Abstract
Background and Purpose
To determine the incidence of neurovascular events as late complications in pediatric brain tumor patients and to evaluate radiation as a risk factor.
Methods
Patients were ascertained using the tumor database of a pediatric tertiary care center. Included patients had a primary brain tumor, age birth to 21 years, initial treatment 1/1/93-12/31/02, and at least two visits with Neuro-Oncology. Radiation exposure included: whole brain, whole brain plus a focal boost, or focal brain. The primary outcome was stroke or transient ischemic attack (TIA).
Results
Of 431 subjects, 14 had 19 events of stroke or TIA over a median follow-up of 6.3 years. The incidence rate was 548/100,000 person-years. Overall, 61.5% of subjects received radiation, including 13/14 subjects with events. Median time from first radiation to first event was 4.9 years. The stroke/TIA hazard ratio for any brain irradiation was 8.0 (95% CI:1.05-62, p=0.045); for Circle of Willis (COW) radiation was 9.0 (95% CI:1.2-70, p=0.035); and for focal non-COW radiation was 3.4 (95% CI:0.21-55, p=0.38).
Conclusions
The incidence of neurovascular events in this population is 100-fold higher than in the general pediatric population and cranial irradiation is an important risk factor. By defining the incidence of this late effect, physicians are better able to counsel parents regarding treatment, monitor patients at risk, and target a population for primary stroke prevention in future studies.
Keywords: childhood brain tumors, stroke, treatment-related stroke
INTRODUCTION
Radiotherapy, an effective therapeutic modality for the treatment of many pediatric brain tumors poses significant risks, particularly to the developing brain (1-3). Cranial irradiation causes late toxicities including neuroendocrine perturbations (4), cognitive deficits (1, 2), cavernous malformations (5), small vessel occlusive disease (1, 6-8), vasculopathy, and stroke (6, 9-11). The population at risk for these complications continues to grow, because brain tumors are the most common solid tumor of childhood and length of survival is increasing (5-year survival of 72%) (12). With improved long-term survival, understanding the late effects of these treatments becomes paramount.
Cranial irradiation-induced late vasculopathy is well documented in the literature, with 47 case reports (10, 13-19) over the past 30 years, and six case series/cohort studies (6, 20-24). Few studies have determined incidence, interval to symptoms, or risk factors compared with a control group. The largest cohort study, from Bowers et al. (21), estimated the incidence and relative risk (RR) of stroke compared with sibling controls in brain tumor survivors (rate:267.6/100,000, RR:29). While this was a landmark study, it did not differentiate between peri-operative and late stroke and relied on self-report of stroke. Brain tumor survivors are at risk for stroke mimics (including migraine, seizure, postictal paralysis, acute demyelinating encephalomyelitis, radiation necrosis, etc.) (25); thus, estimates based on self-reports may inaccurately estimate incidence in this group.
In contrast, Ullrich et al. used magnetic resonance imaging (MRI) to investigate vasculopathy incidence in children with primary brain tumors treated with radiation (22). Because all subjects received radiation, it was not possible to investigate radiation as a risk factor. Two cohort studies reported the physician confirmed stroke incidence in both irradiated and non-irradiated brain tumor survivors (23, 24). Defining the true incidence of stroke in these cohorts may have been hampered by a short follow-up duration (mean 4.6 years), the inclusion of subjects with neurofibromatosis type 1 (NF1) who have a known increased risk of spontaneous and radiation-induced neurovascular disease and stroke, and using both ischemic and hemorrhagic strokes as outcome measures despite their distinctive pathogeneses.
The primary aim of this study was to determine the incidence of physician-diagnosed stroke or TIA as a late complication in pediatric brain tumor patients at a tertiary care center. The secondary aim was to evaluate cranial irradiation as a risk factor for stroke or TIA; we hypothesized that children receiving brain radiation have a higher risk of neurovascular events than non-irradiated brain tumor patients and that radiation to the COW confers the highest risk.
METHODS
For this retrospective cohort study, subjects were identified using the tumor registry of the Division of Oncology at The Children’s Hospital of Philadelphia (CHOP). Potential subjects were under 22 years of age at diagnosis of brain tumor, and started treatment (chemotherapy, radiation or surgery [including biopsy]) between January 1, 1993 and December 31, 2002 at CHOP. Excluded subjects had: fewer than two follow-up visits in the Neuro-Oncology clinic, a disease known to be associated with vascular abnormality (e.g. NF1) (26, 27), intra-operative or peri-operative (within 30 days of surgery) stroke remote from the surgical bed, or a neurovascular event in the setting of disease progression. Patients with peri-/intra-operative strokes within the surgery bed were included, but these strokes were not counted toward the total event number.
