Abstract
An important event during apoptosis is regulated cell condensation known as apoptotic volume decrease (AVD). Ion channels have emerged as essential regulators of this process mediating the release of K+ and Cl−, which together with osmotically obliged water, results in the condensation of cell volume. Using a Grade IV human glioblastoma cell line, we examined the contribution of calcium-activated K+ channels (KCa channels) to AVD after the addition of either staurosporine (Stsp) or TNF-α-related apoptosis-inducing ligand (TRAIL) to activate the intrinsic or extrinsic pathway of apoptosis, respectively. We show that AVD can be inhibited in both pathways by high extracellular K+ or the removal of calcium. However, BAPTA-AM was only able to inhibit Stsp-initiated AVD, whereas TRAIL-induced AVD was unaffected. Specific KCa channel inhibitors revealed that Stsp-induced AVD was dependent on K+ efflux through intermediate-conductance calcium-activated potassium (IK) channels, while TRAIL-induced AVD was mediated by large-conductance calcium-activated potassium (BK) channels. Fura-2 imaging demonstrated that Stsp induced a rapid and modest rise in calcium that was sustained over the course of AVD, while TRAIL produced no detectable rise in global intracellular calcium. Inhibition of IK channels with clotrimazole or 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34) blocked downstream caspase-3 activation after Stsp addition, while paxilline, a specific BK channel inhibitor, had no effect. Treatment with ionomycin also induced an IK-dependent cell volume decrease. Together these results show that calcium is both necessary and sufficient to achieve volume decrease and that the two major pathways of apoptosis use unique calcium signaling to efflux K+ through different KCa channels.
Keywords: apoptosis, cell volume, apoptotic volume decrease, AVD, calcium-activated potassium channels
apoptosis is one form of programmed cell death that allows the elimination of individual cells without engaging an inflammatory response in the surrounding tissue (16). Apoptosis can be observed during development where it participates in sculpting organs and tissues but also in mature organisms where it assures a constant cell population in tissues that constantly replenish some of their cells (27, 32). Two pathways of apoptosis are generally recognized. The intrinsic pathway is initiated within a cell that suffers from nutrient deprivation (28), DNA damage (33), or the generation of free radicals during redox stress (34). These various stressors result in mitochondrial disruption and the release from the mitochondrial membrane of cytochrome c, a key component in the formation of the apoptosome and the activation of caspases (15). Experimentally, a convenient method for reliably initiating the intrinsic pathway of apoptosis is through treatment with staurosporine (Stsp), a potent protein kinase inhibitor, known to cause mitochondrial disruption and the release of cytochrome c into the cytosol (3, 24). By contrast, the extrinsic pathway is initiated when external ligands, such as TNF-α, FasL, or TNF-α-related apoptosis-inducing ligand (TRAIL), bind to their respective transmembrane death receptors and directly activate caspases through adaptor proteins (36).
Despite differences in the signaling that initiates the intrinsic and extrinsic death pathways, both share important morphological and biochemical hallmarks. Importantly, common features that define both forms of apoptosis are cellular condensation, activation of downstream effector caspases, nuclear condensation, and DNA fragmentation (37). Caspase-3 is an effector caspase shared between the intrinsic and extrinsic pathways. During apoptosis initiated by the intrinsic pathway, cytochrome c, after being ejected from the mitochondrial membrane, mediates the assembly of the apoptosome, a large heteromeric protein complex that culminates in the enzymatic activation of caspase-3 (22, 35). The extrinsic pathway, in contrast, has a much more direct route for the activation of caspases. The death receptors are linked to procaspase-8 through the FADD adaptor protein, which upon ligand binding to the receptor, activates caspase-8 that then in turn activates caspase-3 (7).
Cellular condensation is considered to be an early hallmark in apoptosis and has been termed apoptotic volume decrease (AVD) (25). It appears to be a necessary event for apoptosis and one recent study (11) suggests that cell condensation alone can be sufficient to activate caspase-3. Mechanisms that mediate AVD have been actively studied in a number of cell types (6, 29, 41) and have demonstrated an essential role for K+ and Cl− efflux, providing the driving force for cytoplasmic water efflux from the cell.
A number of studies have begun to study the efflux pathways for K+, Cl−, and water that are engaged during apoptosis. These studies relied primarily on channel and transport inhibitors showing that in human pulmonary artery smooth muscle cells (19) and mouse CD4+ T lymphocytes (10) the use of high extracellular potassium or the treatment with various potassium channel inhibitors can inhibit or delay the apoptotic program. The Gardos channel of erythrocytes, now known to be the intermediate-conductance calcium-activated potassium (IK) channel (18), has been shown to be a key regulator of erythrocyte volume and mediator of erythrocyte apoptosis (21) and survival (14).
In the present study, we show that human malignant gliomas express two classes of Ca2+-activated K+ channels (KCa channels) namely IK and large-conductance calcium-activated potassium (BK) channel and that these channels play differential roles in apoptosis. IK mediates AVD in response to activation of the intrinsic pathway, while BK channels are engaged by the extrinsic pathway. Both channels differ markedly with regards to their conductance and Ca2+ sensitivity. IK channels respond to small physiological changes in Ca2+, while BK channels require rather large greater than 500 nM increases in cytosolic Ca2+ to open at the resting membrane potential (38). The differential activation of these two KCa channels in apoptosis appear to be the result of different calcium profiles during induction of apoptosis.
EXPERIMENTAL PROCEDURES
Cell culture.
The glioma cell line D54-MG (World Health Organization, grade IV, glioblastoma multiforme) was a gift from Dr. D. Bigner (Duke University, Durham, NC). Cells were maintained in DMEM/F-12 (GIBCO) containing 2 mm glutamine and supplemented with 7% FBS (Aleken Biological) at 37°C and 10% CO2.
Solutions.
The control NaCl bath solutions (normal bath) contained the following (in mM): 125 NaCl, 5.0 KCl, 10.5 glucose, 32.5 HEPES, and 1 CaCl2. The pH of each solution was adjusted to 7.4 with 10 M NaOH, and the osmolarity of each solution was confirmed with a freezing point micro-osmometer (Fiske Model 210; Fiske Associates; Norwood, MA). The osmolarities of all bath solutions were 300 ± 10 mosM. Pipette solutions contained the following (in mm): 140 KCl, 1 MgCl2, 10 EGTA, and 10 HEPES sodium salt, pH adjusted to 7.2 with Tris base. CaCl2 was added directly to pipette solution the day of use at a concentration of 4.9 mM resulting in a free Ca2+ concentration of ∼150 nM, as calculated by the Maxchelator program (http://maxchelator.stanford.edu; Ca-Mg-ATP-EGTA Calculator v1.0 using constants from NIST database #46 v8).
