Abstract
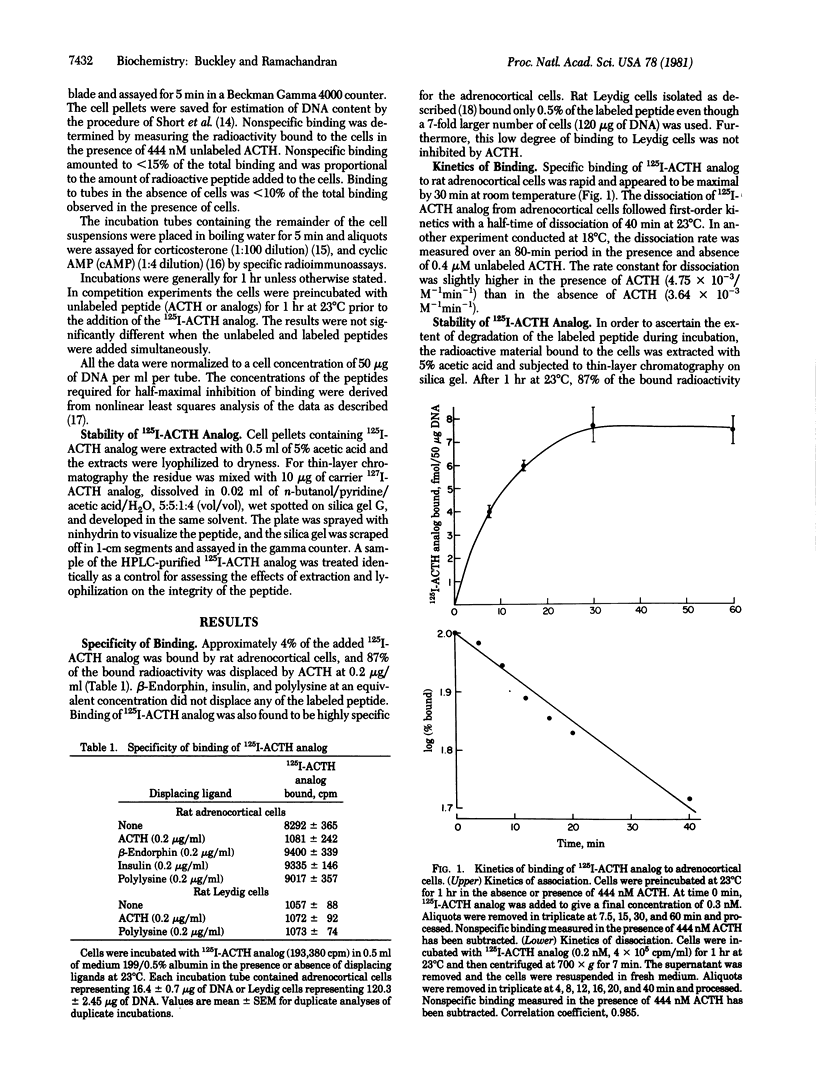
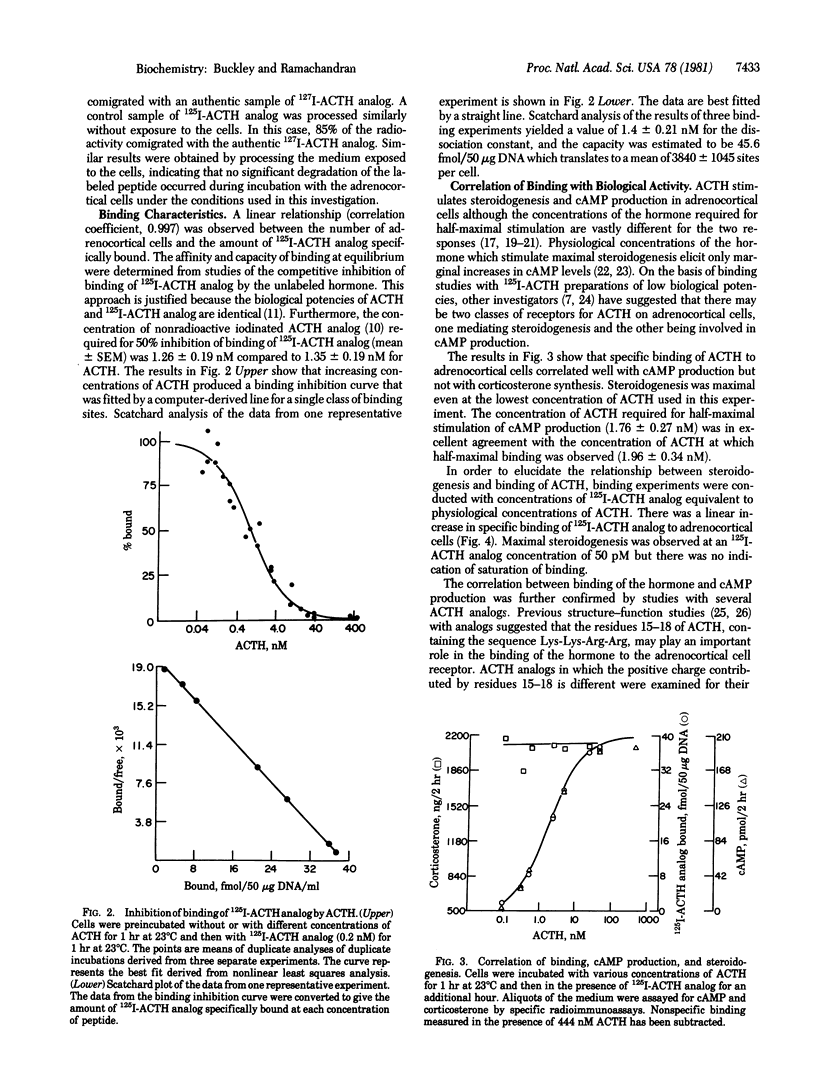
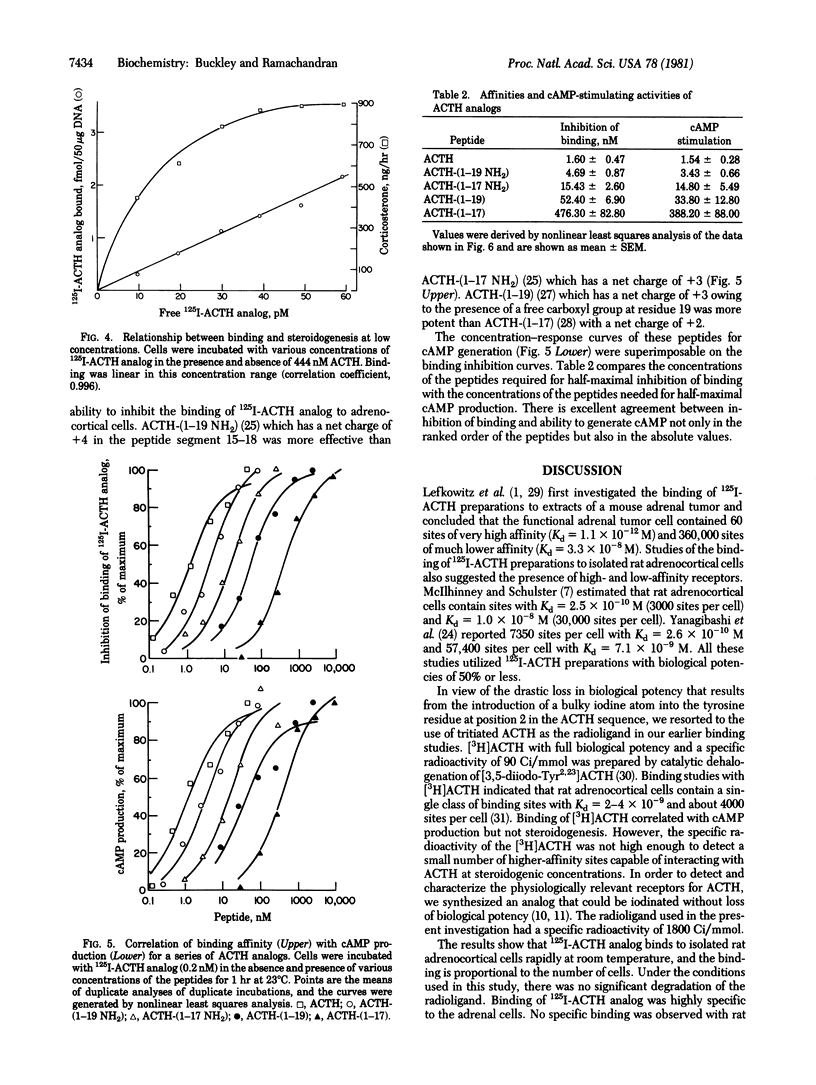
The binding of corticotropin (ACTH) to receptors on isolated rat adrenocortical cells was investigated with the aid of [[125I]ITyr23, Phe2, Nle4]ACTH-(1-38) (125I-ACTH analog) which retained full biological potency and had a specific radioactivity of 1800 +/- 75 Ci/mmol. Binding was highly specific to adrenocortical cells, and the radioactive peptide was displaced by low concentrations of ACTH but not by other basic peptides. Binding was rapid, reversible, and linearly related to the number of cells. 125I-ACTH analog was not significantly degraded by incubation with the cells at 23 degrees C for 1 hr. Scatchard analysis of the binding was compatible with a single class of binding sites with Kd = 1.41 +/- 0.21 nM, and the number of sites was estimated to be 3840 +/- 1045 per cell. The binding curve was superimposable on the concentration-response curve for cyclic AMP. Small, but significant amounts of 125I-ACTH analog were bound at concentrations sufficient for maximal stimulation of steroidogenesis. For a series of ACTH analogs, the concentrations of the peptides required for half-maximal stimulation of cyclic AMP production were in excellent agreement with the concentration required for half-maximal inhibition of binding. These results suggest that the adrenocortical cells contain only one class of ACTH receptors and that stimulation of a small fraction of these receptors (less than 3%) is sufficient for maximal steroidogenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall R. J., Sayers G. Isolated adrenal cells: steroidogenesis and cyclic AMP accumulation in response to ACTH. Arch Biochem Biophys. 1972 Jan;148(1):70–76. doi: 10.1016/0003-9861(72)90116-6. [DOI] [PubMed] [Google Scholar]
- Bristow A. F., Gleed C., Fauchère J. L., Schwyzer R., Schulster D. Effects of ACTH (corticotropin) analogues on steroidogenesis and cyclic AMP in rat adrenocortical cells. Evidence for two different steroidogenically responsive receptors. Biochem J. 1980 Feb 15;186(2):599–603. doi: 10.1042/bj1860599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley D. I., Hagman J., Ramachandran J. A sensitive radioimmunoassay for corticotropin using a fully biologically active 125I-labeled ligand. Endocrinology. 1981 Jul;109(1):10–16. doi: 10.1210/endo-109-1-10. [DOI] [PubMed] [Google Scholar]
