Abstract
BACKGROUND AND PURPOSE
Neuropeptide Y (NPY) is a 36-amino acid polypeptide found abundantly in the central and peripheral nervous systems. NPY exerts a potent depressor effect via the activation of both Y1 and Y2 receptors in the nucleus tractus solitarii (NTS) of rats. However, the precise mechanisms involved in this NPY-mediated action remained unclear.
EXPERIMENTAL APPROACH
Effects of a selective antagonist of Y1 receptors, a PKC inhibitor, a PI3 kinase inhibitor, a NOS inhibitor, an endothelial NOS (eNOS)-selective inhibitor, a neuronal NOS (nNOS)-specific inhibitor or a MAPK inhibitor, on responses to microinjection of NPY into the NTS of Wistar-Kyoto rats were studied to determine the underlying mechanisms. Blood pressure and heart rate were measured and, in NTS, protein phosphorylation assessed by immunohistochemical techniques.
KEY RESULTS
Unilateral microinjection of exogenous NPY (4.65 pmol/60 nL) into the NTS of urethane-anesthetized Wistar-Kyoto rats markedly decreased blood pressure and heart rate. Microinjection of the Y1 receptor antagonist BIBP3226 or the Gi/Go-protein inhibitor, Pertussis toxin, into the NTS attenuated these NPY-induced hypotensive effects. A selective Y1 receptor agonist increased expression of ERK1/2, ribosomal protein S6 kinase (RSK) and the phosphorylation of eNOS. RSK also bound directly to eNOS and induced its phosphorylation at Ser1177. Pretreatment of the NTS with an eNOS inhibitor, but not a nNOS inhibitor, attenuated the NPY-induced hypotensive effects.
CONCLUSIONS AND IMPLICATIONS
Together, these results suggested that NPY-induced depressor effects were mediated by activating NPY Y1 receptor-PKC-ERK-RSK-eNOS and Ca2+-eNOS signalling pathways, which are involved in regulation of blood pressure in the NTS.
Keywords: extracellular signal-regulated kinases1/2, neuropeptide Y, nucleus tractus solitarii, ribosomal protein S6 kinase, nitric oxide
Introduction
The nucleus tractus solitarii (NTS) is located in the dorsal medulla of the brainstem, which is the primary centre for integrating cardiovascular control and other autonomic functions in the CNS. Our previous studies demonstrated that neuropeptide Y (NPY) played an important role in central cardiovascular control (Tseng et al., 1989). However, how NPY exerted its effects on the NTS remained unclear.
NPY was first isolated from porcine brain (Tatemoto et al., 1982) and is a 36-amino acid polypeptide, sharing extensive sequence homology with pancreatic polypeptide (PP) and peptide YY (PYY). PYY and PP are mainly secreted into systemic blood by the gut and pancreas, respectively, and have thus been included in the same peptide family called the Y or NPY family (Michel et al., 1998; Mahaut et al., 2010). In rats, these peptides exert pleiotropic physiological actions, which are mediated by the activation of at least one of the four receptor subtypes (Y1, Y2, Y4 and Y5; receptor nomenclature follows Alexander et al., 2011). All these receptors have been cloned and are seven transmembrane GPCRs. NPY is extensively expressed mainly in the brainstem (NTS, area postrema and dorso-motor nucleus of the vagus) and peripheral sympathetic nerves whose physiological functions include the regulation of blood pressure, appetite and feeding, circadian rhythms, modulation of learning and memory, control of brain endocrine axes and regulation of neural progenitor cell proliferation (Tseng et al., 1989; Hansel et al., 2001; Luquet et al., 2005; Mahaut et al., 2010). Specifically, NPY-containing neurons are present in the paraventricular nucleus (PVN) of the hypothalamus, the ventrolateral medulla (VLM), the NTS, the presynaptic bulbospinal neurons of the brainstem and the sympathetic fibres that innervate blood vessels (Fetissov et al., 2003; Wolak et al., 2003). NPY binds to and activates at least five different NPY receptors (Blomqvist and Herzog, 1997), two of which, the Y1 and Y2 receptors, are abundantly expressed in the mammalian brain (Dumont et al., 1998). The Y1 receptor is thought to be involved in many of the central and peripheral effects exerted by NPY. The NTS is a medullary relay nucleus that transmits both cardiovascular and respiratory signals and contains a high density of Y1 and Y2 receptors (Kask et al., 2002). We and others have demonstrated that microinjection of NPY into the NTS elicits a dose-dependent reduction in blood pressure and heart rate (Tseng et al., 1989), indicating that NPY in the NTS plays a significant part in regulating cardiovascular activity through the activation of NPY receptor-mediated downstream signal transduction.
Microinjection of NG-monomethyl-L-arginine, a NOS inhibitor, into the NTS increased systemic arterial pressure and renal sympathetic nerve activity (Tseng et al., 1996), indicating that the NOS system is involved in central cardiovascular regulation. Our previous study indicated that the insulin-PI3K-Akt-NOS and the adenosine-MEK-ERK-endothelial NOS (eNOS) signalling pathways in the NTS of rats played a significant role in central cardiovascular regulation (Huang et al., 2004; Ho et al., 2008). Notably, NPY has been shown to increase Rho-kinase activity in the mesenteric arterial vascular wall and to decrease NO overproduction in the mesenteric vasculature, making NPY a more effective vasoconstrictor, which counterbalances arterial vasodilatation in portal hypertension (Moleda et al., 2011). Moreover, NPY induction of neuronal proliferation is mediated through PKC, an upstream regulator of ERK1/2 (Gur et al., 2002). However, the signalling mechanisms following NPY receptor activation and NO production in the NTS remain unclear. Thus, we aimed to explore the possible involvement of PI3K/Akt-dependent and/or MEK/ERK signalling pathways in the cardiovascular response to NPY.
In this study, we evaluated the short-term cardiovascular effect of NPY given to the NTS of Wistar-Kyoto rats (WKY) and delineated its underlying molecular signalling mechanism. We found that the NPY-induced depressor effect was mediated by activating the Y1 receptor-PKC-ERK-ribosomal protein S6 kinase (RSK)-eNOS and Ca2+-eNOS signalling pathways, which are involved in the regulation of blood pressure in the NTS.
Methods
Animals
All animal care and research protocols were approved by the Research Animal Facility Committee of Kaohsiung Veterans General Hospital. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). Male WKY rats (weighing 230–300 g; total = 83) were obtained from the National Science Council Animal Facility (Taipei, Taiwan) and were housed in the animal nursery of Kaohsiung Veterans General Hospital (Kaohsiung, Taiwan). The rats were given normal rat chow (Purina, St. Louis, MO, USA) and tap water ad libitum.