Drugs were added directly to bath solutions from stock solutions. Stock solutions of paxilline, clotrimazole, 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34), 2-aminoethoxydiphenyl borate (2-APB), SKF96365, and Stsp were dissolved at no less than 1,000× final concentration in DMSO such that the final concentration of DMSO never exceeded a 1:1,000 dilution, whereas TRAIL and apamin were dissolved at 1,000× final concentration in double-distilled H2O. DMSO at its final concentration (0.1%) did not perturb cell volume nor affect volume regulation (data not shown). Stsp (500 nM) was obtained from LC Laboratories (Woburn, MA), TRAIL (50 ng/ml) was obtained from Millipore (Billerica, MA), TRAM-34 (1 μM) was obtained from TOCRIS Bioscience (Ellisville, MO), and paxilline (2 μM) was obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). All other drugs were purchased from Sigma-Aldrich (St Louis, MO). Clotrimazole was used at 10 μM, 2-APB at 100 μM, SKF96365 at 25 μM, apamin at 300 nM, DIDS at 200 μM, and TEA at 2 mM.
Cell volume measurements.
Cell volumes were measured as previously described (12) by electronic sizing with a Coulter Counter Multisizer 3 (Beckman-Coulter, Miami, FL). The instrument determines the cell volume of cells suspended in solution by measuring the voltage step that occurs when a cell displaces its volume in electrolyte solution as it passes through a small aperture across which a constant current is applied. The aperture size used for these experiments was 100 μm.
To prepare the cells for volume measurements, cells between 2 and 4 days in culture were incubated for 3–5 min with 0.5 mM EGTA dissolved in a standard PBS solution. After pelleting the cells by brief centrifugation, the cells were resuspended in normal bath solution in the presence or absence of pharmacological inhibitors and incubated for 20 min before the beginning of the first baseline measurement. Cell volume measurements were obtained every minute and each measurement was an average of 10,000 cells. Ten baseline readings, during which mean cell volume varied less than 100 fl, were collected before apoptosis was induced. Each experiment was repeated no fewer than 3 times.
Data analysis for volume regulation experiments.
Coulter counter data were collected with Multisizer 3 software and exported to Excel. Time points were rounded to whole minutes, and mean cell volumes were normalized to the average baseline value for that experiment. All data were plotted in Origin 7.0 (MicroCal, Northampton, MA) as means ± SE, where Vt is the volume measured at time t and Vbaseline is the average of the baseline volumes for that experiment. Significance (P) was determined by Student's t-test of relative volumes at 60 min after Stsp addition or 80 min after TRAIL addition.
Western blotting.
Lysates were made in a standard lysis buffer containing 20 mM Tris at pH 7.4, 100 mM NaCl, 1 mM EDTA, and 1% SDS supplemented with 1:100 dilution of protease inhibitors (Sigma) and maintained on ice. Samples were briefly sonicated and centrifuged for 5 min at 12,000 g. Protein concentration of the solution was determined by Bradford using the Dc protein assay from Bio-Rad, and equal amounts of protein were prepared in a 6× sample buffer (60% glycerol, 300 nm Tris at pH 6.8, 12 mm EDTA, 12% SDS, 864 mm 2-mercaptoethanol, and 0.05% bromphenol blue) and loaded into their own individual well of a 4–20% gradient precast SDS-polyacrylamide gel (Bio-Rad). Protein separation was performed at a constant 100 V for ∼90 min. Gels were then transferred at 200 mA for 90 min at room temperature onto PVDF membrane (Millipore, Bedford, MA). Membranes were blocked in blocking buffer (5% nonfat dried milk, 2% BSA, and TBS plus 0.1% Tween 20). The primary antibodies anti-IK (AV35098, Sigma-Aldrich) and anti-BK (clone L6/60, UC-Davis/NeuroMAb Facility, Davis, CA) were used at dilutions of 1:300 and 1:500, respectively. Primary antibodies to cleaved caspase-3 were obtained from Cell Signaling Technology (no. 9664; Beverly, MA) and used at a 1:1,000 dilution. Blots were incubated in primary antibody for 1 h followed by a wash period (four times for 5 min). Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h, followed by another wash period (four times for 5 min), and developed using SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) on a Kodak Image Station 4000MM.
Electrophysiology.
Recordings of whole cell currents were made using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) following standard recording techniques (17). Patch pipettes were made with thin-walled borosilicate glass (TW150F-4; World Precision Instruments, Sarasota, FL) using an upright puller (PP-830; Narishige Instruments, Tokyo, Japan) and had resistances of 3–5 megohms. Current recordings were digitized online at 10 kHz and low pass filtered at 2 kHz using a Digidata 1320 (Axon Instruments). pClamp 9.0 (Axon Instruments) was used to acquire and store data. Series resistance (Rs) was compensated to 80%, reducing voltage errors, and cells with a compensated Rs above 10 MΩ were omitted. D54 cells were cultured on glass coverslips and allowed to adhere overnight. Cells were used between 2 and 4 days in culture.
Calcium imaging.
The AM ester of the calcium indicator dye fura-2 PE3 was purchased from Teflabs (Austin, TX) and resuspended fresh on the day of the experiment in Pluronic F-127 *20% solution in DMSO (Molecular Probes, Grand Island, NY) at a stock concentration of 1.0 μg/μl and sonicated for 5 min. The working solution for cell loading was comprised of 5 ul of the stock solution in 1 ml of 0.1% FBS containing medium (∼6 μM fura-2-AM final concentration). D54-MG glioma cells were plated on 35-mm glass bottom dishes and allowed to grow for at least 48 h under our usual cell culture procedure. The cells were then rinsed three times in serum free medium then incubated in the fura-2 loading solution for 45 min at 37°C. The cells were then rinsed three times with 7% FBS containing medium and allowed to recover for 30 min at 37°C. The cells were then rinsed three times in normal bath solution, transferred to an Olympus Spinning Disc microscope, and allowed to equilibrate for a minimum of 15 min before the start of the experiment. Cells were maintained in a humidified temperature-controlled chamber at 37°C for the duration of the experiment. Images were taken at the 340 and 380 nm excitation wavelengths every 20 s for 60–100 min. A baseline calcium measurement was obtained for 5 min before the addition of either 500 nM Stsp or TRAIL. For analysis, the ratio of 340/380 intensity was taken and normalized to the baseline.
RESULTS
Apoptotic volume decrease requires potassium efflux and is calcium dependent.
