iron deficiency has been shown to be associated with both RLS and neurocognitive function (1). Because we did not collect information on iron deficiency, we employed use of iron-specific supplements as a surrogate measure for iron deficiency in the current analysis.
In a secondary analysis, we also examined the association between RLS and depression based on different definitions: 1) physician-diagnosed depression; 2) either physician-diagnosed depression or regular use of antidepressant medication; 3) physician-diagnosed depression, CESD-10 score ≥10, or GDS-15 score ≥5; and 4) physician-diagnosed depression, regular antidepressant medication use, CESD-10 score ≥10, or GDS-15 score ≥5.
n order to test the robustness of our observations, we conducted several sensitivity analyses by 1) excluding the women who had ever been diagnosed with diabetes or arthritis; 2) excluding women who had ever had confirmed Parkinson's disease; 3) excluding women who had ever been diagnosed with myocardial infarction/angina or stroke by the end of follow-up; and 4) excluding the incident depression cases diagnosed during the period 2002–2004 in case these were subclinical cases preceding the diagnosis of RLS.
Finally, we conducted a meta-analysis of the published data on RLS and depression. We identified relevant studies by searching the MEDLINE, PubMed, Embase (Excerpta Medica Database), and OMIM (Online Medelian Inheritance in Man) databases using the keyword combination “restless legs syndrome or RLS or Ekbom syndrome and depression” for all studies published in English from 1966 through August 2011. Studies without control participants were not considered in the analysis. In addition, the reference lists from the relevant publications were used to identify additional studies. Four clinic-based studies and 14 community-based studies met the inclusion criteria and were included in the meta-analysis (5–8, 11–19, 21, 22, 24–26) (Web Table 1). We used the Q statistic to examine heterogeneity among the studies, and the significance level was set at 0.1. We used random-effects models to calculate the pooled odds ratio because significant heterogeneity was observed. Publication bias was assessed using Begg's test.
We used the SAS statistical package (version 9; SAS Institute Inc., Cary, North Carolina) for the cohort analyses and STATA (version 9.0; StataCorp LP, College Station, Texas) for the meta-analysis. All P values were 2-tailed (P < 0.05).
RESULTS
At baseline, 928 out of 56,399 women reported having ever been diagnosed with RLS. Compared with women without RLS, those with RLS were more likely to be older, to frequently snore, and to have a higher prevalence of major chronic conditions, such as hypertension, elevated cholesterol concentration, stroke, heart diseases, Parkinson's disease, arthritis, and diabetes (Table 1).
Table 1.
Characteristics of Participants According to Restless Legs Syndrome Status at Baseline, Nurses' Health Study, 2002a
|
Physican-diagnosed RLS Status in 2002 |
||||
|---|---|---|---|---|
|
No RLS (n= 55,471) |
RLS (n= 928) |
|||
| % | Mean (SD) | % | Mean (SD) | |
| Age, years | 68.0 (6.9) | 69.6 (6.9) | ||
| Smoking status | ||||
| Never smoker | 46.4 | 45.8 | ||
| Current smoker | 7.2 | 5.6 | ||
| Former smoker | 46.4 | 48.6 | ||
| Caucasian race | 97.5 | 98.5 | ||
| Premenopausal | 0.4 | 0.2 | ||
| Current menopausal hormone use | 34.4 | 36.9 | ||
| Body mass indexb | 26.5 (5.3) | 26.9 (5.3) | ||
| Physical activity, METs/week | 18.5 (22.8) | 18.3 (22.7) | ||
| Alcohol intake, g/day | 6.1 (10.7) | 5.5 (10.8) | ||
| Sleep duration, hours/day | 7.13 (0.98) | 6.96 (0.99) | ||
| Frequent snoring | 20.7 | 26.2 | ||
| Marital status | ||||
| Married/in a partnership | 76.1 | 77.8 | ||
| Widowed | 17.1 | 15.5 | ||
| Separated/divorced/single | 6.8 | 6.7 | ||
| Living alone | 18.2 | 17.0 | ||
| Not involved in any social or community groups | 30.8 | 30.1 | ||
| Presence of chronic conditions during or prior to 2002 | ||||
| Arthritis | 10.2 | 15.5 | ||
| Diabetes | 8.8 | 11.7 | ||
| Hypertension | 52.7 | 59.7 | ||
| Hypercholesterolemia | 63.4 | 71.9 | ||
| Stroke | 2.0 | 3.9 | ||
| Cancer | 16.9 | 18.6 | ||
| Parkinson's disease | 0.3 | 0.7 | ||
| Myocardial infarction/angina | 9.9 | 14.4 | ||
Abbreviations: MET, metabolic equivalent task; RLS, restless legs syndrome; SD, standard deviation.
a All data were standardized to the age distribution of the overall cohort.
b Weight (kg)/height (m)2.
