Abstract
Burkitt lymphoma (BL) has been reported to be strongly associated with Epstein-Barr virus (EBV) infection. The fact that EBV is generally present in cancer cells but rarely found in healthy cells represents an opportunity for targeted cancer therapy. One approach is to activate the lytic replication cycle of the latent EBV. Nuclear factor (NF)-κB is thought to play an essential role in EBV lytic infection. Elevated NF-κB levels inhibit EBV lytic replication. Parthenolide (PN) is a sesquiterpene lactone found in medicinal plants, particularly in feverfew (Tanacetum parthenium). The aim of the present study was to analyze the effect of PN on the survival of Raji EBV-positive lymphoma cells. Raji cells were treated with 0, 4 or 6 μmol/l PN for 48 h. MTT assay and western blot analysis were performed to evaluate the findings. Results showd that PN suppressed the growth of the EBV-positive BL cell line, Raji, and activated the transcription of BZLF1 and BRLF1 by inhibiting NF-κB activity. Most notably, when PN was used in combination with ganciclovir (GCV), the cytotoxic effect of PN was amplified. These data suggest that the induction of lytic EBV infection with PN in combination with GCV may be a viral-targeted therapy for EBV-associated BL.
Keywords: parthenolide, Epstein-Barr virus, lytic replication, Burkitt lymphoma
Introduction
Epstein-Barr virus (EBV) is a member of the γ-herpesvirus family. EBV-associated lymphoid malignancies include a subset of Burkitt lymphoma (BL), acquired immune deficiency syndrome lymphoma, Hodgkin’s lymphoma, post-transplant lymphoma, age-associated B-cell lymphoma, and peripheral T- and natural killer-cell lymphomas (1–3). The EBV life cycle includes distinct latent and lytic genetic programs (4–6). Several therapeutic strategies requiring the activation of EBV lytic genes for tumor cell killing have been described (7,8). The switch from latent to lytic EBV infection is mediated by the expression of two EBV immediate-early (IE) viral proteins, BZLF1 and BRLF1. Lytic EBV replication damages the cancer cells and triggers host immune responses against EBV and the infected cells (9). During lytic infection, EBV encodes viral kinases that phosphorylate the antiviral nucleoside analogue ganciclovir (GCV) to produce its active cytotoxic activity (10). Taken together, these findings suggest that EBV-targeted cancer therapy warrants investigation.
Nuclear factor-κB (NF-κB) is a significant transcriptional factor involved in the regulation of cell apoptosis, cell cycle progression and carcinogenic transformation. Constitutive NF-κB activity has been observed in lymphoma (11). Recent studies have demonstrated that the transforming EBV-encoded latent membrane protein 1 (LMP1) induces constitutive NF-κB activity (12) and that elevated NF-κB levels promote the survival and proliferation of infected cells and inhibit lytic gene promoter activation, lytic protein synthesis and lytic replication (13–16). These findings suggest that inhibiting NF-κB is an effective and novel method for treating EBV-positive malignancies.
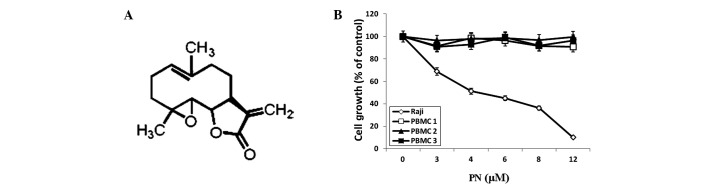
Parthenolide (PN) is a sesquiterpene lactone (17) found in medicinal plants, particularly in feverfew (Tanacetum parthenium). The nucleophilic nature of its methylene-γ-lactone ring and epoxide group enables rapid interactions with biological sites (Fig. 1A). PN is a herbal medicine that has been used to treat migraines and rheumatoid arthritis for centuries. Recently, PN has been identified to have several other properties, including antitumor activity, inhibition of DNA synthesis and inhibition of cell proliferation, in various cancer cell lines (18). In addition, PN sensitizes cancer cells to other antitumor agents (19,20). Accumulating evidence has shown that PN is capable of inhibiting the activity of the NF-κB subunit RelA/p65 by inhibiting the IκB kinase-mediated phosphorylation of IκB (21), suggesting that PN is a novel therapeutic agent for treating EBV-positive lymphoma.