We have previously shown that AVD in D54-MG glioma cells is dependent on a DIDS-sensitive chloride conductance (11). We hypothesized that this rapid chloride efflux occurred with concomitant potassium efflux to drive osmotically obliged water across the membrane during cellular condensation. To test this hypothesis, we used this apoptotic glioma cell model system to identify the responsible potassium efflux pathways during AVD. D54-MG glioma cells were suspended in an isotonic bath solution, and their mean cell volumes were periodically monitored using a Coulter Counter maintained at 37°C. After a 20-min period of equilibration, a 10-min baseline measurement was made before the addition of either 500 nM Stsp or 50 ng/ml TRAIL to induce apoptosis by either the intrinsic or extrinsic pathway, respectively. The addition of Stsp induced a robust, rapid-onset cellular condensation compared with control cells. Beginning ∼3–5 min after Stsp addition, the cell volume decreased by ∼33% in 60 min (Fig. 1A). In contrast, cell condensation induced by the addition of TRAIL required a significant lag time of ∼30 min to begin; however, by 90 min postinduction, the mean cell volume had also condensed by ∼30% (Fig. 1B). To test the requirement of potassium efflux during glioma AVD, apoptosis was also induced in sister cultures suspended in an isotonic bath solution containing high extracellular potassium (100 mM KCl), essentially decreasing the outward directed K+ gradient. Both Stsp- and TRAIL-induced AVD were significantly impaired (80–100% reduction) in high K+ bath solution, suggesting that the efflux of potassium is a critical component required for cell condensation during glioma apoptosis (Fig. 1, A and B).
Fig. 1.
Apoptotic volume decrease is dependent on K+ efflux and Ca2+ but staurosporine (Stsp)- and TNF-α-related apoptosis-inducing ligand (TRAIL)-induced apoptotic volume decrease (AVD) express different sensitivity to BAPTA-AM. A and B: normalized mean cell volumes (MCVs) of D54-MG cells were recorded in the presence of normal bath solution with no drug addition (A) or high extracellular K+ following addition of 500 nM Stsp (A) or 50 ng/ml TRAIL (B). C and D: normalized cell volumes recorded in the absence of bath Ca2+ or chelation of intracellular Ca2+ with BAPTA-AM following addition of 500 nM Stsp (C) or 50 ng/ml TRAIL (D). Data are the mean from three independent experiments. Error bars represent SE with n = 10,000 cells.
We hypothesize that intracellular calcium elevations play an important role during the induction of apoptosis and that they may activate KCa channels to facilitate potassium efflux necessary for volume condensation. As a first step to test this hypothesis, we tested whether AVD was dependent on the presence of calcium. D54-MG cells were initially suspended in an isotonic bath solution that was devoid of any calcium (normal calcium = 1 mM), and apoptosis was initiated as before. Suspension of cells in calcium free bath solution completely abolished AVD induced by either Stsp and TRAIL (Fig. 1, C and D). A caveat to this approach, however, is that the cells were incubated in a zero calcium solution for upwards of 30 min before the apoptotic stimulus is given, which may also deplete intracellular sources of calcium and interrupt normal intracellular signals that may initiate AVD. To further investigate the calcium contribution to AVD, sister cultures were loaded with the Ca2+ chelator BAPTA-AM (50 μM) for 45 min before suspension in a bath solution containing normal calcium and the induction of apoptosis. The presence of BAPTA in cells treated with Stsp significantly attenuated AVD by >70% (Fig. 1C). In contrast, TRAIL-induced AVD was surprisingly unaffected by the presence of intracellular BAPTA (Fig. 1D). These data suggest that the underlying pathways for potassium efflux during Stsp- and TRAIL-induced AVD may rely on completely different mechanisms.
KCa channels are functionally expressed in D54-MG glioma cells.
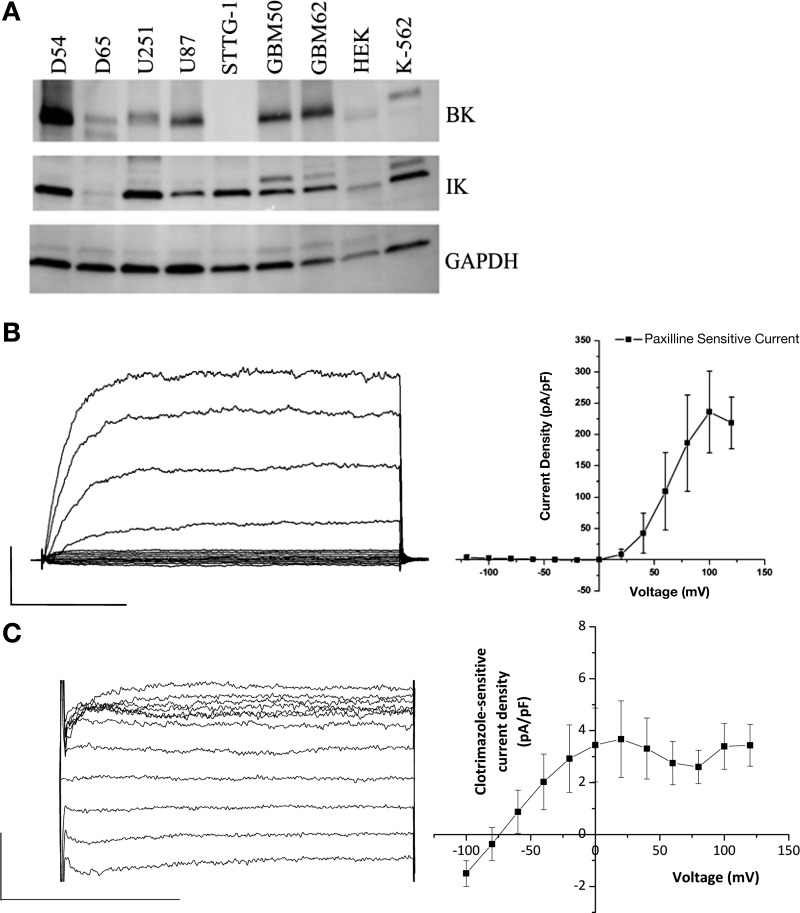
We next sought to determine the complement of KCa channels functionally expressed in D54 glioma cells that may contribute to potassium efflux during AVD. Western blot analysis reveals widespread expression of both BK and IK channels in several glioma cell lines, consistent with previous studies (1) (Fig. 2A). As previously shown (39), small-conductance calcium-activated potassium (SK) channels were not expressed in human glioma cells (data not shown). To test for functional expression, we used whole cell patch-clamp electrophysiology to detect characteristic currents of these channels. Free calcium concentration in the patch pipette was held at 150 nM to ensure detection of IK channels that may be present. Cells were held at a holding potential of −40 mV, close to their normal resting potential, and stepped from the holding potential to −120 to +120 mV in 20-mV increments. Traces before and after addition of specific inhibitors were subtracted to reveal specific drug-sensitive currents. Treatment with paxilline, a specific BK channel inhibitor, revealed a large magnitude outwardly-rectifying current that was only activated at positive potentials, consistent with the activation of BK channels (Fig. 2B). Detection of the smaller conductance IK and SK channels was performed in the continued presence of paxilline to reduce the contribution of BK currents that may mask the relatively smaller currents. Treatment with the specific IK channel inhibitor clotrimazole revealed a smaller, linear, inwardly-rectifying current with an equilibrium potential close to EK+, consistent with recruitment of IK channels (Fig. 2C). Clotrimazole did not significantly change paxilline-sensitive BK currents (at +160 mV, paxilline-sensitive currents were 44.5 ± 9.5 in control vs. 49.4 ± 14.1 pA/pF in clotrimazole). No apamin-sensitive currents were detected in the D54-MG glioma cell line, in agreement with their absence from Western blot analysis (data not shown). The absence of SK channel function in glioma cells has been previously described using whole cell patch-clamp electrophysiology (39).