The subjects’ medical records were searched for: demographic information (age, gender, race), date of diagnosis, tumor pathology, tumor location, dates and location of tumor-directed surgery, date of radiation, chemotherapy administration, date of most recent imaging study, radiation dose and fields (focal, whole brain, or craniospinal), occurrence of stroke or stroke-like symptoms and date of occurrence, presence or absence of relapse and subsequent therapy, and date of death or last visit. All brain MRI reports for eligible subjects were reviewed for the presence of infarction. All brain MRI images for each patient with stroke symptoms were re-reviewed by one neuroradiologist to verify the presence or absence of diffusion-weighted imaging abnormalities. Magnetic resonance angiography (MRA), when available, was reviewed for evidence of large-vessel vasculopathy.
CHOP Institutional review board (IRB) approval with waiver of informed consent was obtained prior to study initiation.
Outcome was defined as a neurovascular event, including ischemic stroke or TIA. Stroke was defined as an acute neurological syndrome with deficits conforming to a vascular territory, and findings on neuroimaging of an acute ischemic infarct corresponding to the clinical deficit. TIA was similarly defined, but lasted less than 24 hours and MRI revealed no acute ischemia. Three independent investigators assessed possible events before reaching consensus.
To estimate the dose of radiation to the COW, the radiation field was categorized one of three ways: whole brain radiation (dose to COW equals whole brain dose); focal radiation to areas of brain not including the COW (e.g. parietal and occipital lobes), in which the dose to COW is zero; and focal radiation to areas including the COW (e.g. hypothalamus, thalamus), in which the dose to COW equals the focal dose. Sites (e.g. temporal and frontal lobes) for which the specific COW dose depended on the specific tumor location within that region were classified in the focal non-COW group. The COW was defined as the internal carotid, middle cerebral, anterior cerebral, anterior communicating, posterior communicating, posterior cerebral, and basilar arteries.
Statistical Analysis
To determine the effect of radiation group exposure (e.g. no radiation, radiation involving the COW, and radiation not involving the COW), the following analyses were performed: (1) dichotomous exposure, three group analysis with chi squared or Fisher’s exact test; (2) continuous exposure, three group analysis with ANOVA, and parametric analysis on radiation dose. Incidence rates were calculated based on the first event only.
Cox proportional hazard models were used to estimate the hazard ratios (HRs) for the primary outcome, neurovascular events (TIA or stroke) for those patients receiving radiation compared with those who did not, with adjustment for potential confounders including age at diagnosis, tumor type, and chemotherapy. Censoring criteria were death, first stroke/TIA, or last documented visit. HRs were further calculated for dose of radiation and dose of radiation to the COW. Statistical tests were performed using SPSS 17.0 and STATA SE 10.0. To identify independent risk factors, a multivariate analysis was performed using a Cox proportional hazards model in a stepwise manner, removing variables based on a threshold p value of 0.20.
RESULTS
Of 649 subjects who met inclusion criteria, 174 (26.8%) were ineligible: 83 with NF1, 58 with one visit, 24 received no treatment, eight had extensive intra-operative or peri-operative stroke remote from the surgical bed, and one had TIA in the setting of disease progression. Charts were unavailable for 44 patients (6.8%). This left 431 (66.4%) eligible patients for analysis.
Two hundred sixty-five subjects (61.5%) received cranial irradiation and 323 underwent surgery, with 79 undergoing surgery in the region of the COW (Table 1). Tumor types were distributed as expected for this population (Supplementary Table 1). Median time-to-death was 1.1 years (S.D.:2.6 years) with 311 (72.2%) subjects remaining alive.
Table 1.