During the 300,155 person-years of follow-up, we identified 1,268 incident cases of clinical depression. The age-adjusted relative risk of clinical depression was 1.66 (95% confidence interval (CI): 1.18, 2.35) for women with RLS versus those without RLS (Table 2). Adjustment for lifestyle factors, the presence of chronic conditions, sleep duration, and frequent snoring attenuated the association (adjusted relative risk = 1.5, 95% CI: 1.1, 2.1). The interactions between RLS and the covariates (age, obesity, sleep duration, and frequent snoring) were not significant (P-interaction > 0.3 for all), and the association between RLS and clinical depression persisted in subgroup analyses stratified according to these variables (data not shown).
Table 2.
Adjusted Relative Risk of Clinical Depressiona According to Baseline Restless Legs Syndrome Status, Nurses' Health Study, 2002–2008
| No RLS |
RLS |
P Value | ||
|---|---|---|---|---|
| RR | 95% CI | |||
| No. of cases | 1,235 | 33 | ||
| Person-years of follow-up | 295,493 | 4,662 | ||
| Crude incidence (per 100,000 person-years) | 418 | 708 | ||
| Age-adjusted RR | 1 | 1.66 | 1.18, 2.35 | 0.004 |
| Multivariate RRb | 1 | 1.58 | 1.11, 2.23 | 0.01 |
| Multivariate RRc | 1 | 1.51 | 1.07, 2.14 | 0.02 |
| Multivariate RRd | 1 | 1.49 | 1.06, 2.10 | 0.02 |
Abbreviations: CI, confidence interval; RLS, restless legs syndrome; RR, relative risk.
aClinical depression was defined as reported regular use of antidepressant medication and physician-diagnosed depression.
bAdjusted for age (years), body mass index (weight (kg)/height (m)2; continuous), ethnicity (Caucasian or other), smoking status (never, past, or current smoker), marital status (married/in a partnership, widowed, or separated/divorced/single), solitary living status (yes/no; binary), regular involvement in a social or community group (yes/no; binary), menopausal status (premenopausal or postmenopausal), menopausal hormone use (never, past, or current user), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, or ≥15 g/day), and physical activity (quintiles).
cFurther adjusted for the presence of diabetes, arthritis, stroke, myocardial infarction, angina, cancer, hypertension, high cholesterol level, or Parkinson's disease (yes/no).
dFurther adjusted for use of iron-specific supplements, sleep duration (≤5, 6, 7, 8, or ≥9 hours per 24-hour period), and snoring frequency (every night, most nights, a few nights per week, occasionally, or almost never).
Women with physician-diagnosed RLS also had a significantly higher CESD-10 score in 2004 and a significantly higher GDS-15 score in 2008 (Table 3). The multivariable-adjusted mean differences were 1.00 (standard error (SE), 0.12) for CESD-10 score and 0.47 (SE, 0.07) for GDS-15 score among women with RLS versus those without RLS (P < 0.0001). Women with RLS had a higher risk of developing clinically relevant depression symptoms (CESD-10 ≥10 or GDS-15 ≥5)—the multivariate-adjusted relative risk of clinically relevant depression symptoms was 1.53 (95% CI: 1.33, 1.76). Considering that 1 item on the CESD-10 (“My sleep was restless”) was sleep-related and may have confounded the association between RLS and CESD-10 score, we excluded this item and recalculated the score based on the other 9 items, yielding relatively lower mean scores (4.08 (SE, 0.01) among women with RLS and 4.73 (SE, 0.11) among women without RLS) and a significant multivariate-adjusted mean difference (0.64 (SE, 0.11); P < 0.0001).
Table 3.