Fig. 2.
D54-MG cells express functional large-conductance calcium-activated potassium channel (BK) and intermediate-conductance calcium-activated potassium channel (IK) channels. A: Western blots of glioma cell lines probed for BK channels, IK channels and GAPDH as a loading control. B and C: whole cell patch-clamp recordings of D54-MG cells with ∼150 nM internal free Ca2+ using a step protocol from a −40-mV holding potential stepping from −120 to +120 mV at 20-mV intervals. B: representative trace of paxilline-sensitive current and average data illustrating BK currents, recorded in the presence of 200 μM DIDS to block chloride currents (n = 7); scale bars represent 100 ms and 5,000 pA. C: representative trace of clotrimazole-sensitive currents and average data illustrating IK currents, recorded in the presence of 2 μM paxilline and 130 mM gluconate to block BK and chloride currents, respectively (n = 4); scale bars represent 50 ms and 150 pA.
Stsp- and TRAIL-induced apoptotic volume decrease engage different calcium-activated potassium channels.
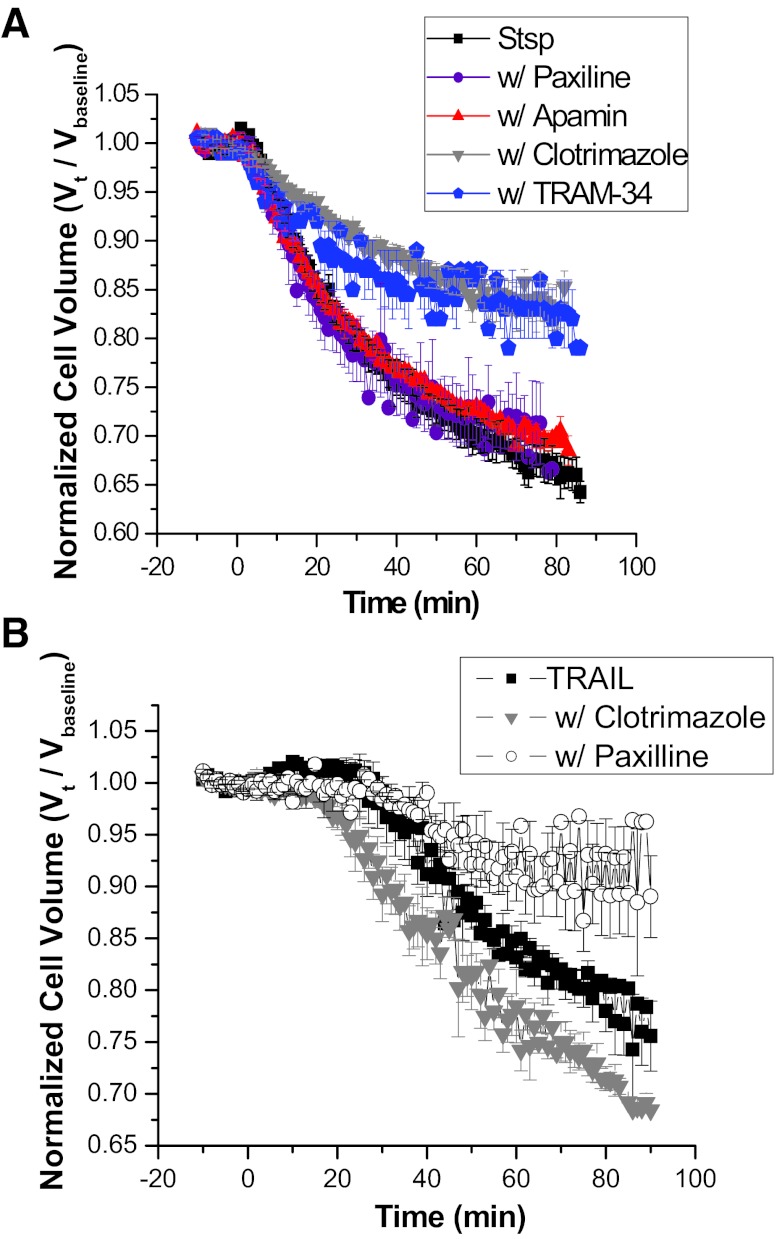
We (31, 39) and others (1, 13) have shown the presence of calcium-activated potassium channels in glioma cells; however, their exact roles in glioma cell biology are poorly understood. To elucidate their role during AVD, we used specific inhibitors to each class of KCa, namely paxilline, apamin, and clotrimazole to block BK, SK, and IK channels, respectively. As expected from the above data suggesting an absence of SK channels, the specific SK channel inhibitor apamin had no effect on Stsp-induced AVD. Paxilline, a potent blocker of BK channels, surprisingly also had no effect on Stsp-induced AVD. Interestingly, clotrimazole, an inhibitor of IK channels, was able to inhibit Stsp-induced cell condensation by over 50% (Fig. 3A). To confirm this finding, we also used TRAM-34, a more specific IK channel inhibitor (42), and obtained similar results. Taken together these data show that the relatively low conductance IK channel is predominantly responsible for potassium efflux during Stsp-induced AVD.
Fig. 3.
Intrinsic and extrinsic pathways of apoptosis use different K+ efflux pathways to achieve AVD. Normalized MCVs of D54-MG cells recorded after the addition of either Stsp (A) or TRAIL (B) in the presence of the indicated KCa channel inhibitors. Data are averaged from three independent experiments. Error bars represent SE of n = 10,000 cells. Stsp-induced AVD relies on IK channels while TRAIL-induced AVD is inhibited by BK channel antagonists.