Demographic and clinical characteristics, overall and by treatment group
|
Radiation to COW N = 221 |
Non-COW Radiation N = 44 |
No Radiation N = 161 |
Total N = 426* |
Pvalue † | |
|---|---|---|---|---|---|
| Female | 94 (42.5%) | 19 (43%) | 71 (44%) | 186(43%) | P= .97 |
| Ethnicity | P=.07 | ||||
| Caucasian: | 165 | 26 | 124 | 315 (73.8%) | |
| African American: | 32 | 13 | 15 | 60 (14.1%) | |
| Hispanic: | 10 | 3 | 5 | 18 (4.2%) | |
| Asian: | 4 | 0 | 6 | 10 (2.3%) | |
| Other: | 6 | 1 | 6 | 13 (3%) | |
| Unknown: | 4 | 1 | 5 | 10 (2.3%) | |
| Median age at diagnosis (SD) years |
8.9 (4.7) | 10.2 (5.5) | 5.4 (5.9) | 7.6 (5.3) | P<.001 |
| Median Age at Radiation (SD) years |
9.1 (4.4) | 10.5 (5.3) | P =.06 | ||
| Median Radiation Dose Gy (IQR) |
55.8 (54- 59.4) |
54 (54-55.8) | P=.02 | ||
| Median Follow up (SD) years |
6.3 (4.4) | 7.3 (4.5) | 6.7 (3.8) | 6.5 (4.2) | P=.3 |
COW:Circle of Willis, Gy:gray, SD:standard deviation, IQR:interquartile range
P-value comparing exposure groups:Radiation to COW, Non-COW radiation, and no radiation
Radiation information was missing for 5 of 431 subjects
There were a total of 19 neurovascular events (8 strokes, 11 TIAs) in 14 subjects (Table 2); thirteen of whom received radiation, 12 to the COW. Median time to first event was 4.9 years (range 32 days - 12.9 years).
Table 2.
Patient Events
| ID | Event | Description | Gender | Diagnosis | COW Dose |
Boost & CS Dose |
Age at XRT (y) |
Time from XRT (y) |
|---|---|---|---|---|---|---|---|---|
| 1 | Stroke | Hemiparesis and dysarthria | Male | Medulloblastoma | 1800 | PF:5580 CS:1800 |
11.5y | 5.9 |
| 2 | 1. TIA | Left hand and mouth anesthesia | Female | Pineal PNET | 5400 | Pineal: | 14.5 | 1. 5.4 |
| 2. Stroke |
Left hemiparesis | 5400 | 2. 7.1 | |||||
| 3. Stroke |
Hand and mouth anesthesia | CS:3600 | 3. 8.3 | |||||
| 3 | Stroke | Sinus venous thrombosis | Male | Suprasellar choriocarcinoma |
5400 | PF:5400 CS:3600 |
16.2 | 0.5 |
| 4 | 1. Stroke |
Right hand paresis | Female | Suprasellar LGG | 5400 | SS:5400 | 14.5 | 1. 5.2 |
| 2. TIAs | Right hand anesthesia/ paresis and aphasia |
CS:0 | 2. 5.2 | |||||
| 5 | Stroke | Dysarthria, lateral gaze paresis, bifacial paresis and quadriparesis |
Female | Pineal ATRT | 5400 | Pineal:5400 CS:0 |
4.2 | 5.9 |
| 6 | 1. Stroke |
Expressive aphasia and left arm weakness |
Female | Hypothalamic LGG |
5400 | SS:5400 | 9.9 | 1. 0.09 |
| 2. TIA | Dizziness, right ptosis and expressive aphasia |
CS:0 | 2. 1.6 | |||||
| 7 | 1. TIA | Left paresis, and dysarthria | Male | 4th ventricle LGG | 3600 | PF:5580 | 14.8 | 1. 5.5 |
| 2. Stroke |
Left hemianesthesia of face, tongue and fingers |
CS:3600 | 2. 11.2 | |||||
| 8 | TIA | Right hand anesthesia and expressive aphasia |
Male | Pineal germinoma | 5040 | Pineal:5040 CS:1800 |
10.7 | 4.9 |