Adjusted Relative Risk of Clinically Relevant Depression Symptomsa According to Baseline Restless Legs Syndrome Status, Nurses' Health Study, 2002–2008
|
No RLS |
RLS |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. | Score | RR | 95% CI | No. | Score | RR | 95% CI | |
| Clinically relevant depression symptoms (CESD-10 score ≥10 or GDS-15 score ≥5) | ||||||||
| No. of cases | 7,292 | 212 | ||||||
| Person-years of follow-up | 299,386 | 4,791 | ||||||
| Crude incidence (per 100,000 person-years) | 2,436 | 4,425 | ||||||
| Age-adjusted RR | 1 | Reference | 1.71* | 1.49, 1.96 | ||||
| Multivariate RRb | 1 | Reference | 1.53* | 1.33, 1.76 | ||||
| CESD-10 score | ||||||||
| Age-adjusted mean score | 4.97 | 4.94, 5.00 | 6.21* | 5.98, 6.44 | ||||
| No. of cases with CESD-10 score ≥10 | 5,775 | 172 | ||||||
| Age-adjusted RR | 1 | Reference | 1.73* | 1.49, 2.02 | ||||
| Multivariate RRb | 1 | Reference | 1.52* | 1.30, 1.77 | ||||
| GDS-15 score | ||||||||
| Age-adjusted mean score | 1.36 | 1.34, 1.38 | 1.91 | 1.78, 2.04 | ||||
| No. of cases with GDS-15 score ≥5 | 2,323 | 71 | ||||||
| Age-adjusted RR | 1 | Reference | 1.82* | 1.44, 2.30 | ||||
| Multivariate RRb | 1 | Reference | 1.70* | 1.34, 2.15 | ||||
Abbreviations: CESD-10, 10-item version of the Center for Epidemiologic Studies Depression Scale; CI, confidence interval; GDS-15, 15-item version of the Geriatric Depression Scale; RLS, restless legs syndrome; RR, relative risk.
* P < 0.0001.
a Depression symptoms were evaluated with the CESD-10 in 2004 and the GDS-15 in 2008. Clinically relevant depression symptoms were defined as CESD-10 score ≥10 or GDS-15 score ≥5.
b Adjusted for age (years), body mass index (weight (kg)/height (m)2; continuous), ethnicity (Caucasian or other), smoking status (never, past, or current smoker), marital status (married/in a partnership, widowed, or separated/divorced/single), solitary living status (yes/no; binary), regular involvement in a social or community group (yes/no; binary), menopausal status (premenopausal or postmenopausal), menopausal hormone use (never, past, or current user), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, or ≥15 g/day), physical activity (quintiles), the presence of diabetes, arthritis, stroke, myocardial infarction, angina, cancer, hypertension, high cholesterol level, or Parkinson's disease (yes/no), use of iron-specific supplements, sleep duration (≤5, 6, 7, 8, or ≥9 hours per 24-hour period), and snoring frequency (every night, most nights, a few nights per week, occasionally, or almost never).
We conducted several sensitivity analyses and obtained similar results. The multivariable-adjusted relative risk was 1.48 (95% CI: 0.99, 2.23) for clinical depression and 1.52 (95% CI: 1.29, 1.79) for clinically relevant depression symptoms after excluding women with histories of diabetes and arthritis, two common conditions that mimic RLS. The multivariable-adjusted relative risk was 1.53 (95% CI: 1.08, 2.16) for clinical depression and 1.54 (95% CI: 1.34, 1.76) for clinically relevant depression symptoms after excluding women who had ever been diagnosed with Parkinson's disease, and relative risks were 1.51 (95% CI: 1.02, 2.23) and 1.53 (95% CI: 1.30, 1.80), respectively, after excluding women who had ever been diagnosed with cardiovascular diseases prior to the endpoint. When we excluded the incident depression cases diagnosed during the period 2002–2004, the multivariable-adjusted relative risk was 1.40 (95% CI: 0.83, 2.33) for clinical depression and 1.70 (95% CI: 1.34, 2.15) for GDS-15 score ≥ 5.
When different definitions of depression were applied, the statistical significance of the association between RLS and depression was unchanged. The multivariable-adjusted relative risks ranged from 1.47 to 1.88 (P < 0.0001 for all) (Table 4) after adjustment for age, lifestyle factors, chronic conditions, sleep duration, and other covariates.
Table 4.