Next we sought to determine whether TRAIL-induced AVD utilized similar mechanisms for decreasing cell volume. In stark contrast to the observations with Stsp-induced AVD, clotrimazole had no effect, while paxilline impaired TRAIL-mediated AVD by ∼50% (Fig. 3B). These data indicate that both intrinsic and extrinsic apoptosis share the common hallmark of apoptotic volume decrease but use different potassium channels to drive cellular condensation.
Apoptosis induced by Stsp or TRAIL have unique calcium signatures.
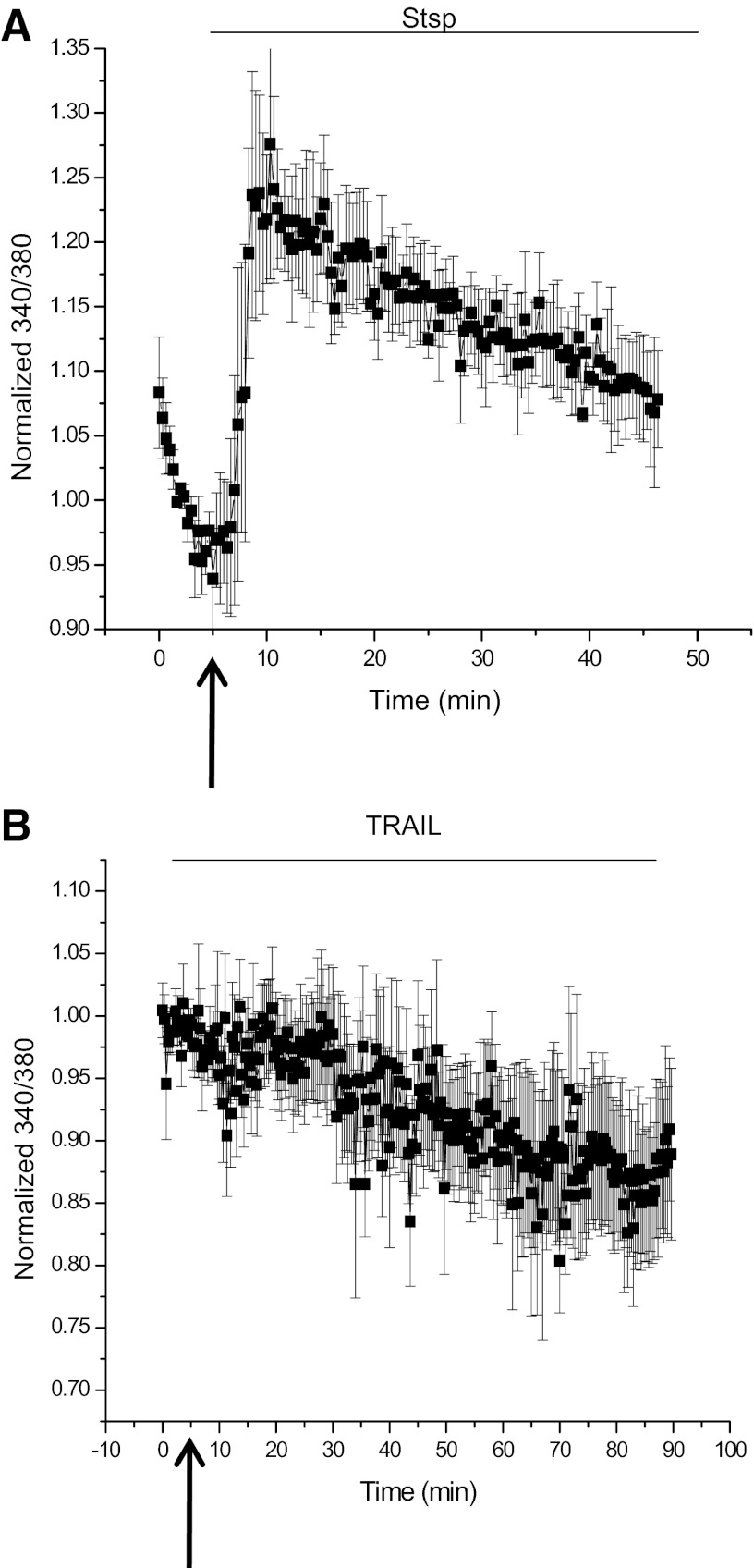
AVD is driven by separate potassium efflux pathways depending on the stimulus. While both intrinsic and extrinsic apoptosis are dependent on calcium, the two pathways have somewhat divergent requirements as seen by the differential effects of BAPTA. To investigate the calcium response induced by addition of Stsp or TRAIL, calcium concentrations were monitored using the ratiometric Ca2+ indicator dye fura-2. D54-MG glioma cells were cultured, loaded with fura-2 dye, and allowed to recover and equilibrate in normal bath solution on an Olympus Spinning Disk microscope maintained at 37°C. Addition of Stsp induced a rapid increase in calcium that was sustained over the course of an hour (Fig. 4A), consistent with the time course of AVD previously observed. Upon addition of TRAIL, however, there was no change in global calcium concentration over the course of 90 min (Fig. 4B), well past the time the cells were previously seen to decrease their volume. These data highlight rather drastic differences in calcium profiles when apoptosis is induced via the intrinsic and extrinsic pathways and emphasize the difference in KCa channels engaged in each.
Fig. 4.
Stsp and TRAIL-induced AVD have different calcium signatures. D54-MG cells were loaded with fura-2 calcium indicator dye and imaged for 5–10 min before induction of apoptosis with either Stsp (A) or TRAIL (B). Images at 340 and 380 nm were obtained every 20 s for the duration of the observed AVD during volume measurements, and the 340/380 ratio was normalized to baseline.
Stsp-induced AVD is dependent on calcium release from intracellular stores and sustained calcium influx from the extracellular space.