| 9 | TIA | Right hemianesthesia and paresis, with aphasia |
Male | Medulloblastoma | Unknown | 13 | 1.7 | |
| 10 | Multiple e TIAs |
Expressive aphasia | Female | Temporal PNET | 0 | Temp:5580 CS:0 |
19.8 | 3.4 |
| 11 | TIA | Facial droop and dysarthria | Female | Medulloblastoma | 2340 | PF:5580 CS:2340 |
3.8 | 0.8 |
| 12 | TIA | Right hand/arm anesthesia and paresis, and aphasia |
Male | Medulloblastoma | 3600 | PF:5580 CS:3600 |
14.9 | 2.4 |
| 13 | TIA | Right arm/leg anesthesia | Female | Right hemisphere LGG |
0 | 0 | ||
| 14 | TIA | Left upper extremity anesthesia | Male | Suprasellar germinoma |
5040 | SS:5040 CS:3600 |
15.8 | 12.9 |
COW:circle of Willis, CS:Craniospinal, XRT:Radiotherapy, Y:years, PF:posterior fossa, TIA:transient ischemic attack, PNET:primitive neuroectodermal tumor, LGG:low-grade glioma, ATRT:atypical teratoid rhabdoid tumor, SS:suprasellar, Temp:temporal lobe
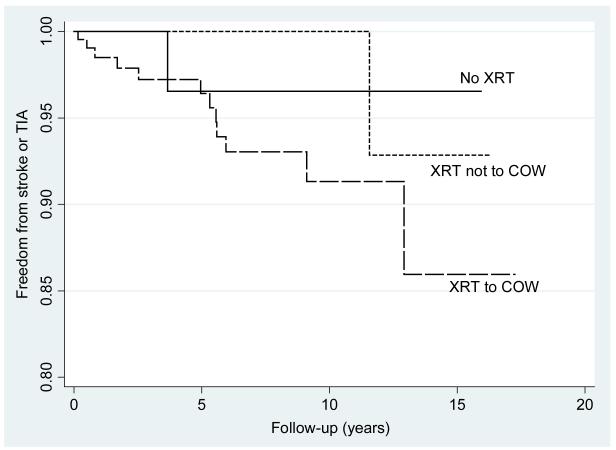
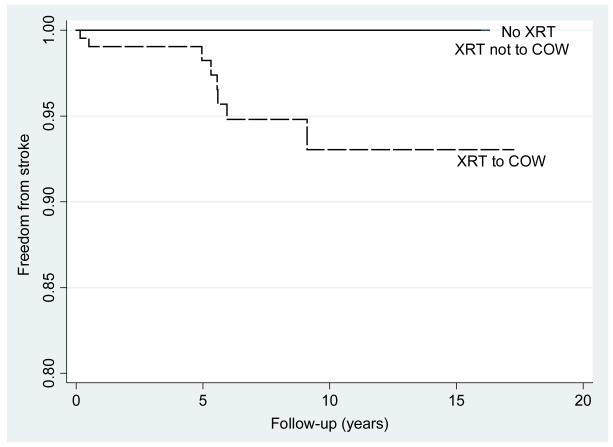
Using only the first event per patient, the overall incidence rate of neurovascular events in this population was 548 per 100,000 (95%CI:324-924/100,000), with 102/100,000 (95%CI:14-721) in the non-radiated group, 347/100,000 (95%CI:49-2461) in those receiving radiation to non-COW areas and 939/100,000 (95%CI:533-16540) in those receiving radiation to the COW (Figure 1a). The incidence rate of stroke alone (i.e. excluding TIAs) was 626/100,000 (95%CI:313-1252,) in those subjects receiving radiation to the COW and zero in subjects receiving either non-COW radiation or no radiation (p≤0.001) (Figure 1b).
Figure 1a.
Nelson-Aalen estimate of stroke or transient ischemic attack (TIA) by radiation (XRT) group: XRT to circle of Willis (COW) (P=0.035), XRT not including the COW (p=0.38), no XRT.
Figure 1b.
Nelson-Aalen estimate of stroke by radiation (XRT) group: XRT to circle of Willis (COW), XRT not including the COW, no XRT. P≤0.001
The HR of stroke or TIA after any cranial irradiation was 8.0 (p=0.045). COW radiation conferred a greater risk (HR=9.0, p=0.035) than focal, non-COW radiation (HR 3.4, p=0.38). Neither surgery (p=0.12) nor chemotherapy (p=0.15) showed a significant association with stroke/TIA in univariate analysis (Tables 3 and 4). However, treatment with chemotherapy was more common in patients who were radiated (p<0.001). Furthermore, radiation plus chemotherapy enhanced stroke rates (p<0.001 for interaction) (Table 4). Stratifying on location of surgery demonstrated no difference between surgery at the COW and non-COW surgery (HR:1.42, p=0.62). For every additional year of age at diagnosis, the risk of stroke or TIA increased by 13% (95% CI:1.02-1.25, p=0.018). Median total and COW radiation doses did not differ between subjects who had stroke/TIA (54.0 Gy median, IQR 54.0-55.8; 52.2 Gy, IQR 18-54, respectively) and those who did not.