Adjusted Relative Risk of Depression According to Baseline Restless Legs Syndrome Status, Using Different Definitions of Depression, Nurses' Health Study, 2002–2008
| Definition of Depression |
No RLS |
RLS |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | Incidence | RR | 95% CI | No. | Incidence | RR | 95% CI | |
| Physician-diagnosed depression | ||||||||
| No. of cases | 1,626 | 44 | ||||||
| Person-years of follow-up | 297,704 | 4,747 | ||||||
| Crude incidence (per 100,000 person-years) | 546 | 927 | ||||||
| Age-adjusted RR | 1 | Reference | 1.65** | 1.22, 2.23 | ||||
| Multivariate-adjusted RRa | 1 | Reference | 1.47* | 1.09, 1.99 | ||||
| Either physician-diagnosed depression or regular use of antidepressant medication | ||||||||
| No. of cases | 3,860 | 131 | ||||||
| Person-years of follow-up | 295,493 | 4,662 | ||||||
| Crude incidence (per 100,000 person-years) | 1,306 | 2,810 | ||||||
| Age-adjusted RR | 1 | Reference | 2.13*** | 1.79, 2.53 | ||||
| Multivariate-adjusted RRa | 1 | Reference | 1.88*** | 1.57, 2.23 | ||||
| Physician-diagnosed depression, CESD-10 score ≥10, or GDS-15 score ≥5 | ||||||||
| No. of cases | 8,469 | 239 | ||||||
| Person-years of follow-up | 295,993 | 4,676 | ||||||
| Crude incidence (per 100,000 person-years) | 2,861 | 5,111 | ||||||
| Age-adjusted RR | 1 | Reference | 1.71*** | 1.50, 1.94 | ||||
| Multivariate-adjusted RRa | 1 | Reference | 1.55*** | 1.36, 1.76 | ||||
| Physician-diagnosed depression, regular antidepressant medication use, CESD-10 score ≥10, or GDS-15 score ≥5 | ||||||||
| No. of cases | 10,057 | 299 | ||||||
| Person-years of follow-up | 295,993 | 4,676 | ||||||
| Crude incidence (per 100,000 person-years) | 3,398 | 6,394 | ||||||
| Age-adjusted RR | 1 | Reference | 1.83*** | 1.63, 2.05 | ||||
| Multivariate-adjusted RRa | 1 | Reference | 1.65*** | 1.47, 1.85 | ||||
Abbreviations: CI, confidence interval; RLS, restless legs syndrome; RR, relative risk.
* P < 0.01; **P < 0.001; ***P < 0.0001.
a Adjusted for age (years), body mass index (weight (kg)/height (m)2; continuous), ethnicity (Caucasian or other), smoking status (never, past, or current smoker), marital status (married/in a partnership, widowed, or separated/divorced/single), solitary living status (yes/no; binary), regular involvement in a social or community group (yes/no; binary), menopausal status (premenopausal or postmenopausal), menopausal hormone use (never, past, or current user), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, or ≥15 g/day), physical activity (quintiles), the presence of diabetes, arthritis, stroke, myocardial infarction, angina, cancer, hypertension, high cholesterol level, or Parkinson's disease (yes/no), use of iron-specific supplements, sleep duration (≤5, 6, 7, 8, or ≥9 hours per 24-hour period), and snoring frequency (every night, most nights, a few nights per week, occasionally, or almost never).
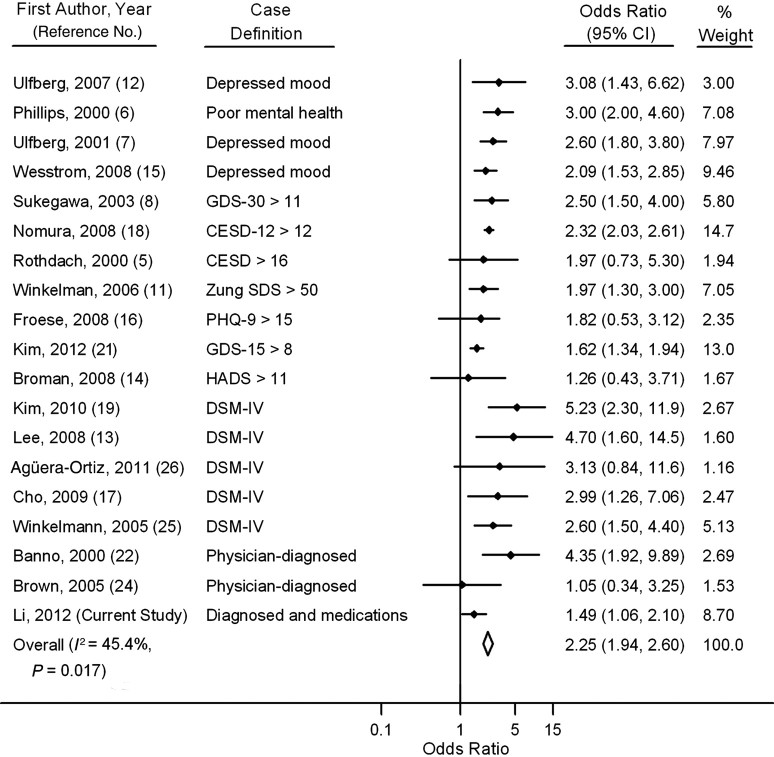
Finally, we conducted a meta-analysis including all published case-control and cross-sectional studies of associations between RLS and clinical depression and/or depressive symptoms. Comparing women with RLS with those without RLS, the pooled odds ratio was 2.32 (95% CI: 2.01, 2.69; P-heterogeneity = 0.04) without inclusion of the present study and 2.25 (95% CI: 1.94, 2.60; P-heterogeneity = 0.02) with the present study included (Figure 1). The pooled odds ratios were similar for community-based and clinical studies (data not shown). To the best of our knowledge, our study provides the first evidence that the presence of RLS is associated with the future risk of developing depression. The funnel plot (Web Figure 2) and results based on Begg's test did not support the existence of publication bias (P = 0.6).