Intra-NTS microinjection and haemodynamic measurements
Rats were anaesthetized with urethane (1.0 g·kg−1 i.p., supplemented with 300 mg·kg−1 i.v. if necessary). A polyethylene catheter was placed in the femoral vein for drug administration. Blood pressure was measured directly through a catheter placed in the femoral artery and connected to a pressure transducer (P23 ID; Gould Electronics, Eichstetten, Germany) and polygraph (RS3800; Gould Electronics). Heart rate was monitored continuously by a tachograph preamplifier (13–4615-65; Gould Electronics). Tracheostomy was performed to maintain airway patency during the experiment. For brainstem nuclei microinjection, the rats were placed in a stereotaxic instrument (Kopf, Tujunga, CA, USA), with the head flexed downward at a 45° angle. The dorsal surface of the medulla was exposed by limited craniotomy, and the rats were rested for at least 1 h before experiments. Single-barrel glass catheters were prepared (0.031 in. OD, 0.006 in. ID; Richland Glass Co., Vineland, NJ, USA) with an external tip diameter of 40 µm. To verify that the needle tip of the glass electrode was exactly in the NTS, L-glutamate (0.154 nmol/60 nL) was microinjected. This would induce a characteristically abrupt fall in blood pressure (≧ −35 mmHg) and heart rate (≧ −50 bpm) if the needle tip was located precisely in the medial site of the intermediate one-third of the NTS, with the coordinates of antero-posterior, 0.0 mm; medio-lateral, 0.5 mm; and vertical, 0.4 mm with the obex as reference (Tseng et al., 1996). The cardiovascular effect of NPY in rat NTS was determined by injection of the following substances: 4.65 pmol of human NPY (Tatemoto et al., 1982) dissolved in normal saline; BIBP3226 (60 pmol) (Rudolf et al., 1994), a selective non-peptide Y1 receptor antagonist; GF109003X (3 pmol), a PKC inhibitor; LY294002 (0.6 pmol) (Vlahos et al., 1994), a PI3 kinase inhibitor; and PD098059 (10 pmol) (Alessi et al., 1995; Mohr et al., 1998), an antagonist of MAPK activation. The vehicle used was 0.2% DMSO in Opti-MEM.
To investigate the effect of pre-administration of BIBP3226, GF109003X, LY294002, L-NAME and PD98059 on the NPY-elicited cardiovascular effects, the rats were first injected with NPY and allowed to recover until their blood pressure and heart rate returned to basal levels. An intra-NTS injection of BIBP3226, GF109003X, LY294002, L-NAME or PD98059 was then given, which was followed 10 min later by another microinjection of the same NPY dose, and the changes in mean blood pressure and heart rate were recorded. The cardiovascular action of the same NPY dose was also observed after 10–90 min. In this study, each injection volume in the NTS was restricted to 60 nL, and microinjections were limited to six to eight per rat (Tseng et al., 1996).
Immunoblotting analysis
The NTS tissue microinjected with BIBP3226, GF109003X or PD98059 was isolated according to a previously established protocol (Ho et al., 2008). Briefly, total protein was prepared by homogenizing the NTS for 1 h at 4°C in lysis buffer and protease inhibitor cocktail and phosphatase inhibitor cocktail 2 (all purchased from Sigma-Aldrich). Protein extracts (20 µg per sample assessed by bicinchoninic acid protein assay; Pierce Chemical Co., IL, USA) were subjected to 6–12.5% SDS–Tris glycine gel electrophoresis and transferred to a PVDF membrane (GE Healthcare, Buckinghamshire, UK). The membranes were blocked and incubated at 4°C overnight with the appropriate antibody: rabbit anti-P-ERK1/2-Thr202Tyr204 (1:1000; Cell Signaling Technology, Denvers, MS, USA), rabbit anti-ERK1/2 (1:1000; Cell Signaling Technology, Beverly, MA, USA), rabbit anti-P-Akt-Ser473 (1:1000; Cell Signaling Technology), rabbit anti-Akt (1:1000; Cell Signaling Technology), mouse anti-P-eNOS-Ser1177 (1:1000; BD Biosciences, San Jose, CA, USA), mouse anti-actin (1:10000; Millipore, Billerica, MA, USA), anti-P-nNOS-Ser1416 (1:1000; Abcam, Cambridge, UK), anti-neuronal NOS (nNOS) (1:2000; Millipore) and mouse anti-eNOS (1:1000; BD Biosciences) were diluted in PBST with 5% BSA. Peroxidase-conjugated anti-mouse (1:10 000) or anti-rabbit (1:10 000) was used as the secondary antibody at room temperature for 1 h and then followed by signal detection using an ECL-Plus protein detection kit (GE Healthcare). Signal intensity was quantitatively measured by densitometry with a Macintosh version of the NIH Image program (NIH, Bethesda, MD, USA).
Immunohistochemistry analysis
After perfusion with 0.9% normal saline, the rat brainstems were fixed immediately in 4% formaldehyde overnight and embedded in paraffin. The brainstems were sectioned coronally at a 5 µm thickness. The sections were deparaffinized, quenched in 3% H2O2/methanol, microwaved in citric buffer (10 mmol·L−1, pH 6.0), blocked in 5% goat serum and incubated with an anti-P-ERK-Thr202Tyr204 (1:100), anti-P-nNOS-Ser1416(1:200), anti-P-eNOS-Ser1177 (1:100) or anti-P-RSK-Thr359Ser363 (1:100) antibody overnight at 4°C. Next, the sections were incubated with biotinylated secondary antibodies (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 h and in AB complex (1:100) for 30 min at room temperature. The sections were visualized with a 3,3′ diaminobenzidine substrate kit (Vector Laboratories) and counterstained with haematoxylin. The sections were then photographed with a microscope mounted with a CCD camera.
Positive cells in the injected NTS and the control, vehicle-injected NTS, were counted at 400× magnification in the same paraffin section and counts across the entire NTS were generated from six sections. An average value from the six sections is presented for each hemisphere. Immunohistochemically detectable protein phosphorylation was visible in the NTS of WKY rats after NPY stimulation and compared with unstimulated (contralateral) control NTS. Paired Student's t-test was used to compare the positive cell numbers in the stimulated NTS and the unstimulated control NTS.
Co-immunoprecipitation (Co-IP) assay
Co-IP was performed as previously described (Alessi et al., 1995; Mohr et al., 1998). Briefly, the NTSs were washed with ice-cold PBS (twice) at the indicated times and were lysed in ice-cold RIPA buffer. The supernatant was incubated with 10 µL of a mouse anti-eNOS (1:100) or an anti-RSK (1:100) monoclonal antibody and agarose ligand (Catch and Release v2.0; Millipore), followed by incubation at 4°C for 1 h. The immune complexes were washed, eluted by boiling in 2X SDS sample buffer, separated by 10% SDS-PAGE and subjected to immunoblotting analysis using anti-eNOS, anti-P-eNOS-Ser1177 (BD Biosciences), anti-RSK and specific anti-P-RSK-Thr359Ser363 antibodies.
In vitro kinase assay
A Co-IP assay using anti-eNOS monoclonal antibody was performed, and an eNOS-RSK complex was eluted (1:100, Catch and Release v2.0; Millipore). The kinase reaction was performed according to the vendor's instructions. The phosphorylation of eNOS was determined by Western blot analysis using anti-eNOS, anti-P-eNOS-Ser1177, anti-RSK and anti-P-RSK-Thr359Ser363 antibodies.
Statistical analysis
All data are expressed as the mean ± SEM. Paired Student's t-test (for comparisons of hypotensive parameters before and after pretreatment), unpaired Student's t-test (for control and study group comparisons) or one-way anova with Scheffé's post hoc comparison was applied to compare group differences. Differences with a probability value P < 0.05 were considered statistically significant.
Materials
Experimental drugs including urethane, L-glutamate, human NPY, the Y1 receptor agonist [ (Leu31,Pro34)-NPY], the Y2 receptor agonist [NPY(13–36) ], Pertussis toxin (PTX), BIBP3226 [R-N2-(diphenylacetyl)-N-(4-hydroxyphenyl)methyl-argininamide] (Gerald et al., 1996), GF109003X, LY294002 (Vlahos et al., 1994), N-nitro-L-arginine methyl ester (L-NAME) (Cheng et al., 2011), PD98059 (Alessi et al., 1995) and DMSO were all obtained from Sigma-Aldrich (Sigma Chemical Co., St. Louis, MO, USA). Anti-P-Akt-Ser473, anti-Akt, anti-P-ERK-Thr202Tyr204, anti-ERK, anti-eNOS, anti-p-RSK-Thr359Ser363 and anti-RSK antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Mouse anti-actin, goat anti-rabbit and goat anti-mouse IgG secondary antibodies were obtained from Sigma-Aldrich.