Figure 1.
(A) Chemical structure of PN. (B) Effects of PN on the growth of Raji cells and normal PBMCs as measured by MTT assay. PN, parthenolide; PBMCs, peripheral blood mononuclear cells.
In this study, we analyzed the effect of PN on the survival of Raji EBV-positive lymphoma cells. We confirmed that PN inhibited RelA/p65 activity and showed that it induced EBV lytic replication in Raji cells, resulting in EBV-positive cell death in vitro. When PN was used in combination with GCV, the anticancer potency of PN was enhanced. These findings indicate that PN may be a novel agent for targeting EBV-associated Burkitt lymphoma.
Materials and methods
Cell culture
The EBV-positive BL cell line, Raji, was obtained from ATCC (Manassas, VA, USA). The cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C and 5% CO2. Peripheral blood mononuclear cells (PBMCs) were purified from the blood donors’ buffy coats by Ficoll gradient centrifugation. This study was conducted in accordance with the Helsinki protocol and approved by the Xiamen University Institutional Review Board.
Reagents and antibodies
PN was purchased from Sigma-Aldrich (St. Louis, MO, USA). PN was dissolved in dimethyl sulfoxide (DMSO; Sigma Chemical Co., St. Louis, MO, USA) as a stock solution of 1 μmol/ml and diluted in the culture medium immediately prior to use. The final concentration of DMSO in all experiments was <0.01%. GCV was purchased from North China Pharmaceutical Group Corporation (Shijiazhuang, Hebei, China) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) was purchased from Sigma. An annexin V fluorescein conjugate (FITC) and propidium iodide (PI) apoptosis detection kit was purchased from Invitrogen (Grand Island, NY, USA). An NF-κB (RelA/p65) transcription factor assay kit was purchased from Cayman Chemical (Ann Arbor, MI, USA). Antibodies against RelA/p65 (1:1000), poly (ADP-ribose) polymerase (PARP; 1:1000), caspase-8 (1:1000), caspase-9 (1:1000), Oct-1 (1:1000) and horseradish peroxidase-conjugated anti-rabbit immunoglobulin (1:3000) were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-β-actin antibody (1:3000) was obtained from Epitomics (Burlingame, CA, USA).
Cell proliferation assay
Raji cells and PBMCs in 180 μl RPMI-1640 were inoculated onto 96-well plates. Four parallel rows of wells were set up for each group. The MTT assay was performed 48 h following treatment with PN at different concentrations in the presence or absence of GCV. The absorbance rate [optical density (OD)] of each well was measured at 570 nm using an enzyme-linked immunosorbent detector (DG3022A). Growth inhibition was determined as a percentage of the control.
Apoptosis detection assays and cell cycle analysis
Raji cells were treated with 0, 4 or 6 μmol/l PN for 48 h. Raji cells were washed and stained with annexin VFITC and PI according to the manufacturer’s instructions. Apoptosis was quantified using a FACSort flow cytometer and CellQuest (BD Biosciences, Mountain View, CA, USA). Apoptosis was also detected via 4′,6-diamidino-2-phenylindole (DAPI) staining. Briefly, Raji cells were washed with phosphate-buffered saline and stained with 1 mg/ml DAPI for 30 min at 37°C. Slides were then washed with phosphate-buffered saline, air dried and covered with coverslips for analysis via fluorescence microscopy using a Leica DM2500 microscope (Buffalo Grove, IL, USA). For cell cycle analysis, the Raji cells were fixed with cold methanol overnight and then treated with PI (2 μg/ml) and RNase prior to flow cytometry.
Caspase-3 assays
Caspase-3 activity was measured in the control and PN-treated Raji cells. Caspase-3 activity assays were performed using a colorimetric substrate according to the manufacturer’s instructions (EMD Chemicals, Gibbstown, NJ, USA). Briefly, Raji cells were lysed, and 50 μg of the resulting cell lysates were added to an assay buffer to a total volume of 90 μl and incubated at 37°C for 10 min. A colorimetric substrate for caspase-3 (final concentration, 200 μmol/l) was then added to the mixture. Absorbance values at 405 nm were recorded for each sample following incubation at 37°C for 2 h.
