Abstract
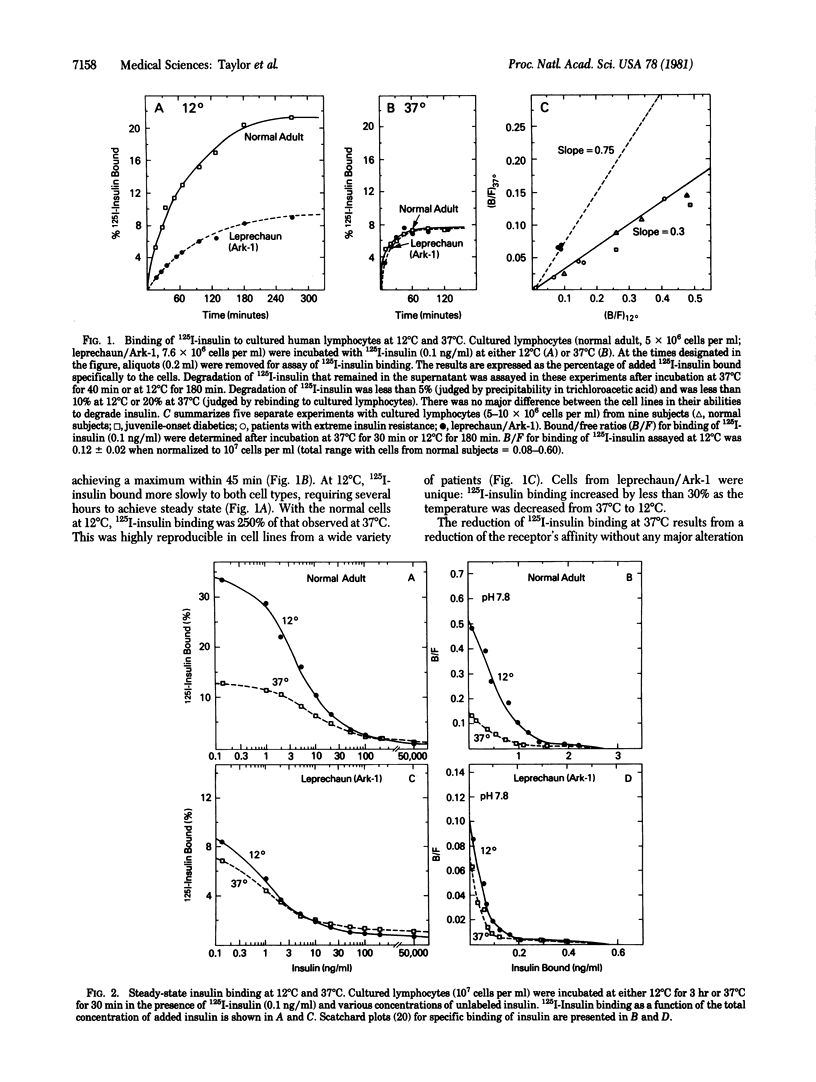
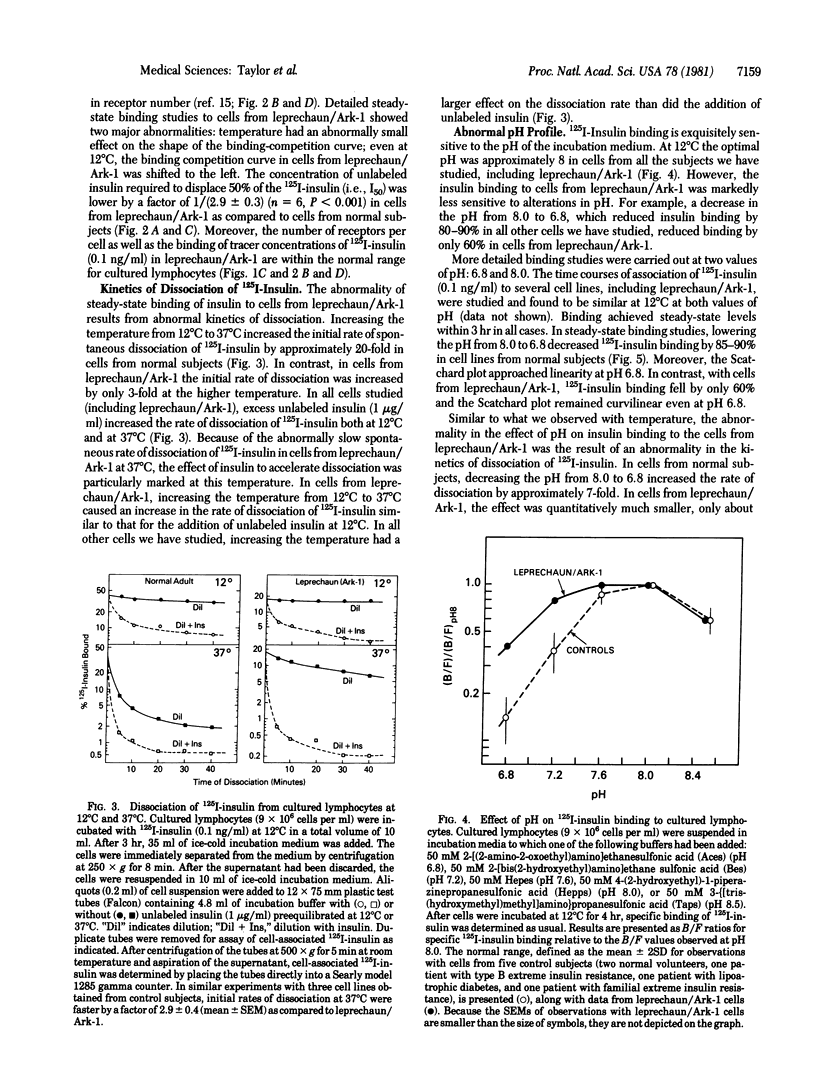
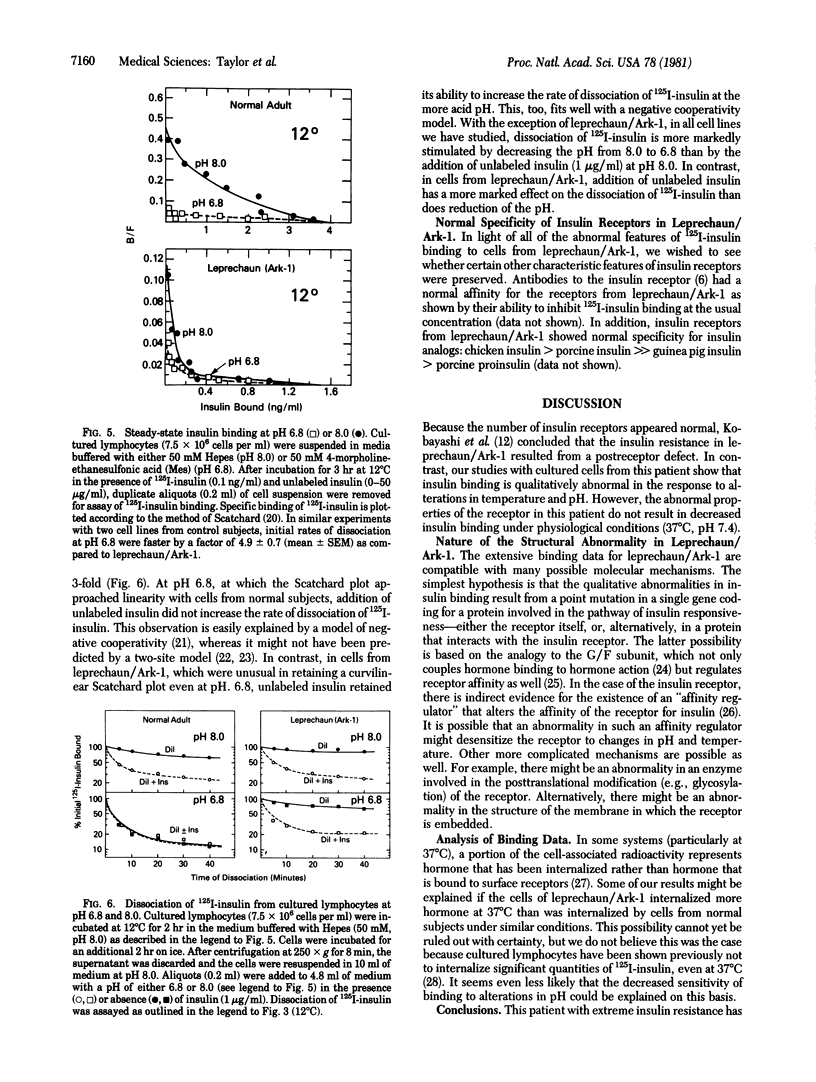
Cultured lymphocytes transformed by Epstein--Barr virus were employed to study insulin receptors from a patient with extreme insulin resistance associated with the syndrome of leprechaunism. With cultured lymphocytes from normal subjects, insulin binding to its receptor is exquisitely sensitive to changes in temperature and pH. In cells from normal subjects, insulin binding was increased by approximately 250% as the temperature was decreased from 37 degrees C to 12 degrees C. In contrast, with cells from the leprechaun, insulin binding was only approximately 30% higher at 12 degrees C than at 37 degrees C. Similarly, insulin binding to cells from the leprechaun was markedly less sensitive to changes in pH, as compared to cells from normal subjects. Binding studies suggested that the number of insulin receptors per cell was within the normal range in this patient. Despite the unusual characteristics of insulin binding in cells from this insulin-resistant patient, the receptors were typical in at least two respects: (i) binding was inhibited normally by antibodies to the receptor; and (ii) the specificity for insulin analogs was normal (chicken insulin greater than porcine insulin much greater than guinea pig insulin greater than porcine proinsulin). This patient has an inborn error affecting insulin receptor function. The receptor's binding function was abnormal in having decreased sensitivity to alterations in temperature and pH. However, the level of insulin binding to cells from the leprechaun was within normal limits. Consequently, the hormonal resistance probably results from a decreased ability of the receptor to couple insulin binding to insulin action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERSON S. A., YALOW R. S. THE PRESENT STATUS OF INSULIN ANTAGONISTS IN PLASMA: OCTOBER 1963. Diabetes. 1964 May-Jun;13:247–259. [PubMed] [Google Scholar]
