Abstract
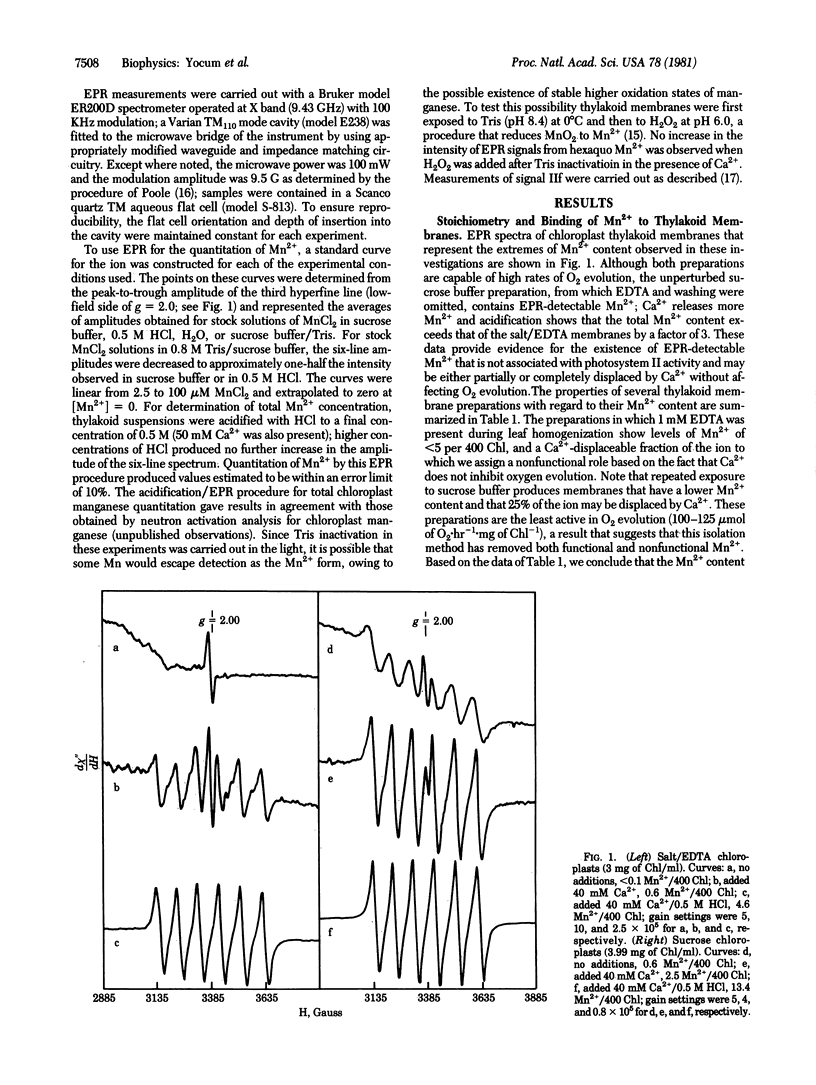
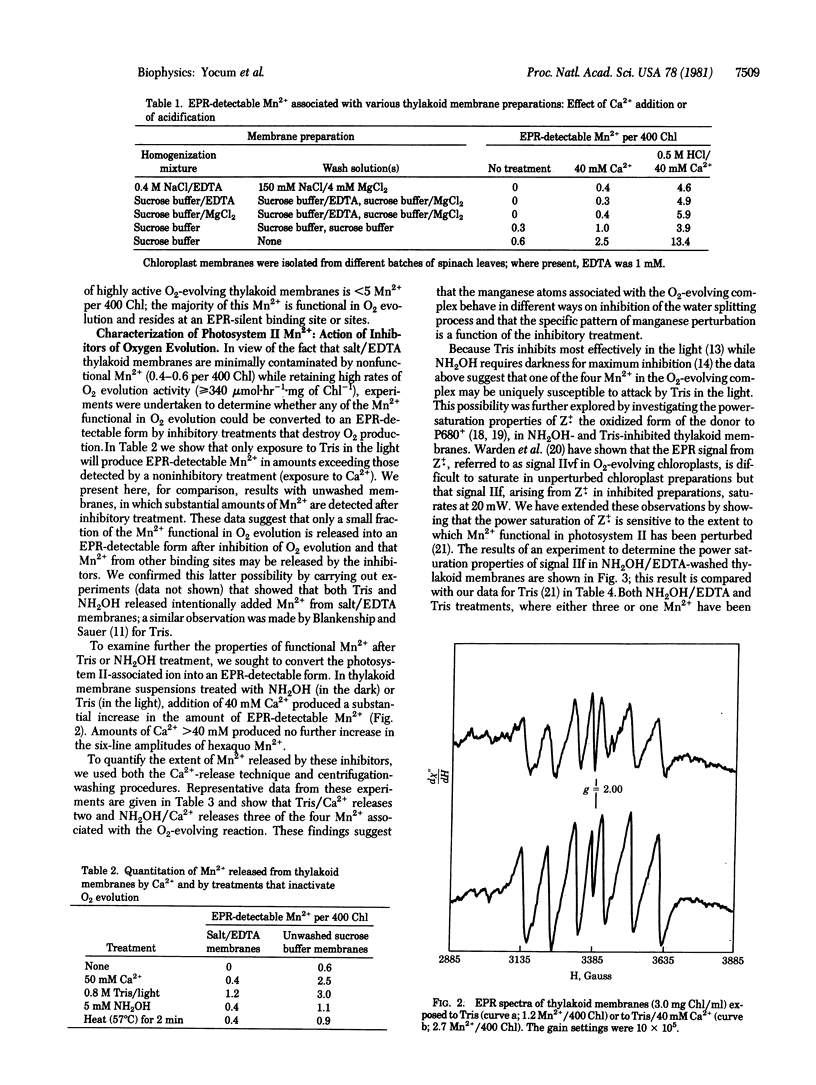
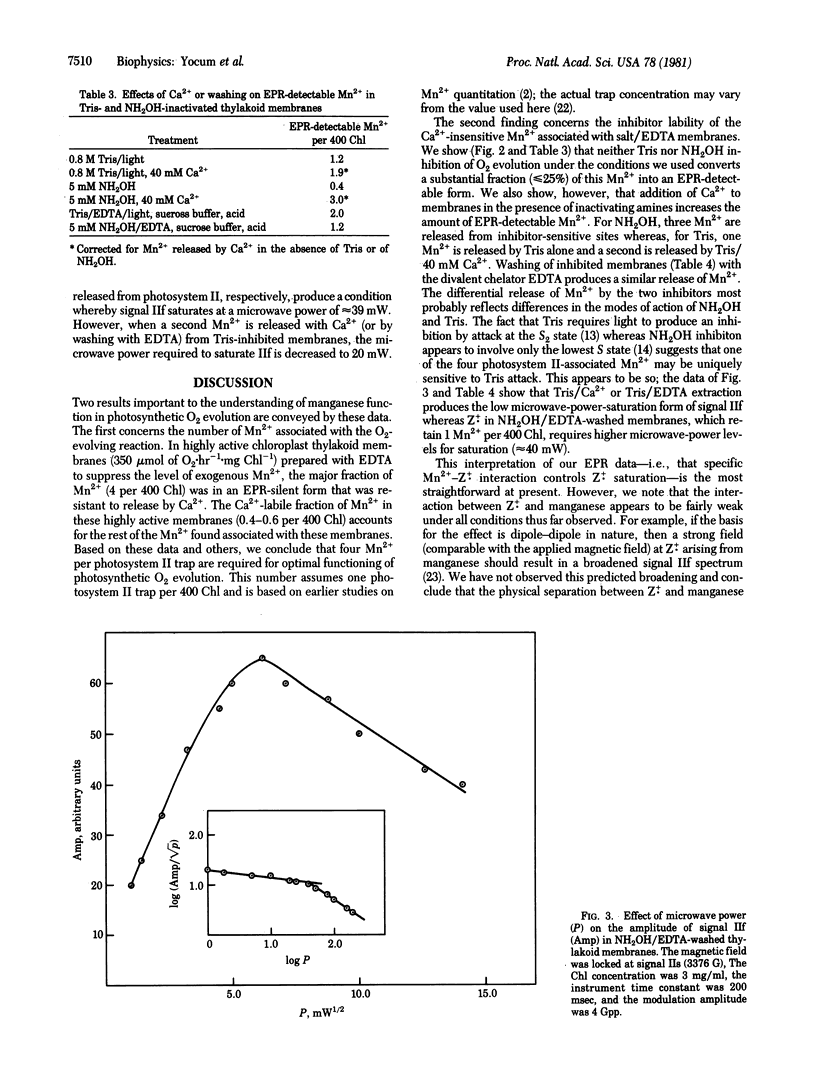
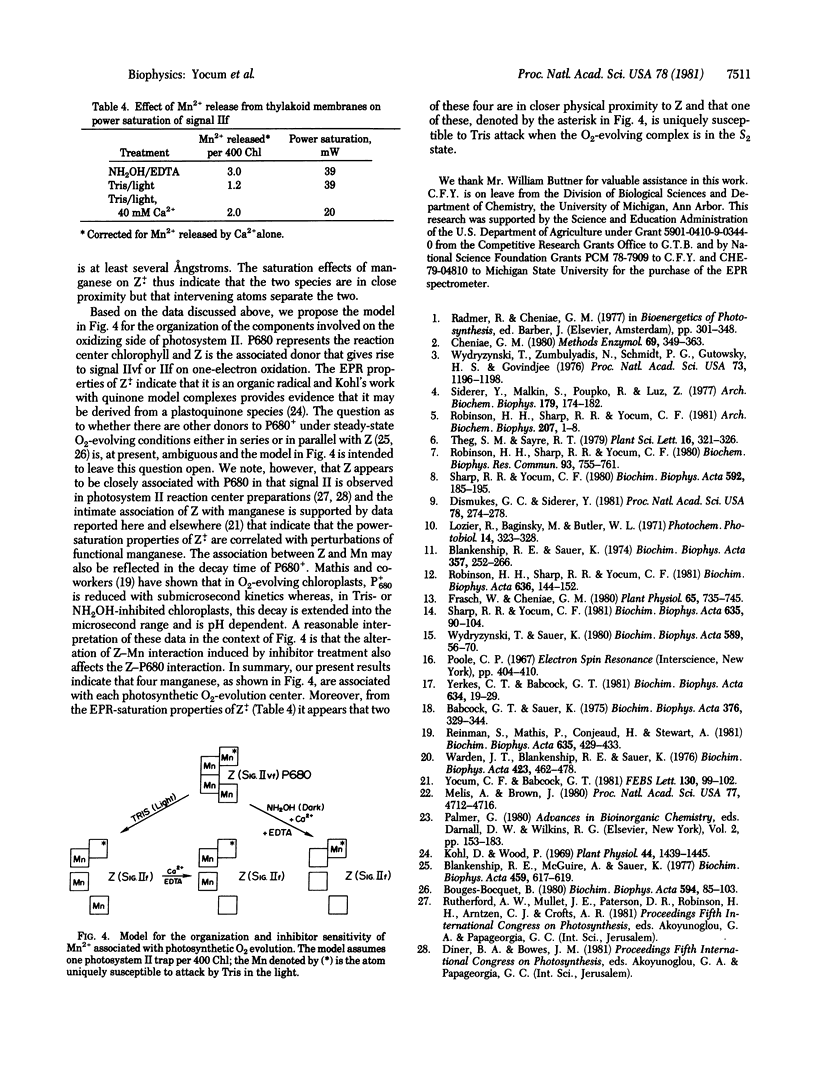
Chloroplast thylakoid membranes isolated in the presence of EDTA retain high rates of O2 evolution (≥340 μmol·h-1·mg chlorophyll-1) but contain no Mn2+ that is detectable by electron paramagnetic resonance (EPR) at room temperature. The total Mn2+ content of these preparations is 4.6 per 400 chlorophylls; 0.6 Mn2+ can be released by addition of Ca2+, a treatment that does not affect O2 evolution. The remaining Mn2+ (4 per 400 chlorophylls) appears to be functionally associated with O2 evolution activity. Inhibition by Tris, NH2OH, or heat will release a small fraction of Mn2+ from these membranes (≈25% with Tris, for example). Addition of Ca2+ further enhances Mn2+ release so that for Tris and for NH2OH, 2 and 3, respectively, Mn2+ per 400 chlorophylls are extracted from the O2-evolving complex. Based on the microwave power-saturation properties of the EPR signal IIf, which arises from an intermediate electron carrier in the water splitting process, it appears that one of the four Mn2+ associated with photosystem II is uniquely sensitive to Tris. A new model is proposed for the organization and inhibitor sensitivity of manganese in the O2-evolving complex.