NF-κB (RelA/p65) activity
Raji cells were incubated with 4 or 6 μmol/l PN for 6, 12 or 24 h. The cells were collected and nuclear proteins were extracted. NF-κB (RelA/p65) activity was detected using a NF-κB (RelA/p65) transcription factor assay kit. The OD of each well was measured at 450 nm using a Model 680 spectrophotometer (Bio-Rad, Philadelphia, PA, USA).
Western blot analysis
Raji cells were incubated with 4 μmol/l PN for 24 h. The nuclear and cytoplasmic extracts were then purified to detect RelA/p65. Preparation of nuclear and cytoplasmic fractions was performed using the NE-PER extraction reagent kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. Briefly, cells were suspended in cytoplasmic extraction reagent I containing 1% protease inhibitor cocktail (Sigma) and 1% phosphatase inhibitor cocktail (Sigma) and then incubated on ice for 10 min. Cytoplasmic extraction reagent II (11 μl) was added to the mixture, vortexed and incubated on ice for 1 min. The sample was centrifuged at maximum speed (13,000 × g) for 10 min at 4°C. The supernatant (cytoplasmic fraction) was transferred to a new tube and stored at −80°C. The pellet was resuspended in 100 μl of ice-cold nuclear extraction reagent and vortexed for 15 sec every 10 min for a total of 40 min. The sample was then centrifuged (13,000 × g) for 10 min at 4°C. The supernatant (nuclear fraction) was transferred to a new tube and stored at −80°C.
Raji cells were treated with 0, 4 or 6 μmol/l PN for 48 h. Whole-cell extracts were purified to detect PARP, caspase-8 and caspase-9. To prepare the whole-cell lysates, the cells were lysed in a lysis buffer (Cell Signaling). Cell lysates were kept on ice for 30 min and centrifuged at 13,000 × g for 10 min at 4°C.
The protein concentration was determined by Bradford assay (Bio-Rad). Sample proteins (50 μg) were solubilized in 1% sodium dodecyl sulfate (SDS) sample buffer and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) on a 4–15% gel (Bio-Rad). Proteins were transferred from the gel onto a polyvinylidene difluoride membrane and immunoblotted with various specific primary antibodies and appropriate horseradish peroxidase-conjugated secondary antibodies. Proteins were visualized using an enhanced chemiluminescence western blotting detection system (Amersham, Sweden). Densitometric digital analysis of protein bands was performed to quantify each protein band using Quantity One 1-D analysis software version 4.1.0 (Bio-Rad). Each protein was normalized by the intensity of the β-actin or Oct-1 housekeeping gene in each sample.
Detection of BZLF1 and BRLF1 mRNA expression in Raji cells using reverse transcriptase polymerase chain reaction (RT-PCR)
Raji cells were collected at 6, 12 and 24 h after incubation with PN 4 μmol/l. BZLF1 and BRLF1 mRNA expression was detected using RT-PCR. The sequences of primers used were GGGACAAGCAAACACCAC (sense) and TTTACACCTGACCCATACC (anti-sense) for BZLF1; CCATACAGGACACAACACCTCA (sense) and ACTCCC GGCTGTAAATTCCT (anti-sense) for BRLF1; GTGGGG CGCCCCAGGCACCA (sense) and CTCCTTAATGTC ACGCACGATTTC (anti-sense) for β-actin. The PCR conditions for BZLF1 were denaturation at 95°C for 2 min; annealing for 30 cycles each at 94°C for 30 sec, 58°C for 30 sec, and 72°C for 60 sec; and an extension at 72°C for 10 min. The PCR conditions for BRLF1 were denaturation at 95°C for 2 min; annealing for 30 cycles each at 94°C for 30 sec, 58°C for 30 sec, and 72°C for 90 sec; and an extension at 72°C for 10 min. The PCR products were subjected to PAGE using a 1.5% agarose gel and visualized using Goldview staining. The fragments were analyzed using the Quantity One 4.52 analysis system (Bio-Rad).
Statistical analysis
Experiments were conducted in triplicate. The results were presented as the means ± standard deviation. Data were processed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA), and statistical differences were determined using analysis of variance followed by a q test. P<0.05 was considered to indicate a statistically significant difference.