- Bar R. S., Muggeo M., Kahn C. R., Gorden P., Roth J. Characterization of insulin receptors in patients with the syndromes of insulin resistance and acanthosis nigricans. Diabetologia. 1980 Mar;18(3):209–216. doi: 10.1007/BF00251918. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Muggeo M., Roth J., Kahn C. R., Havrankova J., Imperato-McGinley J. Insulin resistance, acanthosis nigricans, and normal insulin receptors in a young woman: evidence for a postreceptor defect. J Clin Endocrinol Metab. 1978 Sep;47(3):620–625. doi: 10.1210/jcem-47-3-620. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Schedewie H., Larner J., Olefsky J., Rubenstein A., Fiser R. H., Craig J. W., Elders M. J. Glucose kinetics in leprechaunism: accelerated fasting due to insulin resistance. J Clin Endocrinol Metab. 1980 Nov;51(5):988–994. doi: 10.1210/jcem-51-5-988. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ercole A. J., Underwood L. E., Groelke J., Plet A. Leprechaunism: studies of the relationship among hyperinsulinism, insulin resistance, and growth retardation. J Clin Endocrinol Metab. 1979 Mar;48(3):495–502. doi: 10.1210/jcem-48-3-495. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Jarrett D. B., Roth J. Characterization of antibodies to the insulin receptor: a cause of insulin-resistant diabetes in man. J Clin Invest. 1976 Dec;58(6):1442–1449. doi: 10.1172/JCI108600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P. O., Orci L. Internalization of polypeptide hormones: mechanism, intracellular localization and significance. Diabetologia. 1980 Apr;18(4):263–274. doi: 10.1007/BF00251003. [DOI] [PubMed] [Google Scholar]
- Griffin J. E. Testicular feminization associated with a thermolabile androgen receptor in culutred human fibroblasts. J Clin Invest. 1979 Dec;64(6):1624–1631. doi: 10.1172/JCI109624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon J. T., Kahn C. R., Kempner E. S., Schlegel W. Characterization of the insulin receptor in its membrane environment by radiation inactivation. J Biol Chem. 1980 Apr 25;255(8):3412–3419. [PubMed] [Google Scholar]