Results
NPY modulates hypotensive effects in the NTS
We initially investigated the effects of NPY on the CVS of WKY rats by microinjecting the NTS with NPY, a Y1 receptor agonist or a Y2 receptor agonist. Unilateral microinjection of NPY (4.65 pmol) into the NTS induced significant depressor and bradycardic effects in WKY rats (Figure 1A,B). Similar effects were observed when the same amount (4.65 pmol) of a Y1 receptor agonist or a Y2 receptor agonist was injected. There were no significant differences in the hypotensive effects among the three groups treated with NPY, the Y1 receptor agonist or the Y2 receptor agonist.
Figure 1.
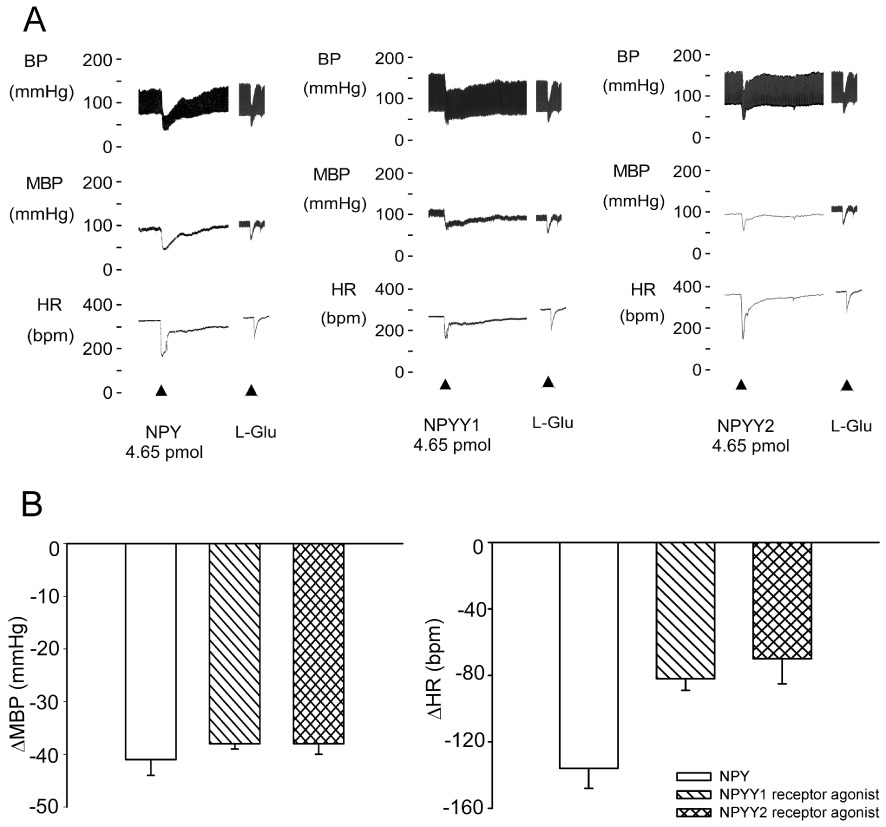
The hypotensive effects of unilateral injection of NPY, Y1 or Y2 receptor agonists into the NTS of WKY rats. (A) Experimental recordings illustrate the cardiovascular effects of NPY, Y1 or Y2 receptor agonists (4.65 pmol) unilaterally microinjected into the NTS. (B) Quantitative data for cardiovascular effects of NPY, Y1 or Y2 receptor agonists. Values are shown as means ± SEM, n= 6. *P < 0.05 significantly different from control group. MBP, mean blood pressure; HR, heart rate.
GPCR and PKC signalling is involved in the NPY-mediated hypotensive effects in the NTS
To determine what NPY receptor-mediated signalling pathway was responsible for these effects, selective NPY receptor antagonists were given to the NTS before NPY injection. As shown in Figure 2A, pre-treatment (10 min) of the NTS with BIBP3226 (60 pmol), a selective Y1 receptor antagonist, attenuated the depressor and bradycardic responses of NPY (P < 0.05, paired t-test; Figure 2A).
Figure 2.
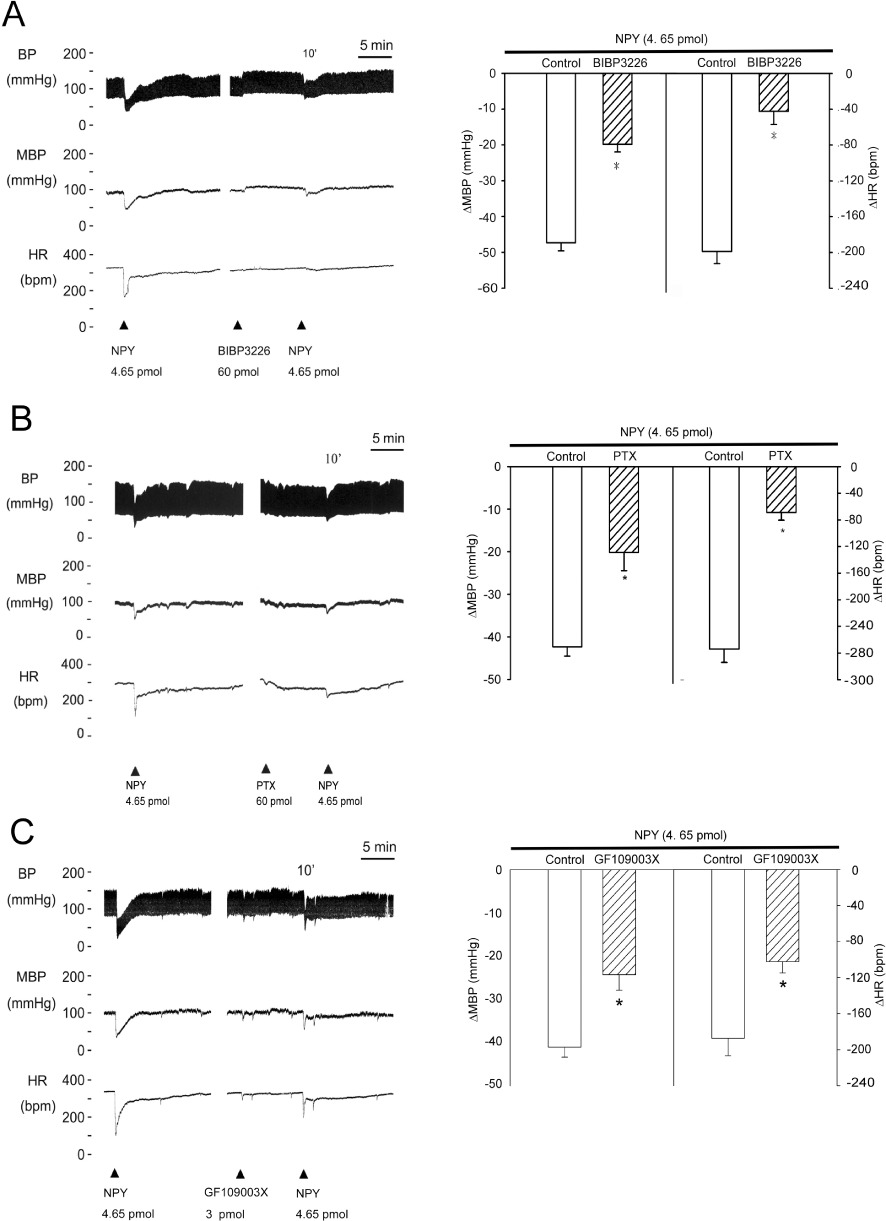
GPCR and PKC signalling were involved in the NPY-mediated depressor effects in the NTS. (A–C) Experimental recordings illustrate the cardiovascular effects and graphs show the hypotensive effects of NPY (4.65 pmol), and the effects of pre-treatment (10 min) of the NTS with BIBP3226 (60 pmol), a selective non-peptide NPYY1 receptor antagonist, PTX (60 pmol) and GF109003X (3 pmol), a PKC inhibitor. The NPY-mediated suppression of mean blood pressure(MBP) and heart rate (HR) was significantly blocked by BIBP3226, PTX and GF109003X. Values are shown as means ± SEM, n= 6. *P < 0.05 significantly different from control group.