Figure 1.
Associations between restless legs syndrome and depression in previously published studies, in the Nurses' Health Study cohort (current study; 2002–2008), and in a pooled meta-analysis of Nurses' Health Study participants (diamond; 2002–2008). Only crude odds ratios were available from the studies by Sukegawa et al. (8), Ulfberg et al. (12), Kim et al. (19), Banno et al. (22), Brown et al. (24), and Agüera-Ortiz et al. (26), and only pooled odds ratios were available from the studies by Winkelman et al. (11) and Broman et al. (14), who reported odds ratios for depression according to the severity of restless legs syndrome. Case definitions of depression included 1) self-reported symptoms of depression (depressed mood or poor mental health) assessed by means of a single question or with an unclear justification for the cutoff score; 2) self-reported symptoms of depression on a scale with a validated cutoff score, such as the 15- or 30-item version of the geriatric depression scale (GDS), the regular or 12-item Center for Epidemiologic Studies Depression scale (CESD), the Zung Self-rating Depression Scale (Zung SDS), the 9-item version of the Personal Health Questionnaire (PHQ), or the Hospital Anxiety and Depression Scale (HADS); 3) depression diagnosed according to the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV); 4) physician- or clinician-diagnosed depression; and 5) physician- or clinician-diagnosed depression and use of antidepressant medication. Based on the Q statistic, P-heterogeneity = 0.017. Bars, 95% confidence interval (CI).
DISCUSSION
In this prospective study, we observed that women with RLS had a higher risk of developing both clinical depression and clinically relevant depressive symptoms. This association appeared to be independent of age, smoking status, physical activity, body mass index, history of several chronic conditions, sleep duration, and snoring.
There are several potential mechanisms that may underlie the association between RLS and risk of depression. Firstly, dopaminergic hypofunction is a common pathophysiologic thread linking RLS and depression, as well as the more severe hypodopaminergic condition of Parkinson's disease. RLS may be related to postsynaptic desensitization that overcompensates during the circadian low point of dopaminergic activity in the evening and night (1). Dopaminergic mesolimbic and mesocortical systems are involved in anhedonia and lack of motivation, two core symptoms of depression (37). In addition, RLS may increase risk of Parkinson's disease (38), and depression is a common nonmotor Parkinson's disease symptom that can antedate the diagnosis of Parkinson's disease (39); thus, a subset of participants with RLS and incident depression may be on an ultimate path toward Parkinson's disease. However, the hypothesized role of the dopaminergic system in the observed RLS-depression relation is complex and remains to be elucidated. Secondly, a common genetic cause for both RLS and depression could be a potential reason. In one recent study, Puschmann et al. (40) observed a family of 5 generations; the 43 individuals displayed signs and symptoms of Parkinson's disease, RLS, essential tremor, or depression, and a considerable number of the affected persons had features of more than 1 of these disorders, implying a potential genetic cause within the family.