- Buckley D. I., Yamashiro D., Ramachandran J. Synthesis of a corticotropin analog that retains full biological activity after iodination. Endocrinology. 1981 Jul;109(1):5–9. doi: 10.1210/endo-109-1-5. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hornsby P. J., Gill G. N. Characterization of adult bovine adrenocortical cells throughout their life span in tissue culture. Endocrinology. 1978 Mar;102(3):926–936. doi: 10.1210/endo-102-3-926. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., McPherson M., Licko V., Ramachandran J. Pituitary corticotropin-inhibiting peptide: properties and use in study of corticotropin action. Arch Biochem Biophys. 1980 May;201(2):411–419. doi: 10.1016/0003-9861(80)90529-9. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pastan I. ACTH-receptor interaction in the adrenal: a model for the initial step in the action of hormones that stimulate adenyl cyclase. Ann N Y Acad Sci. 1971 Dec 30;185:195–209. doi: 10.1111/j.1749-6632.1971.tb45249.x. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pricer W., Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970 Mar;65(3):745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire S., Yamashiro D., Behrens C., Hao Li C. Adrenocorticotropin. 51. Synthesis and properties of analogues of the human hormone with tyrosine residues replaced by 3,5-diiodotyrosine. J Am Chem Soc. 1977 Mar 2;99(5):1577–1580. doi: 10.1021/ja00447a048. [DOI] [PubMed] [Google Scholar]
- Lowry P. J., McMartin C., Peters J. Properties of a simplified bioassay for adrenocorticotrophic activity using the steroidogenic response of isolated adrenal cells. J Endocrinol. 1973 Oct;59(1):43–55. doi: 10.1677/joe.0.0590043. [DOI] [PubMed] [Google Scholar]
- Mackie C., Richardson M. C., Schulster D. Kinetics and dose-response characteristics of adenosine 3',5'-monophosphate production by isolated rat adrenal cells stimulated with adrenocorticotrophic hormone. FEBS Lett. 1972 Jul 1;23(3):345–348. doi: 10.1016/0014-5793(72)80312-0. [DOI] [PubMed] [Google Scholar]