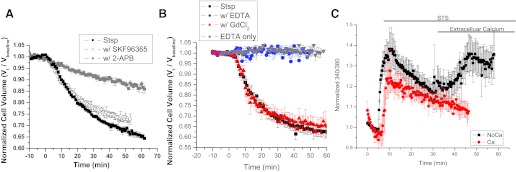
We next sought to determine possible sources for calcium during Stsp-induced, IK-mediated AVD. Initially we loaded the cells for 45 min with 50 μM BAPTA-AM before the experiment. Loading the cells with BAPTA was effective at preventing AVD induced by Stsp, supporting the need for calcium in the process. Next, we used 2-APB to block inositol triphosphate (IP3) receptors to determine the contribution of calcium release from intracellular stores. AVD was reduced by >60% in the presence of 2-APB, suggesting that release of intracellular calcium stores is an essential component in this process (Fig. 5A). This is consistent with the model of Stsp-induced apoptosis where Stsp disrupts the mitochondrial membrane, releasing cytochrome c into the cytoplasm where it can bind IP3 receptors and cause calcium release from intracellular stores (26). However, 2-APB is also known to inhibit calcium entry from the extracellular space through transient receptor potential canonical (TRPC) channels, a channel previously shown to be present and important in glioma cell biology (4, 5). To determine the contribution of TRPC channels during AVD, the TRPC channel inhibitor SKF96365 was used. SKF96365 was unable to significantly inhibit Stsp-induced AVD, suggesting that TRPC-mediated calcium entry does not play a significant role (Fig. 5A). To further elucidate the role extracellular calcium may be playing during AVD, we next used 1 μM GdCl3, a known inhibitor of both TRPC channels and Orai channels responsible for store-operated calcium entry. The presence of GdCl3 was also unable to impair AVD, at least, through the most obvious means of store-operated calcium entry. Finally, 1 mM EDTA was acutely added to the bath solution 1–2 min before the addition of Stsp to chelate the majority of extracellular calcium before induction of apoptosis. In the presence of extracellular EDTA, the cells did not appreciably change their volume after addition of Stsp. (Fig. 5B). Cell volume was not appreciably affected by the acute addition of 1 mM EDTA alone (Fig. 5B). These data suggest that Stsp-induced AVD is mediated by both calcium release from intracellular stores and calcium influx from the extracellular space to sustain cell condensation; however, the exact route of sustained calcium entry remains unknown.
Fig. 5.
Stsp-induced AVD is dependent on Ca2+ from intracellular stores and from the extracellular space. Normalized MCVs of D54-MG cells recorded after addition of 500 nM Stsp in the presence of various calcium source inhibitors; aminoethoxydiphenyl borate (2-APB) a inositol triphosphate receptor inhibitor and SKF96365 to inhibit Trp channels (A) or GdCl3 to inhibit Orai store-operated channels and EDTA to acutely chelate extracellular Ca2+ (B). EDTA without Stsp addition was used as a control. C: D54-MG cells were loaded with fura-2 calcium indicator dye and imaged for 5–10 min before induction of apoptosis with either Stsp in the presence or absence of extracellular calcium. Extracellular calcium was then reapplied. Images at 340 and 380 nm were obtained every 20 s and the 340/380 ratio was normalized to the baseline. Data represent the mean results of three independent experiments. Error bars represent SE with n = 10,000 cells.
To further understand the role of calcium in AVD, we observed Stsp-induced changes in intracellular calcium levels using fura-2. Using similar calcium imaging methods as previously described, we acquired a 5-min baseline and then applied Stsp both in the presence and absence of extracellular calcium. The increase in intracellular calcium was not different in the presence or absence extracellular calcium (Fig. 5C), providing further evidence that AVD is regulated by calcium from intracellular stores. However, when extracellular calcium was replaced, there was an immediate rise in intracellular calcium, implicating store-operated calcium entry in Stsp-induced AVD. We conclude that channel-mediated influx and intracellular store release both play a role in Stsp-induced calcium rises.
IK inhibitors decrease caspase-3 activation during Stsp-induced apoptosis.
Since AVD is a common hallmark of apoptotic events, we next wanted to determine if disruption of cellular condensation would also impair downstream events in the apoptotic cascade. One of the major endpoints of the apoptotic cascade is the activation of the so-called effector caspases, including caspase-3, a common effector caspase between both pathways of apoptosis. D54-MG glioma cell cultures were incubated with vehicle control or specific KCa channel inhibitors in a warm isotonic bath solution for 20 min before the addition of Stsp. Cells were maintained at 37°C and allowed to undergo apoptosis for 5 h. Adherent and nonadherent cells were then collected on ice, and cell lysates were made. Equal amounts of protein were separated using a 20% SDS-PAGE gel, and a Western blot was performed to determine the amount of activated caspase-3 that was generated. After 5 h, the Stsp alone condition showed a robust formation of both the 17- and 19-kDa forms of activated caspase-3. Paxilline was unable to appreciably diminish the amount of cleaved caspase-3 that was formed, and apamin only minimally reduced the amount of cleaved caspase-3. However, the specific IK inhibitors clotrimazole and TRAM-34 showed the largest reduction in the amount of active caspase-3 that was generated in 5 h (Fig. 6). These data indicate that during Stsp-induced apoptosis specific activation of IK channels to facilitate cell condensation is an essential component in the process of efficiently producing activated caspase-3. Inhibition of IK channels attenuates the amount of activated caspase formed or at least slows the process.
Fig. 6.
Caspase-3 activation is inhibited by specific IK antagonist during Stsp-induced AVD. Western blot of lysates made from D54-MG cells exposed to Stsp for 5 h in the presence of KCa channel inhibitors. Specific IK channel antagonists clotrimazole and 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34) reduce the activation of caspase-3 while paxilline and apamin had no effect.
Calcium elevation alone is sufficient to induce IK-mediated cell volume condensation.
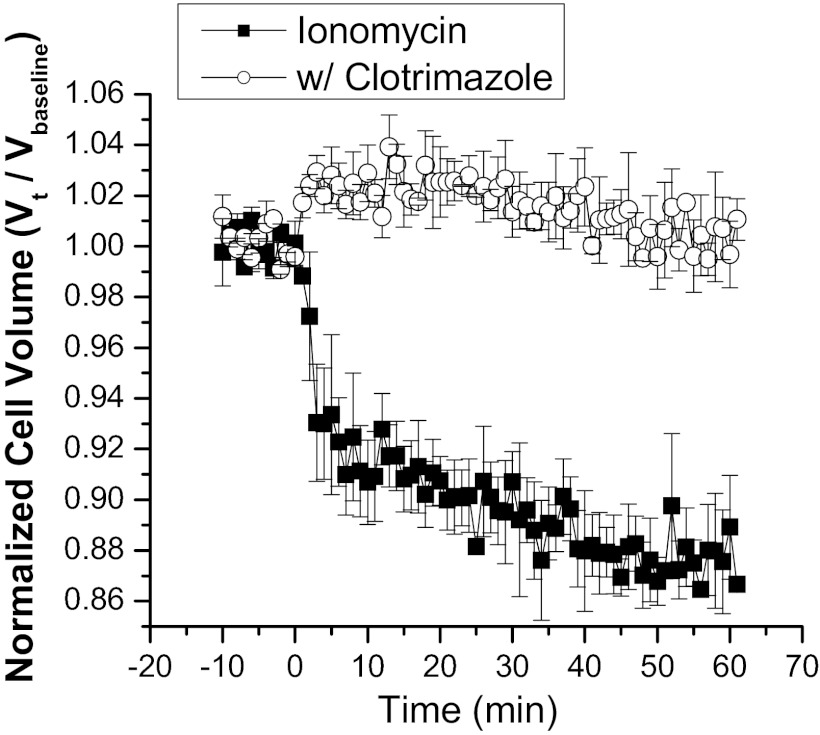
Finally, we wanted to test whether general elevation of intracellular calcium alone was able to induce a concomitant cell volume decrease dependent on the activation of IK channels. The calcium ionophore ionomycin acts as a mobile ion carrier and was added to increase intracellular calcium. D54-MG cells were suspended as before in isotonic bath solution and mean cell volume monitored on a Coulter Counter. Upon addition of 1 μM ionomycin, the cells rapidly responded with a steady cell volume decrease, resulting in an ∼13% volume decrease after 60 min (Fig. 7). This ionomycin-induced cell volume decrease was completely inhibited in the presence of the IK inhibitor clotrimazole. This suggests that one of the direct and immediate targets of global and sustained intracellular calcium increases is the IK channel. Due to K+ efflux, water osmotically leaves the cell resulting in cellular condensation.