The third potential mechanistic link between RLS and depression may be the presence of clinical or subclinical vascular disease: RLS has been related to increased cardiovascular disease risk (11, 41, 42), and vascular factors are associated with clinical presentations of depression in older persons (43). However, our results were unaffected by adjustment for these conditions, making this explanation unlikely. The fourth potential explanation is that because RLS is a bothersome chronic condition (44), persons with RLS may have a distinctly impaired quality of life (1, 2). For example, it is common for persons with RLS to complain of fatigue, disturbed sleep, diminished concentration, and psychomotor agitation (4). Thus, symptoms accompanying RLS may in turn lead to depressive symptoms and/or clinical depression in RLS sufferers. Furthermore, insomnia itself has been well established as a risk factor for incident depression (45), and the sleep disruption common in RLS may predispose RLS patients to depression. In our study, adjustment for sleep duration and frequent snoring did attenuate the association between RLS and depression, implying that the RLS-depression association could be partly explained by the sleep disturbance. We cannot exclude the possibility that women who have been diagnosed with RLS may visit physicians more frequently than others and thus are more likely to have their depression ascertained. Persons with RLS may also be more likely to be diagnosed with depression by physicians attentive to the mental health consequences of chronic illnesses. However, we observed a similar significant association between RLS and increased depression risk in secondary analyses in which different diagnostic criteria for depression were used. Further, when we used the self-reported CESD-10 or GDS-15 score as the outcome, a similar result was seen. The similar significant results obtained using different definitions of depression suggest that our findings were robust. In order to reduce the bias of possible reverse causation, we reexamined the association between RLS and depression after excluding the incident cases occurring between 2002 and 2004, and found similar results.
Our findings are consistent with previous retrospective and cross-sectional observations (5–26). However, the magnitude of the association between RLS and risk of depression observed in the current prospective analysis was smaller than the concurrent odds ratios in previous cross-sectional or retrospective studies, as was shown in the meta-analysis (relative risk = 1.5 vs. odds ratio = 2.3). This is consistent with the notion that retrospective studies and cross-sectional observations could lead to overestimation of the association (46). It has been suggested that depression or antidepressant medication use may increase the risk of RLS (22, 47). Using the prospective study design, we minimized the potential for reverse causation, although we cannot dismiss its existence altogether.
To our knowledge, this was the first prospective study to investigate the association between RLS and risk of incident depression. A key strength of our prospective approach is the ability to avoid recall and selection biases. Another strength is that we collected detailed information on lifestyle and chronic conditions using validated questionnaires, which enabled us to control for the potential confounders that may be associated with both RLS and depression, such as obesity, physical activity, and chronic diseases. However, because of the observational nature of our study design, we cannot exclude the possibility of residual confounding. Thus, we support interventional studies to assess whether treated RLS patients develop less depression than placebo-treated controls.
One limitation is that the RLS assessment in the current study was based on established physician diagnoses. The prevalence of RLS in US women ranges from 6.4% to 11.2%, based on the International RLS Study Group criteria (10, 11, 48, 49). Thus, the RLS prevalence in this study (2.4% before excluding women with depression or depression symptoms at baseline and 1.7% in the current sample) demonstrates the underdiagnosis of RLS. In a large-scale US study published in 2005 that included 16,202 adults aged ≥18 years, only 6.2% of the RLS sufferers (n = 337) reported having received a diagnosis of RLS (49). Similar results were seen in another survey conducted among 6 Western European countries: Only 9% of RLS cases (n = 365) had been diagnosed previously (50). Thus, although RLS was probably underreported in the present study, we could not interview individual patients, because that approach would have been unrealistic given our sample size. Misclassification of depression is another concern because of the possibility of low recognition of depression by physicians, undertreatment of depression, and use of antidepressant medication for indications other than depression. We tried to maximize the specificity of the case definition, accepting as incident cases of depression only women who reported both a diagnosis of depression and the use of antidepressants. This approach increased the specificity of diagnoses but decreased the sensitivity, which would have been more likely to weaken the observed associations. However, as long as the probability of correctly classifying women with an incident case of depression is independent of their diagnosis of RLS (nondifferential misclassification of outcome between women with RLS and women without RLS), the low sensitivity of this strict case definition should not bias relative risk estimates (51).
In conclusion, in this large-scale prospective study, we found that the presence of physician-diagnosed RLS was associated with a higher risk of developing depression in women during 6 years of follow-up. Further studies using more precise diagnostic strategies for identifying RLS and depression in the community are warranted.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Yanping Li, Olivia Ifeoma Okereke, Alberto Ascherio, Xiang Gao); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Fariba Mirzaei, Eilis J. O'Reilly, Alberto Ascherio, Xiang Gao); Division of Sleep Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (John Winkelman, Atul Malhotra); Department of Psychiatry, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Olivia Ifeoma Okereke); and Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Alberto Ascherio).