PTX was microinjected into the NTS to determine whether a Gi/Go-protein-initiated signalling pathway was involved in these NPY-mediated hypotensive responses. Pre-treatment (10 min) of the NTS with PTX (60 pmol) attenuated the depressor and bradycardic responses of NPY (P < 0.05, paired t-test; Figure 2B). Inhibition of PKC by GF109003X, a downstream effector of G-protein, also reduced NPY-elicited depressor and bradycardic effects (P < 0.05, paired t-test; Figure 2C).
MEK-MAPK signalling is involved in NPY-mediated hypotensive effects
Previously, we established that the MAPK-eNOS signalling pathway participated in an adenosine-mediated regulation of hypotensive effects in the NTS (Ho et al., 2008). Consequently, the involvement of the MAPK pathway in NPY-mediated hypotensive responses was examined in this study. Pre-treatment (10 min) of the NTS with PD98059 (10 pmol), a specific MEK1 inhibitor, attenuated the depressor and bradycardic responses induced by NPY in WKY rats (P < 0.05, paired t-test; Figure 3A). Figure 3B shows that there was an approximately twofold increase in the ERK1/2 phosphorylation (P-ERK1/2) level in NPY or Y1 receptor agonist-treated NTS lysate (lanes 2 and 4), which was blocked by PD98059 (lanes 3 and 5). In contrast, the Y2 receptor agonist did not induce phosphorylation of ERK1/2 (lanes 6 and 7). In situ ERK phosphorylation was further supported by immunohistochemical analysis of paraffin sections of the NTS. A significant increase in P-ERK1/2 positive cells was detected in the NTS after NPY injection. Moreover, pre-treatment (10 min) of the NTS with PD98059 abolished ERK phosphorylation after NPY (upper panel, Figure 3C). Similar observations were obtained in the group injected with the Y1 receptor agonist (middle panel, Figure 3C). Consistent with the Western blots, immunohistochemical analysis also showed no phosphorylation of ERK after treatment with the Y2 receptor agonist (lower panel, Figure 3C).
Figure 3.
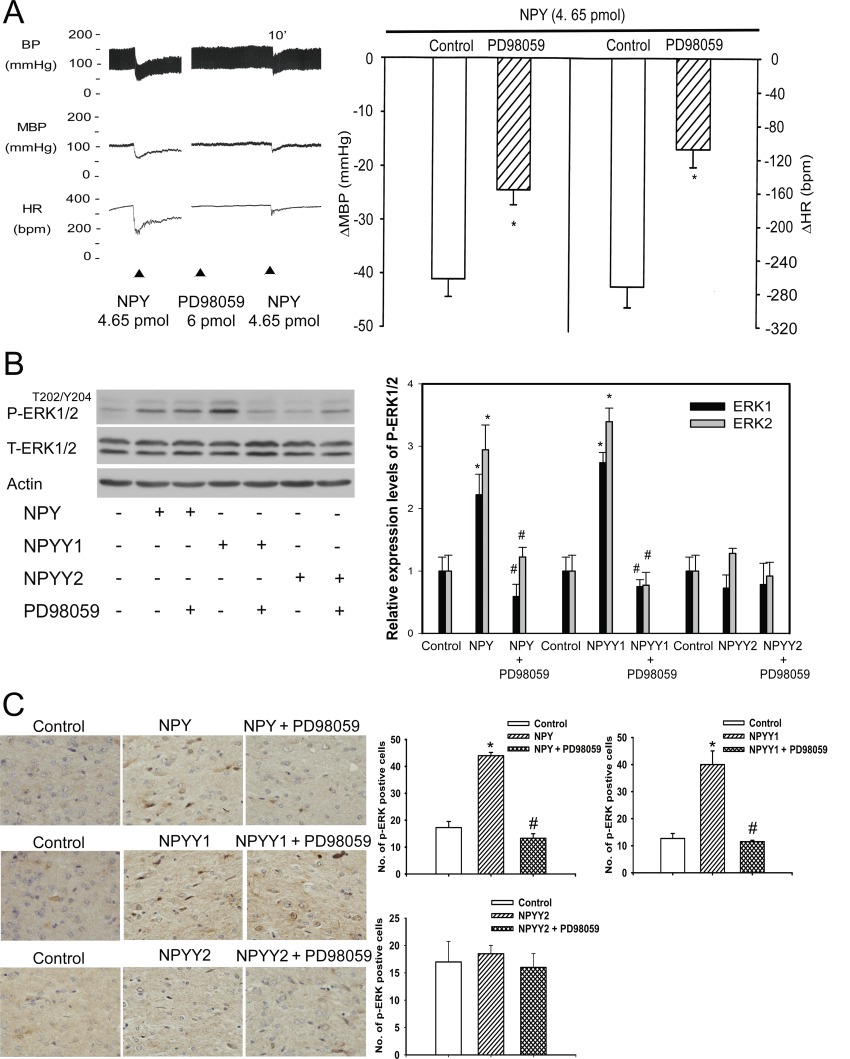
The MEK/ERK signalling pathway was involved in the NPY-mediated depressor effects in the NTS of WKY rats. (A) Experimental recordings illustrate the cardiovascular effects and the graphs summarise the effects of NPY (4.65 pmol),and the effects of pre-treatment (10 min) of the NTS with PD98059 (6 pmol), an antagonist of MAPK activation. (B) Immunoblots of NTS lysates demonstrated that P-ERK1/2 was specifically induced by the Y1 receptor agonist but not the Y2 receptor agonist, and this phosphorylation was blocked by PD98059 treatment. The data are quantitatively represented using bar graphs (right panel). (C) Immunohistochemical validation of the Y1 receptor agonist -mediated P-ERK1/2 in the NTS. P-ERK1/2 positive cells were counted in the stimulated NTS and the contralateral (control), unstimulated NTS at 40× magnification in the same paraffin sections. Statistical analysis was determined by paired Student's t-test., P < 0.01 (n= 6). A quantitative bar graph demonstrated an increase in P-ERK positive cells after injecting NPY and the Y1 receptor agonist, but not after injection of the Y2 receptor agonist. PD98059 pretreatment significantly decreased the number of P-ERK positive cells after injecting NPY and the Y1 receptor agonist; this result was not observed after injecting the Y2 receptor agonist. Data are represented as means ± SEM (n= 6, *P < 0.05 significantly different from control; #P < 0.05 significantly different from NPY, Y1 or Y2 receptor agonists). MBP, mean blood pressure; HR, heart rate.
The Y1 receptor antagonist, BIBP3226 or a PKC inhibitor (GF1009003X) were microinjected into the NTS to determine if they could also block NPY-induced ERK1/2 phosphorylation. Pre-treatment with either compound significantly attenuated NPY-induced ERK1/2 phosphorylation (lanes 2 and 3 of Figure S1A; Figure S1B).
NPY and the Y1 receptor agonist increase phosphorylation of RSK-Thr359Ser363 in rat NTS
Because RSK is one of the downstream targets of the Raf-MEK-ERK protein kinase cascade, the involvement of RSK in NPY-mediated responses was also determined. Figure 4A shows a significant increase in RSK phosphorylation after injection of NPY (P < 0.05; lane 2) and a Y1 receptor agonist (P < 0.05; lane 3). However, no significant increase in RSK phosphorylation was observed after injection of a Y2 receptor agonist (P > 0.05; lane 4) in the NTS. In situ RSK phosphorylation after NPY microinjection was also determined in NTS tissue sections by immunohistochemistry. Figure 4B shows a significant increase in cells with RSK phosphorylation after NPY injection (P < 0.05). These results suggested that the RSK-mediated signalling mechanism might be involved in the cardiovascular regulation of NPY in the NTS of WKY rats.