Table 3.
Unadjusted hazard ratios from Cox regression
| Hazard Ratio | P value | |
|---|---|---|
| Any Brain Radiation | 8.0 (CI:1.05-62) | 0.045 |
| COW Radiation | 9.0 (CI:1.2-70) | 0.035 |
| Non-COW Radiation | 3.4 (CI:.21-55) | 0.38 |
| Surgery | 0.38 (CI:0.13-1.1) | 0.08 |
| Chemotherapy | 2.53 (CI:0.71-9.1) | 0.15 |
| Glioma | 0.23 (CI:0.05-1.03) | 0.055 |
| PNET | 2.74 (CI:0.95-7.9) | 0.063 |
| Age at Diagnosis (annually) | 1.13 (CI:1.02-1.25) | 0.018 |
COW:circle of Willis, PNET:primitive neuroectodermal tumor
Table 4.
Incidence Rates by Treatment Modality
| Treatment Modality | Stroke or TIA |
Total # Subjects |
Incidence* rate |
||
|---|---|---|---|---|---|
| Any Radiation |
Chemotherapy | Surgery | |||
| − | − | − | 0 | 5 | 0 |
| + | − | − | 1 | 31 | 580 |
| − | + | − | 0 | 14 | 0 |
| + | + | − | 4 | 53 | 2400 |
| − | − | + | 0 | 74 | 0 |
| + | − | + | 2 | 52 | 490 |
| − | + | + | 1 | 65 | 220 |
| + | + | + | 6 | 127 | 740 |
TIA:transient ischemic attack,
number
Incidence rate per 100,000 person-years
A stepwise multivariable model including the following candidate variables was performed: age at diagnosis, radiation, chemotherapy, surgery and tumor type. The final model identified radiation to the COW (HR 4.35; 95% CI:0.97-19.6, p=0.055), and chemotherapy (HR 3.38; 95% CI:9.2-12.5, p=0.067), as heightening neurovascular risk. Chemotherapy significantly interacted with radiation (p=<0.001), but an estimate of the interaction between chemotherapy and radiation could not be determined because modeling yielded unstable results.
Of the neurovascular events, 2 were associated with co-morbidities. One occurred in the setting of a sinus venous thrombosis and the other two weeks after Staphylococcus aureus meningitis. To ensure our findings were not altered by these 2 cases, an ad hoc analysis excluding these subjects yielded similar results:HR for COW radiation, 7.6 (95% CI:0.97-59.6, p=0.05), and for non-COW radiation, 3.4 (95% CI:0.22-54, p = 0.39).
Of the 14 subjects who experienced events, 10 were initiated on secondary stroke prevention regimens (Table 5). Despite these interventions, five subjects with TIA experienced repeat events, three of which were stroke.
Table 5.
Neuroimaging Changes and Treatment Prescribed
| ID | Event | MRI | Vascular Territory | MRA | Treatment |
|---|---|---|---|---|---|
| 1 | Stroke | RD right pons | Basilar Artery | Absent bilateral PCAs | Hyperbaric oxygen, steroids, Aspirin |
| 2 | 1. TIA | 1. NA | 1. Aspirin | ||
| 2. Stroke | 2. RD right internal capsule |
MCA:M1 lenticulostriate | 2. Fetal PCA with hypoplastic P1 |
2. Decreased estrogen replacement, added statin |
|
| 3. Stroke | 3. RD left internal capsule |
MCA:M1, lenticulostriate | |||
| 3 | Stroke | RD right frontoparietal lobe |
MCA:M3 | NP | None |
| 4 | 1. Stroke | 1. RD left frontoparietal lobe |
MCA:M3 | Left MCA and ACA high- grade stenosis |
1. Warfarin |
| 2. TIAs | 2. NNA | ||||
| 5 | Stroke | RD pons | Basilar artery perforators | NP | Hyperbaric oxygen, Aspirin, pentoxifylline, Vitamin E |
| 6 | 1. Stroke | 1. RD pons | 1. Basilar artery perforators | 1. Attenuation of basilar and bilateral proximal PCAs |
1. Nimodipine, Aspirin |
| 2. TIA | 2. NNA | 2. Progressive stenosis | 2. Clopidogrel | ||
| 7 | 1. TIA | 1. NA | Left cavernous ICA mild stenosis |
Aspirin | |
| 2. Stroke | 2. RD right pons | 2. Basilar artery perforators | |||
| 8 | TIA | NA | NA | None | |
| 9 | TIA | NA | NA | Aspirin | |
| 10 | Multiple TIAs |
NA | NP | 1.Aspirin 2.Discontinued sumatriptan and estrogen replacement therapy |
|
| 11 | TIA | NA | NP | Aspirin | |
| 12 | TIA | NA | Left AICA narrowed, left SCA narrowed |
Aspirin | |
| 13 | TIA | NA | NP | ||
| 14 | TIA | NA | NP |
MRI:magnetic resonance imaging, MRA:magnetic resonance angiography, RD:restricted diffusion on DWI, PCA:posterior communicating artery, TIA:transient ischemic attack, NA:No abnormality, P1:First branch of the posterior communicating artery, NP:Not performed, MCA:middle cerebral artery, ACA:anterior communicating artery, NNA:No new abnormality, AICA:anterior inferior cerebellar artery, SCA:superior cerebellar artery