This study was supported by grant R01 NS062879-01A2 from the National Institute of Neurological Disorders and Stroke, grant P01 CA87969 from the National Cancer Institute, and grant 5048070-01 from the National Alliance for Research on Schizophrenia and Depression.
None of the sponsors participated in the design of study or in the collection, analysis, or interpretation of the data. Drs. Yanping Li and Xiang Gao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: none declared.
REFERENCES
- 1.Salas RE, Gamaldo CE, Allen RP. Update in restless legs syndrome. Curr Opin Neurol. 2010;23(4):401–406. doi: 10.1097/WCO.0b013e32833bcdd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Earley CJ, Silber MH. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med. 2010;11(9):807–815. doi: 10.1016/j.sleep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Hornyak M. Depressive disorders in restless legs syndrome: epidemiology, pathophysiology and management. CNS Drugs. 2010;24(2):89–98. doi: 10.2165/11317500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Picchietti D, Winkelman JW. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep. 2005;28(7):891–898. [PubMed] [Google Scholar]
- 5.Rothdach AJ, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000;54(5):1064–1068. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 6.Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 7.Ulfberg J, Nyström B, Carter N, et al. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16(6):1159–1163. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 8.Sukegawa T, Itoga M, Seno H, et al. Sleep disturbances and depression in the elderly in Japan. Psychiatry Clin Neurosci. 2003;57(3):265–270. doi: 10.1046/j.1440-1819.2003.01115.x. [DOI] [PubMed] [Google Scholar]
- 9.Sevim S, Dogu O, Kaleagasi H, et al. Correlation of anxiety and depression symptoms in patients with restless legs syndrome: a population based survey. J Neurol Neurosurg Psychiatry. 2004;75(2):226–230. [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips B, Hening W, Britz P, et al. Prevalence, correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129(1):76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 11.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7(7):545–552. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Ulfberg J, Bjorvatn B, Leissner L, et al. Comorbidity in restless legs syndrome among a sample of Swedish adults. Nordic RLS Study Group. Sleep Med. 2007;8(7-8):768–772. doi: 10.1016/j.sleep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Lee HB, Hening WA, Allen RP, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20(1):101–105. doi: 10.1176/jnp.2008.20.1.101. [DOI] [PubMed] [Google Scholar]
- 14.Broman JE, Mallon L, Hetta J. Restless legs syndrome and its relationship with insomnia symptoms and daytime distress: epidemiological survey in Sweden. Psychiatry Clin Neurosci. 2008;62(4):472–475. doi: 10.1111/j.1440-1819.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 15.Wesstrom J, Nilsson S, Sundstrom-Poromaa I, et al. Restless legs syndrome among women: prevalence, co-morbidity and possible relationship to menopause. Climacteric. 2008;11(5):422–428. doi: 10.1080/13697130802359683. [DOI] [PubMed] [Google Scholar]
- 16.Froese CL, Butt A, Mulgrew A, et al. Depression and sleep-related symptoms in an adult, indigenous, North American population. J Clin Sleep Med. 2008;4(4):356–361. [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SJ, Hong JP, Hahm BJ, et al. Restless legs syndrome in a community sample of Korean adults: prevalence, impact on quality of life, and association with DSM-IV psychiatric disorders. Sleep. 2009;32(8):1069–1076. [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura T, Inoue Y, Kusumi M, et al. Prevalence of restless legs syndrome in a rural community in Japan. Mov Disord. 2008;23(16):2363–2369. doi: 10.1002/mds.22274. [DOI] [PubMed] [Google Scholar]
- 19.Kim KW, Yoon IY, Chung S, et al. Prevalence, comorbidities and risk factors of restless legs syndrome in the Korean elderly population—results from the Korean Longitudinal Study on Health and Aging. J Sleep Res. 2010;19(1):87–92. doi: 10.1111/j.1365-2869.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 20.Celle S, Roche F, Kerleroux J, et al. Prevalence and clinical correlates of restless legs syndrome in an elderly French population: the Synapse Study. J Gerontol A Biol Sci Med Sci. 2010;65(2):167–173. doi: 10.1093/gerona/glp161. [DOI] [PubMed] [Google Scholar]
- 21.Kim WH, Kim BS, Kim SK, et al. Restless legs syndrome in older people: a community-based study on its prevalence and association with major depressive disorder in older Korean adults. Int J Geriatr Psychiatry. 2012;27(6):565–572. doi: 10.1002/gps.2754. [DOI] [PubMed] [Google Scholar]