Figure 4.
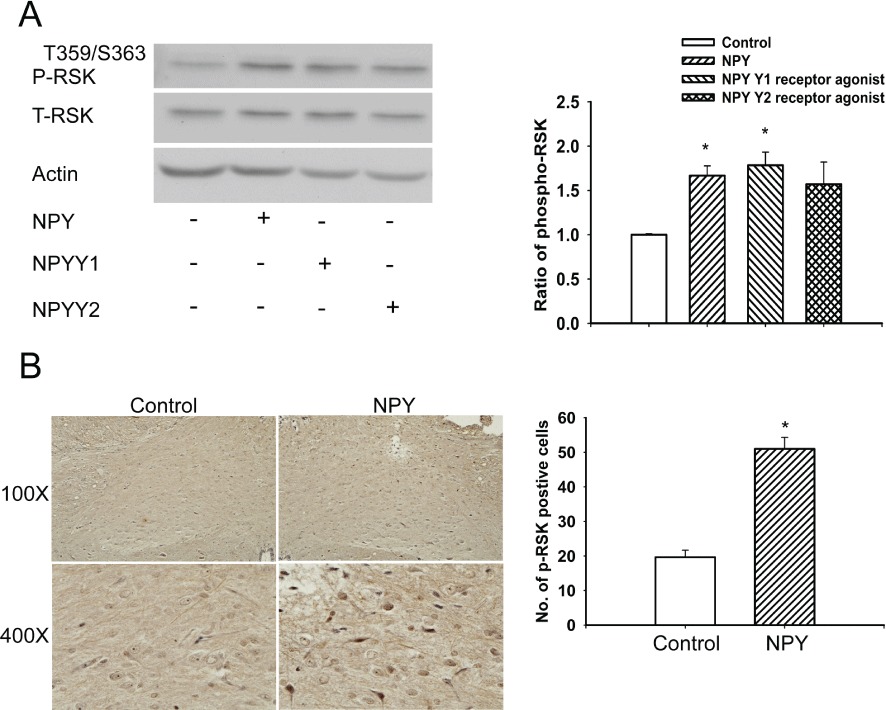
Quantitative immunoblotting analysis of P-RSK in the NTS following treatment with NPY, Y1 or Y2 receptor agonists. (A) A comparative analysis of immunoblots demonstrated an increase in RSK phosphorylation level in the NTS after microinjection of NPY (lane 2) and the Y1 receptor agonist (lane 3). The phosphorylation level of RSK was not increased after microinjection of the Y2 receptor agonist (lane 4). The bar graph summarises these results. (B) P-RSK positive cells were counted in the NPY stimulated and unstimulated (control, contralateral) NTS at 400× magnification in the same paraffin section. Statistical analysis was determined by paired Student's t-test., P < 0.01 (n= 6). Note: the number of P-RSK positive cells is significantly higher in the NTS of WKY rats that received NPY injection when compared with the cell counts in the control unstimulated NTS. Values are shown as means ± SEM, n= 6. *P < 0.05 significantly different from control group.
eNOS is the downstream target of MAPK-RSK signalling activated by NPY
L-NAME, a NOS inhibitor, was microinjected into the NTS to test whether NO production was involved in the hypotensive effects of NPY. Pretreatment of the NTS with L-NAME for 10 min almost abolished the depressor and bradycardic responses to (P < 0.05, paired t-test; Figure 5A). In further experiments, pre-treatment (10 min) of the NTS with with the e-NOS specific inhibitor, L-NIO (6 nmol), significantly attenuated the depressor responses of NPY (P < 0.05, paired t-test; Figure 5B). Similar results were obtained after the Y1 receptor agonist or and the Y2 receptor agonist (Figure S2). However, pretreatment of the NTS with 7-NI (5 pmol), an nNOS specific inhibitor, did not affect the depressor and bradycardic responses to NPY (P > 0.05, paired t-test; Figure 5C).
Figure 5.
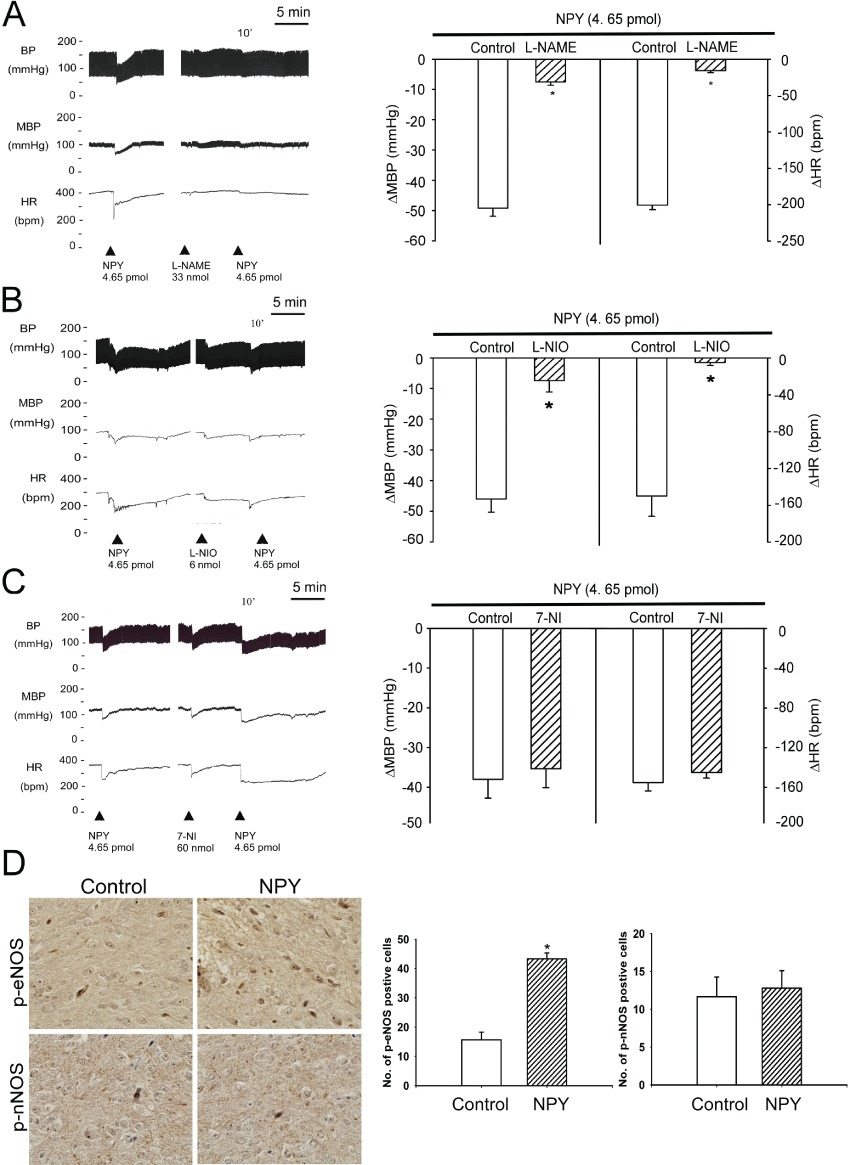
Effects of eNOS on the depressor effects of NPY in the NTS of WKY rats. (A–C) Experimental recordings illustrate the cardiovascular effects of unilateral NTS injection of NPY (4.65 pmol),and the effects of pre-treatment (10 min) of the NTS with the NOS inhibitor L-NAME (33 nmol),the eNOS inhibitor L-NIO (6 nmol) and the nNOS inhibitor 7-NI (60 nmol). The NPY-induced decrease in mean blood pressure (MBP) and heart rate (HR) was markedly blocked by L-NAME and L-NIO, but not by 7-NI. D. In situ qualitative and quantitative analysis of NPYY1-mediated P-eNOS and P-nNOS phosphorylation in the NTS. The P-eNOS or P-nNOS positive cells were counted in the stimulated and unstimulated (contralateral) NTS at 400× magnification in the same paraffin sections. Statistical analysis was determined by paired Student's t-test., P < 0.01 (n= 6). Note: the number of P-eNOS positive cells (not P-nNOS) was significantly higher in the NTS that received NPY injection when compared with the contralateral control NTS. Values are shown as means ± SEM, n= 6. *P < 0.05 significantly different from control group.