- McIlhinney J., Schulster D. The preparation of biologically active 125I-labeled adrenocorticotrophic hormone by a simple enzymic radioiodination procedure utilizing lactoperoxidase. Endocrinology. 1974 May;94(5):1259–1264. doi: 10.1210/endo-94-5-1259. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., Schulster D. Studies on the binding of 125I-labelled corticotrophin to isolated rat adrenocortical cells. J Endocrinol. 1975 Jan;64(1):175–184. doi: 10.1677/joe.0.0640175. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Kong Y. C., Ramachandran J. Steroidogenesis and cyclic adenosine 3',5'-monophosphate accumulation in rat adrenal cells. Divergent effects of adrenocorticotropin and its o-nitrophenyl sulfenyl derivative. J Biol Chem. 1973 Apr 10;248(7):2409–2417. [PubMed] [Google Scholar]
- Nakamura M., Ide M., Okabayashi T., Tanaka A. Relation between steroidogenesis and 3',5'-cyclic AMP production in isolated adrenal cells. Endocrinol Jpn. 1972 Oct;19(5):443–448. doi: 10.1507/endocrj1954.19.443. [DOI] [PubMed] [Google Scholar]
- Ontjes D. A., Ways D. K., Mahaffee D. D., Zimmerman C. F., Gwynne J. T. ACTH receptors and the effect of ACTH on adrenal organelles. Ann N Y Acad Sci. 1977 Oct 28;297:295–313. doi: 10.1111/j.1749-6632.1977.tb41862.x. [DOI] [PubMed] [Google Scholar]
- Perchellet J. P., Shanker G., Sharma R. Regulatory role of guanosine 3',5'-monophosphate in adrenocorticotropin hormone-induced steroidogenesis. Science. 1978 Jan 20;199(4326):311–312. doi: 10.1126/science.202028. [DOI] [PubMed] [Google Scholar]
- RAMACHANDRAN J., CHUNG D., LI C. H. ADRENOCORTICOTROPINS. XXXIV. ASPECTS OF STRUCTURE--ACTIVITY RELATIONSHIPS OF THE ACTH MOLECULE. SYNTHESIS OF A HEPTADECAPEPTIDE AMIDE, AN OCTADECAPEPTIDE AMIDE, AND A NONADECAPEPTIDE AMIDE POSSESSING HIGH BIOLOGICAL ACTIVITIES. J Am Chem Soc. 1965 Jun 20;87:2696–2708. doi: 10.1021/ja01090a030. [DOI] [PubMed] [Google Scholar]