Keywords: photosynthesis, electron paramagnetic resonance
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Sauer K. The rapid component of electron paramagnetic resonance signal II: a candidate for the physiological donor to photosystem II in spinach chloroplasts. Biochim Biophys Acta. 1975 Feb 17;376(2):329–344. doi: 10.1016/0005-2728(75)90025-0. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E., McGuire A., Sauer K. Rise time of EPR signal IIvf in chloroplast photosystem II. Biochim Biophys Acta. 1977 Mar 11;459(3):617–619. doi: 10.1016/0005-2728(77)90062-7. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E., Sauer K. Manganese in photosynthetic oxygen evolution. I. Electron paramagnetic resonance study of the environment of manganese in Tris-washed chloroplasts. Biochim Biophys Acta. 1974 Aug 23;357(2):252–266. doi: 10.1016/0005-2728(74)90065-6. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Kinetic models for the electron donors of photosystem II of photosynthesis. Biochim Biophys Acta. 1980 Dec;594(2-3):85–103. doi: 10.1016/0304-4173(80)90006-3. [DOI] [PubMed] [Google Scholar]
- Dismukes G. C., Siderer Y. Intermediates of a polynuclear manganese center involved in photosynthetic oxidation of water. Proc Natl Acad Sci U S A. 1981 Jan;78(1):274–278. doi: 10.1073/pnas.78.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch W. D., Cheniae G. M. Flash Inactivation of Oxygen Evolution: IDENTIFICATION OF S(2) AS THE TARGET OF INACTIVATION BY TRIS. Plant Physiol. 1980 Apr;65(4):735–745. doi: 10.1104/pp.65.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl D. H., Wood P. M. On the Molecular Identity of ESR Signal II Observed in Photosynthetic Systems: The Effect of Heptane Extraction and Reconstitution With Plastoquinone and Deuterated Plastoquinone. Plant Physiol. 1969 Oct;44(10):1439–1445. doi: 10.1104/pp.44.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Brown J. S. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinman S., Mathis P., Conjeaud H., Stewart A. Kinetics of reduction of the primary donor of photosystem II. Influence of pH in various preparations. Biochim Biophys Acta. 1981 Apr 13;635(2):429–433. doi: 10.1016/0005-2728(81)90040-2. [DOI] [PubMed] [Google Scholar]
- Robinson H. H., Sharp R. R., Yocum C. F. Effect of manganese on the nuclear magnetic relaxivity of water protons in chloroplast suspensions. Biochem Biophys Res Commun. 1980 Apr 14;93(3):755–761. doi: 10.1016/0006-291x(80)91141-9. [DOI] [PubMed] [Google Scholar]
- Robinson H. H., Sharp R. R., Yocum C. F. On the origin of light-induced changes in the proton magnetic relaxation rate of chloroplast thylakoid membrane suspensions. Arch Biochem Biophys. 1981 Mar;207(1):1–8. doi: 10.1016/0003-9861(81)90001-1. [DOI] [PubMed] [Google Scholar]
- Robinson H. H., Sharp R. R., Yocum C. F. Topology of NH2OH induced Mn(II) release from chloroplast thylakoid membranes. Biochim Biophys Acta. 1981 Jul;636(2):144–152. doi: 10.1016/0005-2728(81)90087-6. [DOI] [PubMed] [Google Scholar]
- Sharp R. R., Yocum C. F. Factors influencing hydroxylamine inactivation of photosynthetic water oxidation. Biochim Biophys Acta. 1981 Mar 12;635(1):90–104. doi: 10.1016/0005-2728(81)90010-4. [DOI] [PubMed] [Google Scholar]
- Sharp R. R., Yocum C. F. Field-dispersion profiles of the proton spin-lattice relaxation rate in chloroplast suspensions. Effect of manganese extraction by EDTA, Tris, and hydroxylamine. Biochim Biophys Acta. 1980 Aug 5;592(1):185–195. doi: 10.1016/0005-2728(80)90124-3. [DOI] [PubMed] [Google Scholar]
- Siderer Y., Malkin S., Poupko R., Luz Z. Electron spin resonance and photoreaction of Mn(II) in lettuce chloroplasts. Arch Biochem Biophys. 1977 Feb;179(1):174–182. doi: 10.1016/0003-9861(77)90101-1. [DOI] [PubMed] [Google Scholar]
- Warden J. T., Blankenship R. E., Sauer K. A flash photolysis ESR study of photosystem II signal IIvf, the physiological donor to P-680+. Biochim Biophys Acta. 1976 Mar 12;423(3):462–478. doi: 10.1016/0005-2728(76)90201-2. [DOI] [PubMed] [Google Scholar]
- Wydrzynski T., Sauer K. Periodic changes in the oxidation state of manganese in photosynthetic oxygen evolution upon illumination with flashes. Biochim Biophys Acta. 1980 Jan 4;589(1):56–70. doi: 10.1016/0005-2728(80)90132-2. [DOI] [PubMed] [Google Scholar]
- Wydrzynski T., Zumbulyadis N., Schmidt P. G., Gutowsky H. S., Govindjee Proton relaxation and charge accumulation during oxygen evolution in photosynthesis. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1196–1198. doi: 10.1073/pnas.73.4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes C. T., Babcock G. T. Surface charge asymmetry and a specific calcium ion effect in chloroplast photosystem II. Biochim Biophys Acta. 1981 Jan 14;634(1):19–29. doi: 10.1016/0005-2728(81)90124-9. [DOI] [PubMed] [Google Scholar]