In addition, immunohistochemical sections of NPY-treated NTS showed a significant increase in the number of P-eNOSS1177 positive cells compared with their counterparts (upper panel, Figure 5D). No significant difference was found in the number of P-nNOSS1416 positive cells in both the control and the NPY-treated NTS (lower panel, Figure 5D). These results indicate that eNOS may play a role in NPY-modulated depressor and bradycardic responses in the NTS.
RSK binds and phosphorylates eNOS at Ser1177
Although the phosphorylation of eNOS at Ser1177 increased NO production in the NTS (Nakata et al., 2005), it remained unclear whether RSK functionally regulates eNOS activity through direct phosphorylation at Ser1177. In order to identify a specific kinase responsible for eNOS phosphorylation at Ser1177, we utilized two bioinformatic websites – the NetPhosK 1.0 Server (http://www.cbs.dtu.dk/services/NetPhosK) and the Kinase Phos 2.0 (http://kinasephos2.mbc.nctu.edu.tw) – to predict interrelated proteins. The software predicted a high probability of such phosphorylation by RSK. We then performed Co-IP assays to analyse whether RSK was bound to eNOS. The NTS lysate was immunoprecipitated with anti-eNOS or anti-P-RSK-Thr359Ser363 antibody and was then probed with total-eNOS and P-RSK antibodies. The results showed that P-RSK co-immunoprecipitated with eNOS (Figure 6A, lanes 2 and 3). To show that RSK directly phosphorylated eNOS at Ser1177, we incubated purified eNOS protein with RSK immunoprecipitated from NTS lysate in the presence of ATP. Figure 6B shows the time-dependent increase in Ser1177 phosphorylation of eNOS by RSK.
Figure 6.
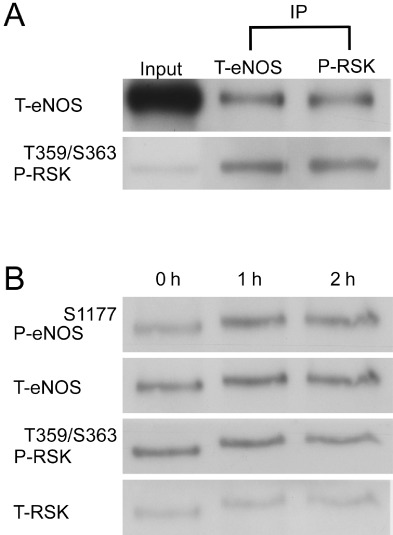
Phosphorylation of eNOS at Ser1177 by RSK in the NTS. (A) Antibodies against total eNOS (T-eNOS) and phosphorylated-RSK-Thr359Ser363 (P-RSK) were used in the NTS protein lysates for Co-IP of eNOS and RSK. The immunoblots demonstrated the direct interaction between eNOS and P-RSK. (B) The RSK-associated kinase activity was determined using eNOS as a substrate. A time-dependent phosphorylation of eNOS (P-eNOS-Ser1177) by P-RSK is demonstrated by autoradiography. RSK-mediated phosphorylation of eNOS persisted for at least 2 h.
The PI3K-Akt signalling pathway is not involved in NPY-induced depressor and bradycardic effects
Previously, we reported that PI3K-Akt signalling is involved in the insulin-mediated cardiovascular effect in the NTS (Huang et al., 2004). In this study, we determined whether PI3K-Akt signalling also contributed to NPY-induced depressor and bradycardic effects. Pretreatment with LY294002 followed by NPY injection did not affect the depressor and bradycardic responses induced by NPY (P > 0.05, paired t-test; Figure S3A). Western blot analysis for Akt phosphorylation and Akt expression using phospho-Akt-Ser473 antibody and specific Akt antibody showed no difference in Akt phosphorylation after injection of NPY (P > 0.05; Figure S3B, lane 3), the Y1 receptor agonist (P > 0.05; Figure S3B, lane 3) or the Y2 receptor agonist (P > 0.05; Figure S3B, lane 4), all compared with control.
Discussion and conclusions
In this study, we have provided evidence that the microinjection of NPY or a Y1 receptor agonist into the NTS induced depressor and bradycardic effects in normotensive WKY rats (Figure 1). NPY and a Y1 receptor agonist both activated the PKC/MEK/ERK/RSK/eNOS/NO and Ca2+/eNOS signalling pathways in the regulation of hypotensive functions by the NTS. Similarly, microinjection of a Y2 receptor agonist induced depressor and bradycardic effects in the NTS. We further demonstrated that the underlying mechanism of Y2 receptor-induced hypotensive effects did not involve the MEK/ERK/RSK pathway. NPY exerts portal hypotensive effects and ameliorates the hyperdynamic circulation in ascitic cirrhotic rats (Moleda et al., 2011). However, other reports indicated that NTS injection of NPY and the Y1 receptor agonist, [Leu31, Pro34]-NPY, induced a dose-dependent decrease in blood pressure and heart rate, which were counteracted by a subsequent injection of NPY (Yang et al., 1993).
All NPY receptors are Gi-protein coupled receptors, and NPY receptor-mediated ERK phosphorylation is blocked by the G-protein inhibitor, PTX (Mullins et al., 2002). Comsisitent with this, we found that PTX pretreatment significantly attenuated the hypotensive responses to NPY (Figure 2). Y1 receptors have been detected on islet cells of kidneys and NPY induced a rapid and transient phosphorylation of ERK1/2. Our data demonstrated that PD98059 inhibited the activation of ERK1/2 by NPY and the Y1 receptor agonist (Figure 3). In the periphery, Y1 receptors are localized to pancreatic islet cells and have been suggested to promote beta cell replication via the activation of ERK1/2 signalling pathway upon NPY binding (Jin et al., 2009). In the CNS, NPY induced neuronal precursor proliferation via PKC (Hansel et al., 2001). Our data demonstrated that NPY and the Y1 receptor agonist, but not the Y2 receptor agonist, stimulated ERK1/2 phosphorylation immediately after injection into the NTS (Figure 3). This finding has established a Y1 receptor-specific functional PKC-ERK signalling cascade in the NTS.