- Imperato-McGinley J., Peterson R. E., Sturla E., Dawood Y., Bar R. S. Primary amenorrhea associated with hirsutism, acanthosis nigricans, dermoid cysts of the ovaries and a new type of insulin resistance. Am J Med. 1978 Aug;65(2):389–395. doi: 10.1016/0002-9343(78)90838-0. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Olefsky J. M., Elders J., Mako M. E., Given B. D., Schedwie H. K., Fiser R. H., Hintz R. L., Horner J. A., Rubenstein A. H. Insulin resistance due to a defect distal to the insulin receptor: demonstration in a patient with leprechaunism. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3469–3473. doi: 10.1073/pnas.75.7.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggeo M., Ginsberg B. H., Roth J., Neville D. M., Jr, De Meyts P., Kahn C. R. The insulin receptor in vertebrates is functionally more conserved during evolution than insulin itself. Endocrinology. 1979 May;104(5):1393–1402. doi: 10.1210/endo-104-5-1393. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Insulin binding to the human lymphocyte receptor. Evaluation of the negative cooperativity model. J Biol Chem. 1977 Aug 25;252(16):5828–5834. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y. Evidence for interdependent action of glucagon and nucleotides on the hepatic adenylate cyclase system. J Biol Chem. 1974 Jan 10;249(1):59–65. [PubMed] [Google Scholar]
- Rosenberg A. M., Haworth J. C., Degroot G. W., Trevenen C. L., Rechler M. M. A case of leprechaunism with severe hyperinsulinemia. Am J Dis Child. 1980 Feb;134(2):170–175. doi: 10.1001/archpedi.1980.02130140044014. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Schilling E. E., Rechler M. M., Grunfeld C., Rosenberg A. M. Primary defect of insulin receptors in skin fibroblasts cultured from an infant with leprechaunism and insulin resistance. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5877–5881. doi: 10.1073/pnas.76.11.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelbroeck M., Van Obberghen E., De Meyts P. Thermodynamics of the interaction of insulin with its receptor. J Biol Chem. 1979 Aug 25;254(16):7736–7740. [PubMed] [Google Scholar]
- West R. J., Leonard J. V. Familial insulin resistance with pineal hyperplasia: metabolic studies and effect of hypophysectomy. Arch Dis Child. 1980 Aug;55(8):619–621. doi: 10.1136/adc.55.8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]