Fig. 7.
Ionomycin-induced volume decrease can be inhibited by clotrimazole. Normalized cell volumes recorded after addition of the Ca2+ ionophore ionomycin. Ionomycin alone induced a steady volume decrease over time that could be blocked by the IK antagonist clotrimazole. Data are the mean of three independent experiments. Error bars represent SE with n = 10,000 cells.
DISCUSSION
In this study, we show that KCa channels are the essential potassium efflux pathways mediating the apoptotic volume decrease that precedes programmed cell death in D54-MG glioma cells. Unexpectedly we found that the two possible apoptotic pathways, intrinsic and extrinsic, engage different KCa channels, namely IK and BK channels, respectively. Both pathways depend on K+ efflux since disruption of the outward K+ gradient was sufficient to abolish AVD regardless of whether we activated the intrinsic or extrinsic death cascade. Furthermore, calcium was similarly required for both apoptotic pathways as the complete absence of calcium from the bath solution prevented AVD. However, loading the cells with the membrane permeable Ca2+ buffer BAPTA-AM before induction of apoptosis revealed different requirements for calcium during AVD induced via the intrinsic or extrinsic pathways. Stsp-induced AVD was essentially inhibited in the presence of BAPTA (Fig. 1C), while TRAIL-induced AVD was completely insensitive to the presence of BAPTA. These data suggest that the underlying ion channels may differ in their Ca2+ sensitivity and importantly their relationship to the source of the Ca2+ increase. We suggest that global calcium changes are required to engage IK channels whereas focal changes are sufficient to activate BK. The inability of BAPTA to diminish TRAIL-induced AVD (Fig. 1D) and the requirement for relatively high calcium concentration for activation suggest that the BK channels recruited for AVD are located close to their calcium source, such that the calcium is able to act on the channels before BAPTA is able to buffer the calcium elevation. Indeed, this proximity requirement for BK channels to be located near their calcium source has been well established in neuronal literature (2) as well as glioma cells (40).
The differential role of IK and BK as mediators of AVD was most specifically revealed by the use of specific pharmacological inhibitors. Stsp was used to induce the intrinsic pathway of apoptosis, which induced a rapid and sustained global calcium signal and triggered opening of IK channels to efflux potassium during AVD. Treatment with either clotrimazole or the more specific TRAM-34 to inhibit IK channels was able to significantly impair cell condensation, suggesting that IK channels are a major pathway for potassium efflux engaged in this pathway. In contrast, the use of TRAIL to induce the extrinsic pathway of apoptosis used BK channels instead of IK channels for the efflux of potassium required for AVD. Treatment with clotrimazole was unable to impair TRAIL-induced AVD, while the specific BK inhibitor paxilline showed a reduction in cell condensation. Curiously, no global changes were detected when TRAIL was applied. Taken together with the BAPTA data discussed above, this suggests that the BK channels are indeed located close to their calcium source and were able to effectively function as mediators of potassium efflux during volume decrease while global calcium levels appeared relatively unaltered.
To our knowledge, this is the first report on a role for KCa channels as mediators of apoptosis in human gliomas. Also, and perhaps more significantly, this is to our knowledge the first report that the different apoptosis pathways have dramatically different calcium profiles and activate different downstream KCa channels to achieve apoptotic volume decrease. The relationships between the calcium profiles and electrophysiological properties of the KCa channels involved are interesting and, in hindsight, not altogether surprising. The IK channel, engaged during Stsp-induced apoptosis, is voltage independent and requires much lower levels of calcium to be activated. However, because their single channel conductance is considerably lower than that of BK channels (38), they may require the prolonged global calcium increases observed to flux sufficient potassium ions to drive volume decrease. In contrast, during TRAIL-induced apoptosis, there were no significant global changes of calcium observed. This suggests that the BK channels engaged during AVD in this pathway must receive transient localized calcium signals that provide sufficient elevations of calcium within microdomains that escape our detection. These microdomains of calcium must be high enough to allow for the opening of the channel at resting membrane potential. As the BK channel has a much larger single channel conductance (38), it is possible then that these channels are able to flux sufficient potassium ions during their transient openings to effectively achieve volume decrease and are not dependent on sustained calcium increases. Furthermore, glioma cells express an isoform of BK channels that is characterized by enhanced sensitivity to intracellular calcium (23). Additionally, IP3 receptors colocalize with BK channels in glioma cells (40). Therefore, it is likely that calcium increases at microdomain levels could activate BK channels. Alternatively, BK channels could be activated by an auxiliary protein, therefore bypassing the need for membrane depolarization and high intracellular calcium levels, as seen in LNCaP prostate cancer cells (43). Further experiments are required to confirm the mechanism of BK channel activation.
While AVD is always observed in response to an apoptotic stimulus, we also wanted to assure that AVD and hence the activity of these KCa channels are necessary for apoptosis to proceed. Our studies probing for cleaved caspase-3 suggest that inhibition of IK channels in the presence of Stsp reduces the amount of cleaved caspsase-3 indicating a rescue from the intrinsic death pathway when IK-mediated condensation was inhibited. Correlative observations have been made in CD4+ T lymphocytes (10), erythrocytes (14, 21), and K-562 erythroleukemia cells (21) where intracellular calcium increases induced apoptosis in an IK-mediated manner. Specific inhibitors to or genetic knockdown of IK channels was able to prevent AVD and downstream apoptotic events; i.e., phosphatidylserine translocation, in these cell types, similar to what we have observed of caspase-3 activation in our glioma cell model. These findings are also conceptually in agreement with a previous complementary study (11) examining the necessity and sufficiency of AVD in gliomas. In this study, AVD was inhibited by preventing the efflux of Cl−, which also inhibited the cleavage of caspase-3. Hence, AVD appears necessary for apoptosis to occur. Our data therefore suggests that Cl− and K+ efflux is required to accomplish water secretion and hence AVD. Blockade of either anion or cation efflux prevents the volume change and hence curtails apoptosis.