NO is synthesized by eNOS and nNOS in the NTS and has been proposed to induce depressor and bradycardic responses in the NTS of rats (Tseng et al., 1996). In addition, NPY was shown to inhibit electrically stimulated release of noradrenaline in the rat hypothalamus via enhanced production of NO (Bitran et al., 1999), which suggested the participation of NO in these effects of NPY (Tseng et al., 1989). In our study, intra-NTS microinjection of NPY produced depressor and bradycardic effects, and pretreatment of the NTS with the NOS inhibitor L-NAME attenuated the cardiovascular responses to NPY (Figure 5). Of note, NPY-mediated cardiovascular effects in the NTS were attenuated by L-NIO and not by 7-NI, implying that eNOS, rather than nNOS, was involved in the reversal of the hypotensive effects (Figure 5). eNOS is a constitutive and strictly Ca2+/calmodulin-dependent enzyme. When the intracellular calcium concentration increases, caveolin is displaced by calcium-calmodulin, which results in stimulatory phosphorylation at Ser1177 of eNOS (Fleming et al., 2001; Cheng et al., 2011). Our present result also showed that the Ca2+ signalling pathway participates in NPY-mediated changes in blood pressure (Figure S4). Intra-NTS eNOS gene delivery also induced a depressor response in SHR (Tai et al., 2004). Paton et al. demonstrated that eNOS-generated NO in the NTS played a role in the control of baroreflex gain and arterial pressure (Waki et al., 2006), and NPY up-regulated P-eNOS-Ser1177 via unknown mechanisms (Robich et al., 2010). NPY-containing neurons are present in different regions of the brainstem (PVN, VLM and NTS). Fetissov et al. indicated that the density of Y1 receptors seems to predominate in the rat brain with higher expression levels than the other subtypes (Fetissov et al., 2003). Furthermore, Michalkiewicz et al. utilized NPY transgenic male rats, overexpressing the endogenous peptide under its natural promoter, via a Y1 receptor-mediated mechanism. In these mice, endogenous NPY exerted a potent antihypertensive function, and its enhanced signalling ameliorated NO deficiency hypertension. The results presented here suggest that endogenous NPY functions within the CNS to protect the CVS in situations of chronic hyperexcitation and hypertension (Michalkiewicz et al., 2005).
To better elucidate the signalling cascade involving the NPY-mediated depressor and bradycardic responses, we utilized bioinformatics tools and predicted the possible interactions among the key signalling molecules examined in this study, including RSK and eNOS. We were able to confirm a direct interaction between RSK and eNOS through a Co-IP assay (Figure 6A). Our results showed that RSK directly increases P-eNOS-Ser1177 in a time-dependent manner in vitro and identified that RSK is a key factor in the activation of eNOS (Figure 6). RSK is located downstream of the Raf-MEK-ERK protein kinase cascade and contains two functional kinase domains: an N-terminal kinase that phosphorylates its substrates and a C-terminal kinase that is involved in the activation of RSK itself (Fisher and Blenis, 1996). More importantly, a previous report suggested that eNOS could be increased via the adenosine-ERK1/2-eNOS signalling pathway in normotensive rats (Ho et al., 2008). Our experimental evidence supports this notion of NPY-promoted hypotensive and bradycardic effects via the ERK-RSK-eNOS/NO pathway. However, the limitation of this study is due to the use of direct interaction between RSK and eNOS through a Co-IP assay (Figure 6A). Using complex mixtures instead of purified proteins allows examination of indirect protein–protein interaction and obviates the need for lengthy purification procedures, but it also makes it difficult to conclude that two proteins under study bind to each other directly. Also, immunoprecipitation as it is normally performed does not provide quantitative data regarding the affinity or stoichiometry of an interaction. Based on the limitation, we will use the proximity ligation assay (in situ PLA) in the future. It is a technology that extends the capabilities of traditional immunoassays to include direct detection of proteins, protein interactions and modifications with high specificity and sensitivity. Protein targets can be readily detected and localized with single molecule resolution and objectively quantified in unmodified cells and tissues.
In conclusion, our study indicated that, in the NTS, NPY induced eNOS activity and produced depressor and bradycardic responses through not only the Y1 receptor-PKC-ERK-RSK pathway but also the Ca2+-eNOS signalling pathway (Figure 7). This is the first study to demonstrate the mechanism by which NPY regulates depressor effects in the NTS. Our findings suggest novel insights into the CNS regulation of BP and may be helpful in the further development of treatments for hypertension.
Figure 7.
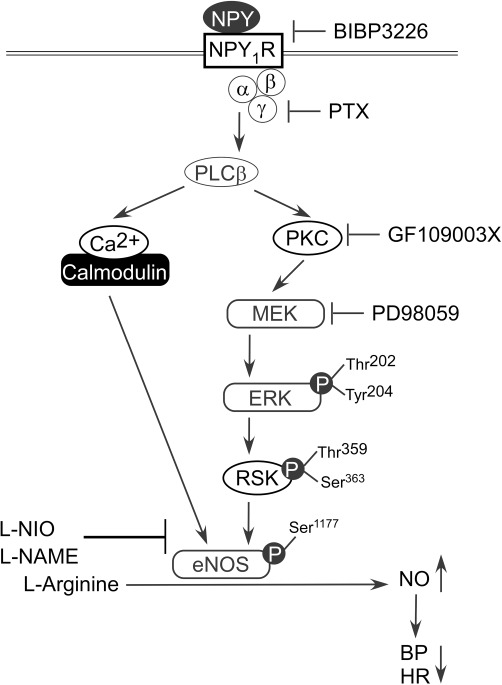
The proposed NPY signalling pathway for the regulation of blood pressure (BP) and heart rate (HR) in the NTS. Microinjection of the Y1 receptor antagonist BIBP 3226, the Gi/Go-protein inhibitor PTX, the PKC inhibitor GF109003X, the MEK inhibitor PD98059 or the eNOS selective inhibitor L-NIO into the NTS attenuated the cardiovascular responses to NPY in WKY rats. Our data suggested that central regulation of blood pressure by NPY is exerted through the Y1 receptor-PKC-ERK-RSK-eNOS-NO signalling axis.
Acknowledgments
The authors gratefully acknowledge the technical assistance and the invaluable input and support of Ms. Yi-Shan Wu and Mr. Bo-Zone Chen. This work was supported by funding from the National Science Council (NSC98-2321-B-075B-002, NSC99-2321-B-075B-002 and NSC100-2321-B-075B-002) and Kaohsiung Veterans General Hospital (VGHKS 100–101) to Dr. C.J. Tseng.
Glossary
- Co-IP
co-immunoprecipitation
- eNOS
endothelial nitric oxide synthase
- L-NAME
N-nitro-L-arginine methyl ester
- NPY
neuropeptide Y
- NTS
nucleus tractus solitarii
- P
phosphorylated
- PVN
paraventricular nucleus
- VLM
ventrolateral medulla
- WKY
Wistar-Kyoto rats
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Inhibition of NPY-induced ERK1/2phosphorylation by a Y1 receptor antagonist or a PKCinhibitor. (A, B) Immunoblotting analysis demonstrated NPY-inducedP-ERK1/2 in the NTS (lane 2 in A and B), and this phosphorylationwas blocked by either the Y1 receptor antagonist(BIBP3226, lane 3, A) or the PKC inhibitor (GF109003X, lane 3, B).The ratio represented the fold-change over the control level ofP-ERK1/2, which was normalized to the level of total ERK1/2.*P < 0.05 versus lane 1, #P< 0.05 versus lane 2.
Figure S2 Effects of eNOS on the depressoreffects of Y1 or Y2 receptor agonists in theNTS of WKY rats. (A, B) Experimental recordings illustrate thecardiovascular effects induced by unilateral NTS injection ofY1 or Y2 receptor agonists (4.65 pmol), with the effects of pre-treatment (10 min) of the NTS with the eNOS inhibitor L-NIO (6 nmol). The NPY-induced decrease in mean blood pressure (MBP) and heart rate (HR) is markedly blocked by L-NIO.
Figure S3 The PI3K/Akt pathway was not involvedin the NPY-mediated depressor effects. (A) The cardiovasculareffects of NPY (4.65 pmol) were not affected by pre-treatment ofthe NTS with of the PI3K inhibitor LY294002 (6 pmol). (B) Theimmunoblot showed the P-Akt proteins after treatment with NPY oragonists of Y1 and Y2 receptors, and theeffect of pre-treatment of the NTS with LY294002. The bar graphdemonstrated no increase in P-Akt level after injecting NPY,Y1 or Y2 receptor agonists. Data arerepresented as means ± SEM (n = 4,*P < 0.05 vs. control).