Vascular imaging by MRA was available in a subset of 82 patients, and demonstrated a higher incidence of large-vessel vasculopathy in those with neurovascular events (6/8, [75%]) than in the remainder of the cohort (7/72 [9.7%], p<0.0001) (Table 5). These abnormalities consisted of moyamoya (4) and stenosis (9). All subjects with vasculopathy on MRA received cranial irradiation.
DISCUSSION
Our study demonstrates a markedly increased risk of stroke and TIA in pediatric brain tumor survivors. The observed stroke/TIA rate of 548/100,000 represents a 100-fold increase over the rate of 2-8/100,000 in the general pediatric population (28, 29) and is greater than double that found in a questionnaire-based study of brain tumor survivors (21). We found that cranial irradiation is the major risk factor for later neurovascular events. As similarly reported by others, radiation exposure to the large intracranial vessels (COW) conferred the highest risk; not only were neurovascular event rates highest in this group, but stroke only occurred in those patients receiving radiation to the COW (24).
Radiation induces a large spectrum of changes leading to vascular injury, including accelerated atherosclerosis and vascular insufficiency (7-8, 18, 30-31). Prior reports have focused on small vessel injury-induced stroke; however, our findings of stroke occurring only in those subjects receiving radiation to the COW and the significantly higher associated large-vessel stenosis identified on MRA in stroke patients support a mechanism involving both large and small intracranial vessel injury.. Future studies are needed to investigate neuroimaging techniques that may identify patients with early vasculopathy or perfusional abnormalities, who are at highest risk of stroke; as these patients may benefit from primary prevention measures.
In univariate analysis, glioma demonstrated a trend toward protection against neurovascular events, PNET showed a trend toward an increased risk, and older age at diagnosis was significantly related to increased risk. These effects disappeared in multivariate analysis, likely reflecting the important role radiation plays as a risk factor. Radiation is infrequently used in glioma treatment, but craniospinal radiotherapy is standard practice for PNET. It is also common practice to delay radiation exposure in children younger than 3 years of age. Radiation therapy heightened the risk of other factors; chemotherapy, an independent risk factor in multivariate analysis, interacted with radiation to increase risk. While certain alkylating chemotherapy agents such as cisplatin have been reported to increase the risk of ischemic stroke (32, 33), more work is needed to understand if an interaction between chemotherapy and radiation exists, or if the finding is a surrogate for more aggressive treatment.
In our cohort, we found that 35.7% (5/14) of patients with stroke/ TIA had repeat events even after initiation of secondary stroke prevention. This finding demonstrates the inadequacy of secondary stroke prevention modalities and highlights the need for more effective primary stroke prevention in this population.
The study data were subject to the limitations and biases of a retrospective analysis, including the limitations of the registry. Collecting the data by chart review enabled cross-referencing of events with neuroimaging, and eliminated recall bias. The possibility of selection bias remains, given the subjects were enrolled at a tertiary care center; however, most children with brain tumors are referred to tertiary care centers, thus the study population is likely representative of the general pediatric brain tumor population.
This single-institution study was underpowered to evaluate certain risk factors, including tumor type, tumor location, surgery, or chemotherapy type. Furthermore, for simplicity, we grouped patients according to radiation location, although this represents an oversimplification of the true dose of radiation to the COW. Misclassification of stroke is highly unlikely because the clinical symptoms correlated with diffusion restriction on MRI. However, TIAs may have been misclassified as seizures or migraines, and therefore undercounted, or, alternatively, seizures and migraines may have been misclassified as TIAs. This is an unavoidable problem when using TIA as an outcome, and the separate analysis evaluating only stroke was performed to obviate this possible bias.