These findings are likely applicable to other cell types that must engage K+ and Cl− channels to condense their volume. However, different cells are likely to engage molecularly and biophysically different channel types. Indeed, during neuronal apoptosis, cortical neurons use the delayed rectifier channel Kv2.1 to efflux potassium as revealed by several studies (30, 44, 45). Pulmonary smooth muscle cells have been shown to use both BK channels (8, 20) as well as 4-AP-sensitive voltage-gated K+ channels (9) during different models of apoptosis. As observed in the present study, the specific K+ channel engaged during AVD may depend on the specific stimulus given and pathways activated in other cell types.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01-NS-031234 and R01-NS-036692 (to H. Sontheimer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.B.M., K.L.T., V.A.C., and H.S. conception and design of research; M.B.M., K.L.T., and V.A.C. performed experiments; M.B.M., K.L.T., and V.A.C. analyzed data; M.B.M., K.L.T., V.A.C., and H.S. interpreted results of experiments; M.B.M., K.L.T., and V.A.C. prepared figures; M.B.M., K.L.T., and V.A.C. drafted manuscript; M.B.M., K.L.T., V.A.C., and H.S. edited and revised manuscript; M.B.M., K.L.T., V.A.C., and H.S. approved final version of manuscript.
REFERENCES
- 1.Abdullaev IF, Rudkouskaya A, Mongin AA, Kuo YH. Calcium-activated potassium channels BK and IK1 are functionally expressed in human gliomas but do not regulate cell proliferation. PLos One 5: e12304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314: 615–620, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bertrand R, Solary E, O'Connor P, Kohn KW, Pommier Y. Induction of a common pathway of apoptosis by staurosporine. Exp Cell Res 211: 314–321, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Bomben VC, Sontheimer H. Disruption of Transient Receptor Potential Canonical Channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia 58: 1145–1156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomben VC, Turner KL, Barclay TT, Sontheimer H. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. J Cell Physiol 226: 1879–1888, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bortner CD, Hughes FM, Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem 272: 32436–32442, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Denault JB, Salvesen GS. Caspases: keys in the ignition of cell death. Chem Rev 102: 4489–4500, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Eid T, Williamson A, Lee TS, Petroff OA, de Lanerolle NC. Glutamate and astrocytes–key players in human mesial temporal lobe epilepsy? Epilepsia 49, Suppl 2: 42–52, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Ekhterae D, Platoshyn O, Krick S, Yu Y, McDaniel SS, Yuan JX. Bcl-2 decreases voltage-gated K+ channel activity and enhances survival in vascular smooth muscle cells. Am J Physiol Cell Physiol 281: C157–C165, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Elliott JI, Higgins CF. IKCa1 activity is required for cell shrinkage, phosphatidylserine translocation and death in T lymphocyte apoptosis. EMBO Rep 4: 189–194, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernest NJ, Habela CW, Sontheimer H. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J Cell Sci 121: 3–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol 288: C1451–C1460, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fioretti B, Castigli E, Micheli MR, Bova R, Sciaccaluga M, Harper A, Franciolini F, Catacuzzeno L. Expression and modulation of the intermediate-conductance Ca2+-activated K+ channel in glioblastoma GL-15 cells. Cell Physiol Biochem 18: 47–56, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Foller M, Bobbala D, Koka S, Boini KM, Mahmud H, Kasinathan RS, Shumilina E, Amann K, Beranek G, Sausbier U, Ruth P, Sausbier M, Lang F, Huber SM. Functional significance of the intermediate conductance Ca2+-activated K+ channel for the short-term survival of injured erythrocytes. Pflügers Arch 460: 1029–1044, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Green DR, Reed JC. Mitochondria and apoptosis. Science 281: 1309–1312, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Haanen C, Vermes I. Apoptosis and inflammation. Mediators Inflamm 4: 5–15, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA 94: 11651–11656, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krick S, Platoshyn O, Sweeney M, Kim H, Yuan JX. Activation of K+ channels induces apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol 280: C970–C979, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Krick S, Platoshyn O, Sweeney M, McDaniel SS, Zhang S, Rubin LJ, Yuan JX. Nitric oxide induces apoptosis by activating K+ channels in pulmonary vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 282: H184–H193, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol Cell Physiol 285: C1553–C1560, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Chang Y, Reinhart PH, Sontheimer H, Chang Y. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci 22: 1840–1849, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA 97: 9487–9492, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol 5: 1041–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Meier P, Finch A, Evan G. Apoptosis in development. Nature 407: 796–801, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Mercille S, Massie B. Induction of apoptosis in nutrient-deprived cultures of hybridoma and myeloma cells. Biotechnol Bioeng 44: 1140–1154, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Okada Y, Shimizu T, Maeno E, Tanabe S, Wang X, Takahashi N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J Membr Biol 209: 21–29, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci 23: 4798–4802, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ransom CB, Sontheimer H. BK channels in human glioma cells. J Neurophysiol 85: 790–803, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 109, Suppl: S97–107, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med 12: 440–450, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Slater AF, Stefan C, Nobel I, van den Dobbelsteen DJ, Orrenius S. Signalling mechanisms and oxidative stress in apoptosis. Toxicol Lett 82–83: 149–153, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol 144: 281–292, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorburn A. Death receptor-induced cell killing. Cell Signal 16: 139–144, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Van Cruchten S, Van Den BW. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 31: 214–223, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol 8: 321–329, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Weaver AK, Bomben VC, Sontheimer H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 54: 223–233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver AK, Olsen ML, McFerrin MB, Sontheimer H. BK channels are linked to IP3-receptors via lipid rafts: A novel mechanism for coupling [Ca2+]1 to ion channel activation. J Biol Chem 282: 31568, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei L, Xiao AY, Jin C, Yang A, Lu ZY, Yu SP. Effects of chloride and potassium channel blockers on apoptotic cell shrinkage and apoptosis in cortical neurons. Pflügers Arch 448: 325–334, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA 97: 8151–8156, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466: 513–516, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 278: 114–117, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Zaks-Makhina E, Kim Y, Aizenman E, Levitan ES. Novel neuroprotective K+ channel inhibitor identified by high-throughput screening in yeast. Mol Pharmacol 65: 214–219, 2004 [DOI] [PubMed] [Google Scholar]