Figure S4 TheCa2+/calmodulin signalling pathway participatedin the NPY-mediated hypotensive effects. (A) Experimentalrecordings illustrate the cardiovascular effects of NPY (4.65 pmol)unilaterally injected into the NTS, and the effects ofpre-treatment (10 min) of the NTS with with a calmodulinantagonist, W-7 (0.1 nmol). (B) The quantitative graphsdemonstrated that pretreatment of W-7 attenuates NPY-mediateddepressor effects. NPY was injected in the absence (open columns)or presence (black columns) of W-7. Values are shown as meandifferences ± SEM, n = 6. *P< 0.05 versus control group.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran M, Tapia W, Eugenin E, Orio P, Boric MP. Neuropeptide Y induced inhibition of noradrenaline release in rat hypothalamus: role of receptor subtype and nitric oxide. Brain Res. 1999;851:87–93. doi: 10.1016/s0006-8993(99)02123-x. [DOI] [PubMed] [Google Scholar]
- Blomqvist AG, Herzog H. Y-receptor subtypes – how many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- Cheng P-W, Lu P-J, Chen S-R, Ho W-Y, Cheng W-H, Hong L-Z, et al. Central nicotinic acetylcholine receptor involved in Ca2+-calmodulin-endothelial nitric oxide synthase pathway modulated hypotensive effects. Br J Pharmacol. 2011;163:1203–1213. doi: 10.1111/j.1476-5381.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- Fetissov SO, Xu ZQ, Byrne LC, Hassani H, Ernfors P, Hokfelt T. Neuropeptide Y targets in the hypothalamus: nitric oxide synthesizing neurones express Y1 receptor. J Neuroendocrinol. 2003;15:754–760. doi: 10.1046/j.1365-2826.2003.01051.x. [DOI] [PubMed] [Google Scholar]
- Fisher TL, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, et al. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- Gur G, Bonfil D, Safarian H, Naor Z, Yaron Z. Pituitary adenylate cyclase activating polypeptide and neuropeptide Y regulation of gonadotropin subunit gene expression in tilapia: role of PKC, PKA and ERK. Neuroendocrinology. 2002;75:164–174. doi: 10.1159/000048234. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Ho WY, Lu PJ, Hsiao M, Hwang HR, Tseng YC, Yen MH, et al. Adenosine modulates cardiovascular functions through activation of extracellular signal-regulated kinases 1 and 2 and endothelial nitric oxide synthase in the nucleus tractus solitarii of rats. Circulation. 2008;117:773–780. doi: 10.1161/CIRCULATIONAHA.107.746032. [DOI] [PubMed] [Google Scholar]
- Huang HN, Lu PJ, Lo WC, Lin CH, Hsiao M, Tseng CJ. In situ Akt phosphorylation in the nucleus tractus solitarii is involved in central control of blood pressure and heart rate. Circulation. 2004;110:2476–2483. doi: 10.1161/01.CIR.0000145116.75657.2D. [DOI] [PubMed] [Google Scholar]
- Jin HK, Bae JS, Furuya S, Carter JE. Amyloid beta-derived neuroplasticity in bone marrow-derived mesenchymal stem cells is mediated by NPY and 5-HT2B receptors via ERK1/2 signalling pathways. Cell Prolif. 2009;42:571–586. doi: 10.1111/j.1365-2184.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Mahaut S, Dumont Y, Fournier A, Quirion R, Moyse E. Neuropeptide Y receptor subtypes in the dorsal vagal complex under acute feeding adaptation in the adult rat. Neuropeptides. 2010;44:77–86. doi: 10.1016/j.npep.2009.10.001. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalkiewicz M, Zhao G, Jia Z, Michalkiewicz T, Racadio MJ. Central neuropeptide Y signaling ameliorates N(omega)-nitro-L-arginine methyl ester hypertension in the rat through a Y1 receptor mechanism. Hypertension. 2005;45:780–785. doi: 10.1161/01.HYP.0000153953.69799.f2. [DOI] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, et al. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Mohr S, McCormick TS, Lapetina EG. Macrophages resistant to endogenously generated nitric oxide-mediated apoptosis are hypersensitive to exogenously added nitric oxide donors: dichotomous apoptotic response independent of caspase 3 and reversal by the mitogen-activated protein kinase kinase (MEK) inhibitor PD 098059. Proc Natl Acad Sci USA. 1998;95:5045–5050. doi: 10.1073/pnas.95.9.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moleda L, Trebicka J, Dietrich P, Gäbele E, Hellerbrand C, Straub RH, et al. Amelioration of portal hypertension and the hyperdynamic circulatory syndrome in cirrhotic rats by neuropeptide Y via pronounced splanchnic vasoaction. Gut. 2011;60:1122–1132. doi: 10.1136/gut.2010.226407. [DOI] [PubMed] [Google Scholar]
- Mullins DE, Zhang X, Hawes BE. Activation of extracellular signal regulated protein kinase by neuropeptide Y and pancreatic polypeptide in CHO cells expressing the NPY Y(1), Y(2), Y(4) and Y(5) receptor subtypes. Regul Pept. 2002;105:65–73. doi: 10.1016/s0167-0115(01)00388-3. [DOI] [PubMed] [Google Scholar]
- Nakata S, Tsutsui M, Shimokawa H, Tamura M, Tasaki H, Morishita T, et al. Vascular neuronal NO synthase is selectively upregulated by platelet-derived growth factor: involvement of the MEK/ERK pathway. Arterioscler Thromb Vasc Biol. 2005;25:2502–2508. doi: 10.1161/01.ATV.0000190663.88143.97. [DOI] [PubMed] [Google Scholar]
- Robich MP, Matyal R, Chu LM, Feng J, Xu SH, Laham RJ, et al. Effects of neuropeptide Y on collateral development in a swine model of chronic myocardial ischemia. J Mol Cell Cardiol. 2010;49:1022–1030. doi: 10.1016/j.yjmcc.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf K, Eberlein W, Engel W, Wieland HA, Willim KD, Entzeroth M, et al. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur J Pharmacol. 1994;271:R11–R13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- Tai MH, Hsiao M, Chan JY, Lo WC, Wang FS, Liu GS, et al. Gene delivery of endothelial nitric oxide synthase into nucleus tractus solitarii induces biphasic response in cardiovascular functions of hypertensive rats. Am J Hypertens. 2004;17:63–70. doi: 10.1016/j.amjhyper.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y – a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Tseng CJ, Mosqueda-Garcia R, Appalsamy M, Robertson D. Cardiovascular effects of neuropeptide Y in rat brainstem nuclei. Circ Res. 1989;64:55–61. doi: 10.1161/01.res.64.1.55. [DOI] [PubMed] [Google Scholar]
- Tseng CJ, Liu HY, Lin HC, Ger LP, Tung CS, Yen MH. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension. 1996;27:36–42. doi: 10.1161/01.hyp.27.1.36. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Waki H, Murphy D, Yao ST, Kasparov S, Paton JF. Endothelial NO synthase activity in nucleus tractus solitarii contributes to hypertension in spontaneously hypertensive rats. Hypertension. 2006;48:644–650. doi: 10.1161/01.HYP.0000238200.46085.c6. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Yang SN, Narvaez JA, Bjelke B, Agnati LF, Fuxe K. Microinjections of subpicomolar amounts of NPY(13-36) into the nucleus tractus solitarius of the rat counteract the vasodepressor responses of NPY(1-36) and of a NPY Y1 receptor agonist. Brain Res. 1993;621:126–132. doi: 10.1016/0006-8993(93)90307-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


