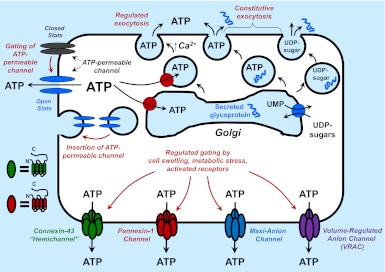
given the established roles for extracellular ATP and other nucleotides as mediators of paracrine or autocrine signaling in most tissues, much current research in the purinergic arena seeks to define the mechanisms by which intracellular nucleotides are exported to extracellular compartments. (See Ref. 6 for a concise but comprehensive summary of ATP release biology.) Although direct cytolysis is an obvious source of extracellular ATP in the setting of traumatic tissue injury, several pathways can be mobilized to release nucleotides from intact cells (Fig. 1). These include exocytosis of nucleotides compartmentalized within 1) the Golgi-derived vesicles used for constitutive secretion of glycoproteins from most cells; or 2) the vesicles and granules used for Ca2+-dependent regulated secretion of neurotransmitters and hormones from specialized secretory cells and tissues that include neurons, glia, platelets, endocrine glands, and exocrine glands. Most mammalian cell types also exhibit an increased rate of ATP export in response to various non-lytic mechanical stimuli, which include hypotonic stress-induced cell swelling, direct deformation of the surface membrane, fluid shear stress, or agonists for G protein-coupled receptors that regulate membrane-cytoskeletal rearrangements. Volume- or mechanical strain-sensitive ATP release in some cells can involve the exocytotic mechanisms noted above (1). In contrast, the ATP release response of other cells to swelling or mechanical stress is mediated by increased activities of a variety of functionally defined conductances or molecularly identified channels that are permeable to nucleotides (6, 9).
Fig. 1.
Exocytotic versus conductive pathways for export of ATP into extracellular compartments. The top part of the figure illustrates the compartmentalization of ATP and nucleotide-sugars, such as UDP-glucose, into the Golgi apparatus (and endoplasmic reticulum; not shown). Intra-Golgi ATP and nucleotide-sugars play critical roles as substrates for protein-folding chaperones and the enzymes that glycosylate secreted proteins. Residual ATP and nucleotides within the Golgi-derived secretory vesicles are released by the constitutive exocytotic pathways utilized for protein secretion. ATP can be actively accumulated within specialized secretory granules or vesicles that are mobilized during Ca2+-dependent regulated exocytosis. Cytosolic ATP can also be released via conductive pathways that involve 1) stimulus-induced gating of various ATP-permeable channels from closed to open states; and/or 2) upregulated trafficking of ATP-permeable channels from intracellular stores to the plasma membrane. The bottom part of the figure illustrates the four major types of channels that can function in conductive ATP release in various cell types. Also shown are the known topographies of individual connexin and pannexin protein subunits and the hexameric states of functional connexin subunit-based hemichannels and pannexin subunit-based channels. The depiction of maxi-anion channels or VRAC-type volume-regulated anion channels as tetrameric assemblies is purely speculative.
Studies in diverse cell types have identified or implicated four types of channels that can function as ATP efflux conduits: 1) nonjunctional hemichannels assembled as hexamers of various connexin-family subunits (2); 2) channels composed of hexameric assemblies of pannexin protein subunits (4); 3) volume-regulated anion channels (VRAC) (3); and 4) maxi-anion channels (9). The latter two have been extensively characterized by electrophysiology and pharmacology but the protein(s) that form the functional channels remain molecularly undefined. Both VRAC and maxi-anion channels are anion-selective, large conductance pathways readily gated in response to hypotonic stress. Differential sensitivity to various pharmacological inhibitors is useful for assessing the contribution from VRAC versus maxi-anion channels to anion efflux. In particular, the robust and selective suppression by extracellular Gd3+ of maxi-anion channel activity provides a hallmark criterion. In a study described in this issue of American Journal of Physiology-Cell Physiology, Islam et al. (5) have used functional and pharmacological criteria to demonstrate that maxi-anion channels comprise a major pathway for swelling-induced ATP efflux in the murine L929 fibrosarcoma cell model.