- Rae P. A., Gutmann N. S., Tsao J., Schimmer B. P. Mutations in cyclic AMP-dependent protein kinase and corticotropin (ACTH)-sensitive adenylate cyclase affect adrenal steroidogenesis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1896–1900. doi: 10.1073/pnas.76.4.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. A., Schimmer B. P. Iodinated derivatives of adrenocorticotropic hormone. Preparation of iodinated alpha 1-24-ACTH with full hormonal activity. J Biol Chem. 1974 Sep 10;249(17):5649–5653. [PubMed] [Google Scholar]
- Ramachandran J., Behrens C. Preparation and characterization of specifically tritiated adrenocorticotropin. Biochim Biophys Acta. 1977 Feb 28;496(2):321–328. doi: 10.1016/0304-4165(77)90314-2. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Lee V., Li C. H. Stimulation of lipolysis and cyclic AMP accumulation in rabbit fat cells by human growth hormone. Biochem Biophys Res Commun. 1972 Jul 25;48(2):274–279. doi: 10.1016/s0006-291x(72)80046-9. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Sairam M. R. The effects of interstitial cell-stimulating hormone, its subunits, and recombinants on isolated rat Leydig cells. Arch Biochem Biophys. 1975 Mar;167(1):294–300. doi: 10.1016/0003-9861(75)90465-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. J., Long J. A., Ramachandran J. Effects of antiserum to adrenocorticotropin on adrenal growth and function. Endocrinology. 1978 Feb;102(2):371–378. doi: 10.1210/endo-102-2-371. [DOI] [PubMed] [Google Scholar]
- Rees L. H., Cook D. M., Kendall J. W., Allen C. F., Kramer R. M., Ratcliffe J. G., Knight R. A. A radioimmunoassay for rat plasma ACTH. Endocrinology. 1971 Jul;89(1):254–261. doi: 10.1210/endo-89-1-254. [DOI] [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez J. M., Dazord A., Morera A. M., Bataille P. Interactions of adrenocorticotropic hormone with its adrenal receptors. Degradation of ACTH-1-24 and ACTH-11-24. J Biol Chem. 1975 Mar 10;250(5):1683–1689. [PubMed] [Google Scholar]
- Short E. V., Jr, Warner H. R., Koerner J. F. The effect of cupric ions on the indole reaction for the determination of deoxyribonucleic acid. J Biol Chem. 1968 Jun 25;243(12):3342–3344. [PubMed] [Google Scholar]
- Yanagibashi K., Kamiya N., Lin G., Matsuba M. Studies on adrenocorticotropic hormone receptor using isolated rat adrenocortical cells. Endocrinol Jpn. 1978 Dec;25(6):545–551. doi: 10.1507/endocrj1954.25.545. [DOI] [PubMed] [Google Scholar]


