Abstract
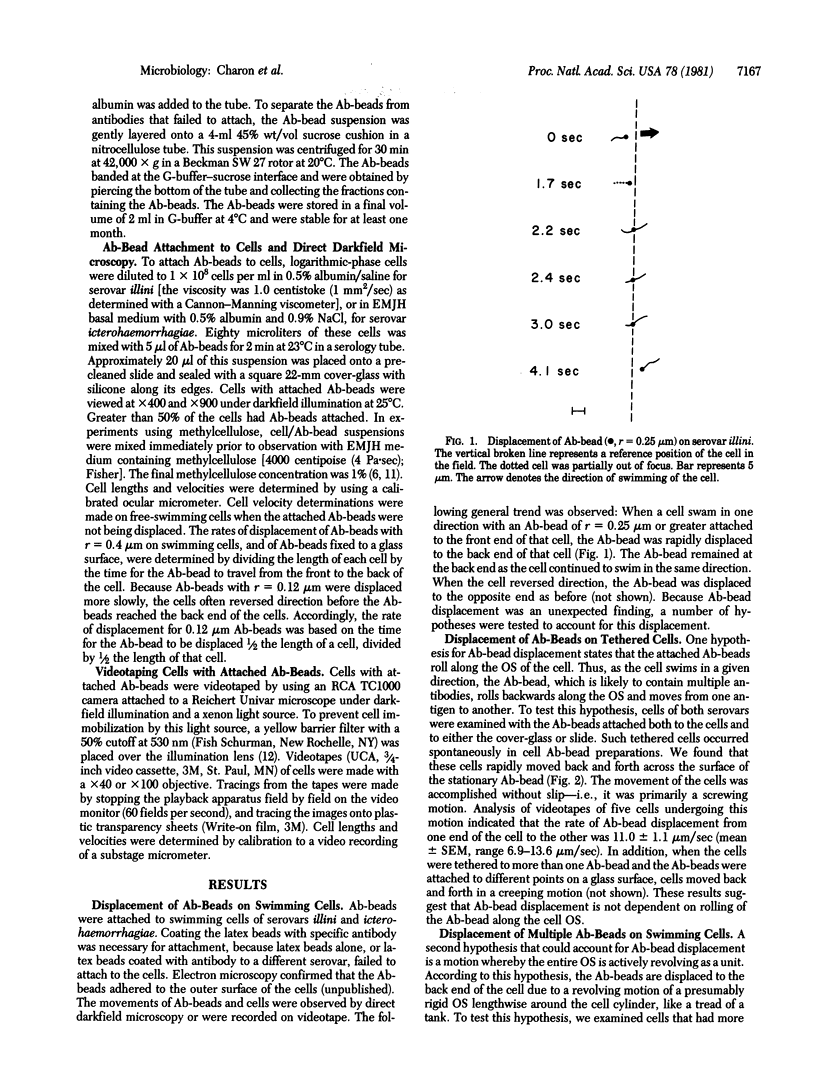
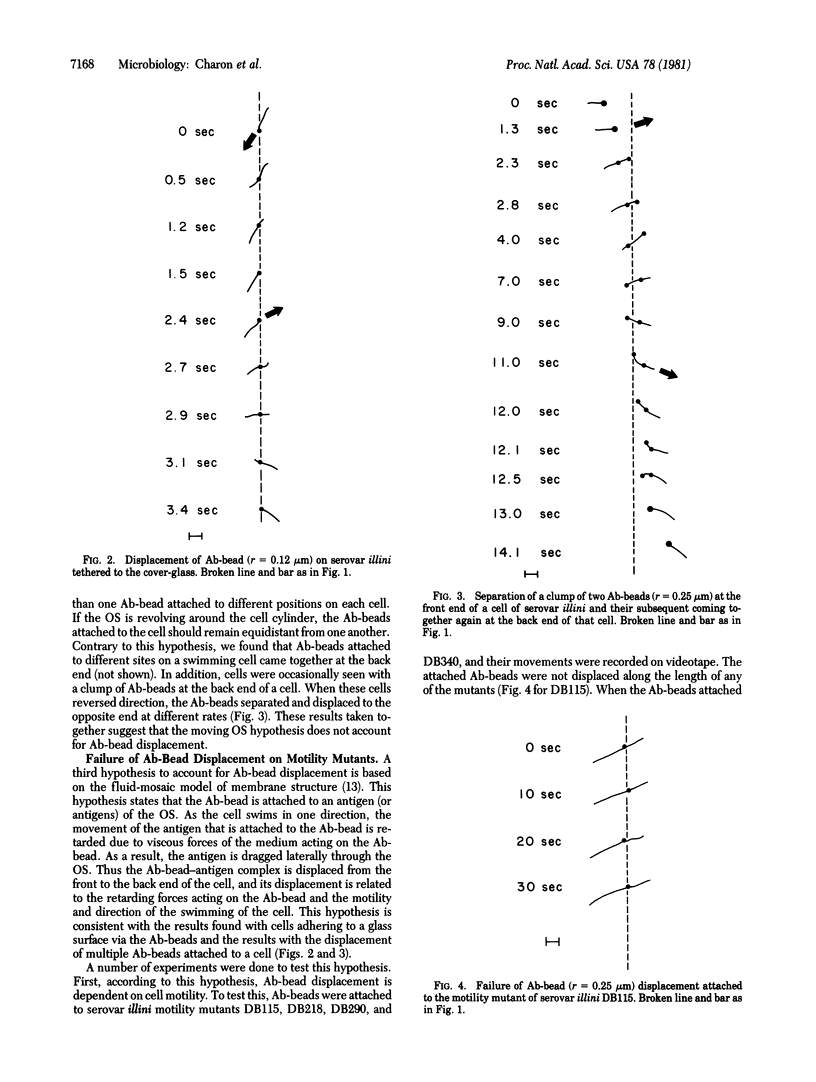
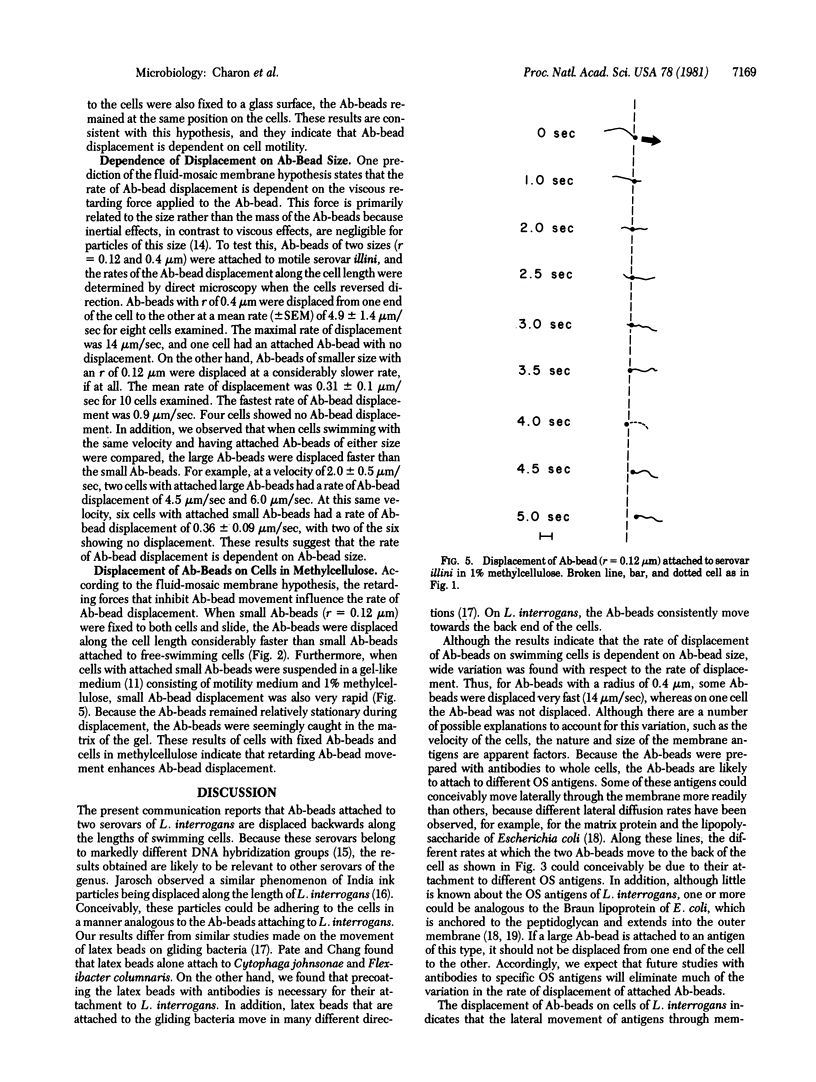
Antibody-coated latex beads (Ab-beads) were attached to Leptospira interrogans serovars illini 3055 and icterohaemorrhagiae SC1157. The movement of the Ab-beads relative to the motion of the cells was observed by direct darkfield microscopy or was recorded on videotape. When the Ab-beads were attached to the front end of motile cells, the Ab-beads were displaced towards the back end of the cells. When the cells reversed direction, the Ab-beads also reversed direction. A number of hypotheses were proposed and tested to account for this Ab-bead displacement. The one best supported by the evidence states that the Ab-beads are attached to antigens of the outer membrane sheath. These antigens are dragged laterally through the sheath due to the forward motion of the cells and the retarding forces of the medium acting on the beads. The results obtained provide information on the nature of the outer membrane sheath of L. interrogans, the basis for certain movements of spirochetes, and insight on how spirochetes attach to eukaryotic cells and tissues. In addition, the results indicate that antigens can move laterally through membranes as rapidly as 11 micrometers/sec.