- 22.Banno K, Delaive K, Walld R, et al. Restless legs syndrome in 218 patients: associated disorders. Sleep Med. 2000;1(3):221–229. doi: 10.1016/s1389-9457(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 23.Saletu B, Anderer P, Saletu M, et al. EEG mapping, psychometric, and polysomnographic studies in restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) patients as compared with normal controls. ((suppl)).Sleep Med. 2002;3:S35–S42. doi: 10.1016/s1389-9457(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 24.Brown LK, Dedrick DL, Doggett JW, et al. Antidepressant medication use and restless legs syndrome in patients presenting with insomnia. Sleep Med. 2005;6(5):443–450. doi: 10.1016/j.sleep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Winkelmann J, Prager M, Lieb R, et al. “Anxietas tibiarum”: depression and anxiety disorders in patients with restless legs syndrome. J Neurol. 2005;252(1):67–71. doi: 10.1007/s00415-005-0604-7. [DOI] [PubMed] [Google Scholar]
- 26.Agüera-Ortiz L, Perez MI, Osorio RS, et al. Prevalence and clinical correlates of restless legs syndrome among psychogeriatric patients. Int J Geriatr Psychiatry. 2011;26(12):1252–1259. doi: 10.1002/gps.2674. [DOI] [PubMed] [Google Scholar]
- 27.Happe S, Reese JP, Stiasny-Kolster K, et al. Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med. 2009;10(3):295–305. doi: 10.1016/j.sleep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 28.König HH, Bernert S, Angermeyer MC. Health status of the German population: results of a representative survey using the EuroQol questionnaire [in German] Gesundheitswesen. 2005;67(3):173–182. doi: 10.1055/s-2005-857991. [DOI] [PubMed] [Google Scholar]
- 29.Lucas M, Mirzaei F, O'Reilly EJ, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011;93(6):1337–1343. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 31.Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Inc; 2000. [Google Scholar]
- 32.Berwick DM, Murphy JM, Goldman PA, et al. Performance of a five-item mental health screening test. Med Care. 1991;29(2):169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 34.Sheikh JI, Yesavage JA. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 35.Lyness JM, Noel TK, Cox C, et al. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157(4):449–454. [PubMed] [Google Scholar]
- 36.Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-III-R (SCID) New York, NY: New York State Psychiatric Institute; 1986. [Google Scholar]
- 37.Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- 38.Gao X, Schwarzschild MA, O'Reilly EJ, et al. Restless legs syndrome and Parkinson's disease in men. Mov Disord. 2010;25(15):2654–2657. doi: 10.1002/mds.23256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poewe W. Non-motor symptoms in Parkinson's disease. ((suppl 1)).Eur J Neurol. 2008;15:14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 40.Puschmann A, Pfeiffer RF, Stoessl AJ, et al. A family with Parkinsonism, essential tremor, restless legs syndrome, and depression. Neurology. 2011;76(19):1623–1630. doi: 10.1212/WNL.0b013e318219fb42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkelman JW, Shahar E, Sharief I, et al. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 42.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32(5):589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60(12):1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Hening WA, Allen RP, Chaudhuri KR, et al. Clinical significance of RLS. ((suppl 18)).Mov Disord. 2007;22:S395–S400. doi: 10.1002/mds.21665. [DOI] [PubMed] [Google Scholar]
- 45.Jaussent I, Bouyer J, Ancelin ML, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34(8):1103–1110. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willett W. Nutritional Epidemiology. 2nd. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 47.Page RL, II, Ruscin JM, Bainbridge JL, et al. Restless legs syndrome induced by escitalopram: case report and review of the literature. Pharmacotherapy. 2008;28(2):271–280. doi: 10.1592/phco.28.2.271. [DOI] [PubMed] [Google Scholar]
- 48.Gao X, Schwarzschild MA, Wang H, et al. Obesity and restless legs syndrome in men and women. Neurology. 2009;72(14):1255–1261. doi: 10.1212/01.wnl.0000345673.35676.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 50.Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in Western Europe: prevalence and characteristics. Sleep Med. 2010;11(1):31–37. doi: 10.1016/j.sleep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.