By itself, this demonstration simply adds L929 cells to the growing list of cells that utilize these channels for conductive ATP release. However, Islam et al. also observed that L929 cells expressed Panx1 mRNA, as well as functional characteristics that typify cells expressing Panx1 channels, such as enhanced permeability to cationic ethidium+ and propidium+ dyes and carbenoxolone (CBX)-inhibitable ATP efflux in response to cell swelling. Panx1 channels have been implicated in swelling-induced ATP efflux. Seminario-Vidal et al. (10) recently reported that hypotonic stress triggers robust ATP release in primary murine tracheal epithelial (MTE) cells isolated from wild-type mice but much less export in MTE cells from Panx1-knockout mice (10). Given the overlapping expression and functions of maxi-anion channels and Panx1 channels in L929 cells, Islam et al. addressed the intriguing question of whether the Panx1 gene product might comprise a molecularly defined component of the maxi-anion channel.
This could seem unlikely because permeability to organic cations, such as ethidium+, has been considered a hallmark of Panx1 channels. In contrast, maxi-anion channels are strictly anion-selective. However, recent studies have indicated that certain defining characteristics of Panx1-based channels may be less clear-cut than previously assumed. Ma et al. (7) have proposed that Panx1 channels are highly selective for anions. This raises questions regarding the interpretation of the enhanced ethidium+ or propidium+ fluxes that have been correlated with activation of Panx1 channels in several cell types. Here again, recent reports have described ethidium+/propidium+ influx responses that can be dissociated from the expression or activity of Panx1 channels (8). Thus, widely expressed channels/transporters other than Panx1 per se may act as the conduit for organic cation fluxes, with Panx1 protein complexes acting as regulators of such permeability pathways.
Given this background, Islam et al. employed both siRNA and pharmacological approaches to assess possible links between Panx1 and maxi-anion channels in L929 cells. Notably, Panx1 siRNA induced partial (30–50%) suppression of the swelling-induced ATP release measured in cell populations while having no effect on the maxi-anion currents assayed in single patch-clamped cells. The investigators also compared the effects of probenecid (which targets Panx1 channels) and Gd3+ (which targets maxi-anion channels) on both hypotonic stress-stimulated ATP efflux and single-channel currents. While probenecid partially inhibited ATP release (by 20–30%), it had no effect on the induced currents. In contrast, Gd3+ suppressed both ATP release and anion current (∼40% at 50 μM; >90% at 500 μM). When added together, probenecid and Gd3+ produced additive inhibition of swelling-induced ATP efflux. These observations indicate that Panx1 channels and maxi-anion channels comprise separate and parallel pathways for ATP efflux. A corollary conclusion is that Panx1 proteins do not comprise a molecularly defined part of the maxi-anion channel entity. Parallel studies revealed that L929 cells also express mRNA for pannexin-2 and connexin-43 (Cx43), but that treatment of L929 cells with Panx2 and Cx43 siRNA had no effect on either ATP efflux or maxi-anion type currents.
Thus, maxi-anion channels have well-defined function but still lack a molecular form. Regardless, the approach used by Islam et al. verifies that most cells are unlikely to place all their mechanical stress-induced ATP efflux “eggs” in a one basket. Murine L929 fibrosarcoma cells utilize maxi-anion channels as a predominant pathway and Panx1 channels as a minor route (5). In contrast, murine tracheal epithelial cells are characterized by a reversed pattern with gated Panx1 channels accounting for ∼80% of the ATP release response to hypotonic stress with Panx1-independent mechanisms (perhaps, maxi-anion channels?) facilitating the remaining efflux (10). Deciphering the cell- and tissue-specific contexts for predominant utilization of particular ATP efflux channels—which are at least superficially similar at the functional level—constitutes an intriguing and likely challenging question for future investigation.
GRANTS
This work was supported in part by National Institutes of Health Grant R01-GM-36387.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
G.R.D. drafted the manuscript; edited and revised the manuscript; approved the final version of the manuscript.
REFERENCES
- 1.Boudreault F, Grygorczyk R. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol 561: 499–513, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol 119: 511–520, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29: 7092–7097, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islam MR, Uramoto H, Okada T, Sabirov RZ, Okada Y. Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells. Am J Physiol Cell Physiol (July 11, 2012). doi:10.1152/ajpcell.00459.2011. [DOI] [PubMed]
- 6.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8: 359–373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma W, Compan V, Zheng W, Martin E, North RA, Verkhratsky A, Surprenant A. Pannexin 1 forms an anion-selective channel. Pflügers Arch 463: 585–592, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol 186: 6553–6561, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal 1: 311–328, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O'Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286: 26277–26286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]