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Zachar Z., Hayes N. S. Concanavalin A-mediated affinity film for Treponema pallidum. Infect Immun. 1980 Jan;27(1):260–263. doi: 10.1128/iai.27.1.260-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Turner L. Movement of microorganisms in viscous environments. Nature. 1979 Mar 22;278(5702):349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- Blakemore R. P., Canale-Parola E. Morphological and ecological characteristics of Spirochaeta plicatilis. Arch Mikrobiol. 1973;89(4):273–289. doi: 10.1007/BF00408895. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Carleton O., Charon N. W., Allender P., O'Brien S. Helix handedness of Leptospira interrogans as determined by scanning electron microscopy. J Bacteriol. 1979 Mar;137(3):1413–1416. doi: 10.1128/jb.137.3.1413-1416.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P. J., Twigg G. I. Leptospiral motility. Nature. 1974 Jul 19;250(463):260–261. doi: 10.1038/250260a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967 Jul;94(1):27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser A., Adrian M. Les spirochètes: sens de l'enroulement. Ann Microbiol (Paris) 1978 Apr;129(3):351–360. [PubMed] [Google Scholar]
- Macnab R., Koshland D. E., Jr Bacterial motility and chemotaxis: light-induced tumbling response and visualization of individual flagella. J Mol Biol. 1974 Apr 15;84(3):399–406. doi: 10.1016/0022-2836(74)90448-3. [DOI] [PubMed] [Google Scholar]
- SINGER J. M., PLOTZ C. M., GOLDBERG R. THE DETECTION OF ANTI-GLOBULIN FACTORS UTILIZING PRE-COATED LATEX ARTICLES. Arthritis Rheum. 1965 Apr;8:194–202. doi: 10.1002/art.1780080203. [DOI] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J., Koppel D. E. Lateral mobility in reconstituted membranes--comparisons with diffusion in polymers. Nature. 1980 Jan 24;283(5745):346–350. doi: 10.1038/283346a0. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Calcium-sensitive modulation of Ig capping: evidence supporting a cytoplasmic control of ligand-receptor complexes. J Exp Med. 1976 Jan 1;143(1):15–31. doi: 10.1084/jem.143.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]