Survivors of pediatric brain tumors are at much higher risk for neurovascular events than the general pediatric population, and radiation, particularly to the COW, is a major risk factor. Multi-center prospective studies are necessary to investigate other risk factors (hypertension, hyperglycemia, hypertriglyceridemia, etc.), primary stroke prevention, and tools to identify high-risk patients early, such as perfusion MRI and MRA. Furthermore, it is important to pursue treatment options that enable reduced radiation doses or replace radiation entirely. Preventing stroke and vasculopathy will help reduce the lifelong toll of childhood brain tumor treatment in this vulnerable population.
Supplementary Material
Acknowledgments
Funding:University of Pennsylvania Department of Neurology Patient-Oriented Research Program
Footnotes
Disclosures:None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 2.Fouladi M, Gilger E, Kocak M, Wallace D, Buchanan G, Reeves C, et al. Intellectual and functional outcome of children 3 years old or youner who have CNS malignancies. J Clin Oncol. 2005;23:7152–60. doi: 10.1200/JCO.2005.01.214. [DOI] [PubMed] [Google Scholar]
- 3.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. 2007. Ann Neurol. 2007;62:515–20. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 4.Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97:663–73. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 5.Burn S, Gunny R, Phipps K, Gaze M, Hayward R. Incidence of cavernoma development in children after radiotherapy for brain tumors. J Neurosurg. 2007;106:379–83. doi: 10.3171/ped.2007.106.5.379. [DOI] [PubMed] [Google Scholar]
- 6.Fouladi M, Lagston J, Mulhern R, Jones D, Xiong X, Yang J, et al. Silent Lacunar Lesions Detected by Magnetic Resonance Imaging of Children with Brain Tumors: A Late Sequela of Therapy. J Clin Oncol. 2000;18:824–83. doi: 10.1200/JCO.2000.18.4.824. [DOI] [PubMed] [Google Scholar]
- 7.Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity-molecular and cellular mechanisms. Br J Cancer. 2001;85:1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9:180–188. doi: 10.1097/01.nrl.0000080951.78533.c4. [DOI] [PubMed] [Google Scholar]
- 9.Bitzer M, Topka H. Progressive Cerebral Occlusive Disease After Radiation Therapy. Stroke. 1995;26:131–136. doi: 10.1161/01.str.26.1.131. [DOI] [PubMed] [Google Scholar]
- 10.Grenier Y, Tomita T, Marymont MH, Byrd S, Burrowes DM. Late Postirradiation Occlusive Vasculopathy in Childhood Medulloblastoma. J Neurosurg. 1998;89:460–464. doi: 10.3171/jns.1998.89.3.0460. [DOI] [PubMed] [Google Scholar]
- 11.Rogers LR. Cerebrovascular complications in cancer patients. Neurol Clin. 2003;21:167–192. doi: 10.1016/s0733-8619(02)00066-x. [DOI] [PubMed] [Google Scholar]
- 12.CBTRUS [Accessed September 21, 2011];CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. Central Brain Tumor Registry of the United States website. 2010 www.cbtrus.org.
- 13.Nishizawa S, Ryu H, Yokoyama T, Ninchoji T, Shimoyama I, Yamamoto S, et al. Post-irradiation Vasculopathy of Intracranial Major Arteries in Children. Neurol Med Chir. 1991;31:336–341. doi: 10.2176/nmc.31.336. [DOI] [PubMed] [Google Scholar]
- 14.Muthukrishnan A, Bajoghli M, Mountz JM. Delayed Development of Radiation Vasculopathy of the Brain Stem Confirmed by F-18 FDG PET in a Case of Anaplastic Astrocytoma. Clin Nucl Med. 2007;32:527–531. doi: 10.1097/RLU.0b013e31806469ef. [DOI] [PubMed] [Google Scholar]
- 15.Maher CO, Raffel C. Early Vasculopathy Following Radiation in a Child with Medulloblastoma. Pediatr Neurosurg. 2000;32:255–258. doi: 10.1159/000028947. [DOI] [PubMed] [Google Scholar]
- 16.Brant-Zawadzki M, Anderson M, DeArmond SJ, Conley FK, Jahnke RW. Radiation-Induced Large Vessel Occlusive Vasculopathy. AJR. 1980;134:51–55. doi: 10.2214/ajr.134.1.51. [DOI] [PubMed] [Google Scholar]
- 17.Epstein MA, Packer RJ, Rorke LB, Zimmerman RA, Goldwein JW, Sutton LN, et al. Vascular Malformation with Radiation Vasculopathy after Treatment of Chiasmatic/Hypothalamic Glioma. Cancer. 1992;70:887–893. doi: 10.1002/1097-0142(19920815)70:4<887::aid-cncr2820700427>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Penagaricano JA, Linskey ME, Ratanatharathorn V. Accelerated Cerebral Vasculopathy after Radiation Therapy to the Brain. Neurol India. 2004;52:482–486. [PubMed] [Google Scholar]
- 19.Salih ISM, Higgins NJN, Warburton EA, Baron JC. Lacunar Stroke Attributable to Radiation-Induced Intracranial Arteriopathy. European Journal of Neurology. 2007;14:937–939. doi: 10.1111/j.1468-1331.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- 20.Omura M, Aida N, Sekido K, Kakehi M, Matsubara S. Large Intracranial Vessel Occlusive Vasculopathy after Radiation Therapy in Children: Clinical Features and Usefulness of Magnetic Resonance Imaging. Int J Radiation Oncology Biol Phys. 1997;38:241–249. doi: 10.1016/s0360-3016(97)82497-2. [DOI] [PubMed] [Google Scholar]
- 21.Bowers DC, Liu Y, Leisenring W, McNeil E, Stovall M, Gurney JG, et al. Late Occurring Stroke Among Long-Term Survivors of Childhood Leukemia and Brain Tumors: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277–5282. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 22.Ullrich NJ, Robertson R, Kinnamon DD, Scot RM, Kieran MW, Turner CD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68:932–938. doi: 10.1212/01.wnl.0000257095.33125.48. [DOI] [PubMed] [Google Scholar]
- 23.Bowers DC, Mulne AF, Reisch JS, Elterman Rd, Munoz L, Booth T, et al. Nonperioperative Strokes in Children with Central Nervous System Tumors. Cancer. 2002;94:1094–1101. [PubMed] [Google Scholar]
- 24.Haddy N, Mousannif A, Tukenova M, Guibout C, Grill J, Dhermain F, et al. Relationship between the brain radiation dose for the treatment of childhood cnacer and the risk of long-term cerebrovascular mortality. Brain. 2011;134:1362–72. doi: 10.1093/brain/awr071. [DOI] [PubMed] [Google Scholar]
- 25.Shellhaas RA, Smith SE, O’Tool E, Licht DJ, Ichord RN. Mimics of childhood stroke: characteristics of a prospective cohort. Pediatrics. 2006;118:704–9. doi: 10.1542/peds.2005-2676. [DOI] [PubMed] [Google Scholar]
- 26.Hilal SK, Solomon GE, Gold AP, Carter S. Primary cerebral arterial occlusive disease in children: Part II. Neurocutaneous syndromes. Radiology. 1971;99:87–93. doi: 10.1148/99.1.87. [DOI] [PubMed] [Google Scholar]
- 27.Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology. 2005;8:553–5. doi: 10.1212/01.WNL.0000150544.00016.69. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal NS, Johnston C, Wu YW, Sidney S, Fullerton HJ. Imaging Data Reveal a Higher Pediatric Stroke Incidence Than Prior US Estimates. Stroke. 2009;40:3415–3421. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch JK, Hirtz DG, deVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 30.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 31.Bowers DC, McNeil DE, Liu Y, Yasui Y, Stovall M, Gurney JG, et al. Stroke as a late treatment effect of Hodgkin’s Disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:6508–15. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 32.Meattini I, Scotti V, Pescini F, Livi L, Sulprizio S, Palumbo V, et al. Ischemic stroke during cisplatin-based chemotherapy for testicular germ cell tumor: case report and review of the literature. J Chemother. 2010;22:134–6. doi: 10.1179/joc.2010.22.2.134. [DOI] [PubMed] [Google Scholar]
- 33.Li SH, Chen WH, Tang Y, Rau KM, Chen YY, Huang TL, et al. Incidence of ischemic stroke post-chemotherapy: a retrospective review of 10,963 patients. Clin Neurol Neurosurg. 2006;108:150–6. doi: 10.1016/j.clineuro.2005.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.