Results
PN inhibited Raji cell growth
Results of the MTT assay analysis showed that PN inhibited Raji cell growth in a dose-dependent manner (P<0.05 compared with the control group), with a half maximal inhibitory concentration value of 5.07 μmol/l, but had no effect on normal PBMCs (Fig. 1B).
PN induced apoptosis and affects the cell cycle in Raji cells
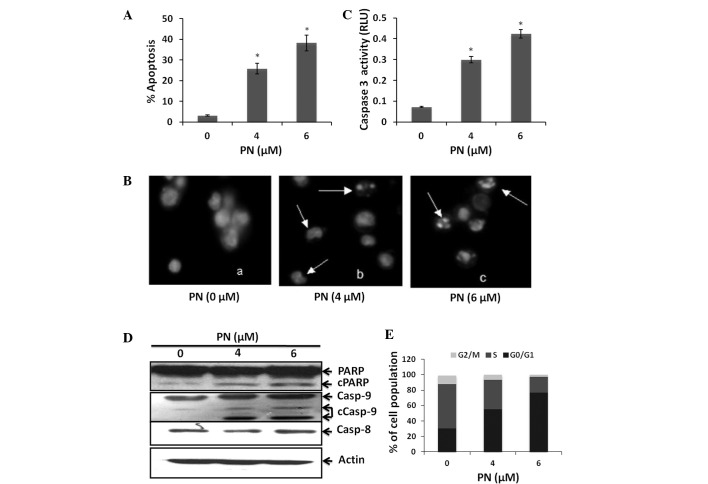
To investigate whether PN is capable of inhibiting Raji cell growth by inducing cell death, we treated Raji cells with 0, 4 or 6 μmol/l PN for 48 h. Induction of cell apoptosis was examined using both an annexin VFITC and PI assay and DAPI staining. Our results revealed that PN induced Raji cell apoptosis in a dose-dependent manner (P<0.05 compared with the control group; Fig. 2A and B). To verify that these cells underwent apoptosis, we measured the generation of caspase-3 activity. Raji cells treated with PN had increased caspase-3 activity (Fig. 2C). In addition, a known caspase substrate, poly- (ADP-ribose) polymerase, was cleaved after PN treatment (Fig. 2D). Furthermore, we found that PN activated caspase-9 but not caspase-8 (Fig. 2D). These results suggest that PN induced Raji cell apoptosis via the mitochondrial pathway.
Figure 2.
Induction of apoptosis and effects of PN on the cell cycle in Raji cells. Raji cells treated with 0, 4 or 6 μmol of PN for 48 h. (A) Annexin VFITC assay (*P<0.05 vs. control group). (B) DAPI staining (x40), arrows point to PN-treated Raji cells with irregular and shrunken nuclei. (C) Caspase-3 activity was measured in the control and PN-treated Raji cells (*P<0.05 vs. control group). (D) Cell extracts were subjected to immunoblotting for PARP, caspase-8 and caspase-9 cleavage. (E) Effects of PN on cell cycle profile. The percentages of cells in G0–G1, S and G2-M phases are shown (n=3). PN, parthenolide; PARP, Poly (ADP-ribose) polymerase.
To determine whether the inhibition of Raji cell proliferation induced by PN was associated with changes in cell cycle progression, a cell cycle analysis was performed on Raji cells treated with PN. Treatment with 4 and 6 μmol/l PN significantly increased the cell population in the G0/G1 phase by 24.9 and 46.5%, respectively, and decreased the cell population in the S phase by 19.2 and 36.7%, respectively (P<0.05 compared with the control group; Fig. 2E).
PN inhibited NF-κB activity in Raji cells
To determine whether PN has an effect on NF-κB activity in Raji cells, we used a NF-κB transcription factor assay kit to detect NF-κB DNA-binding activity. Our results indicated that the RelA/p65-DNA binding activity gradually decreased between 6 and 24 h after the addition of PN (Fig. 3A). We also investigated the expression of RelA/p65 in the cytoplasm and the nucleus separately using western blot analysis. As shown in Fig. 3B, RelA/p65 expression in the nucleus of these cells was reduced following incubation with PN.
Figure 3.
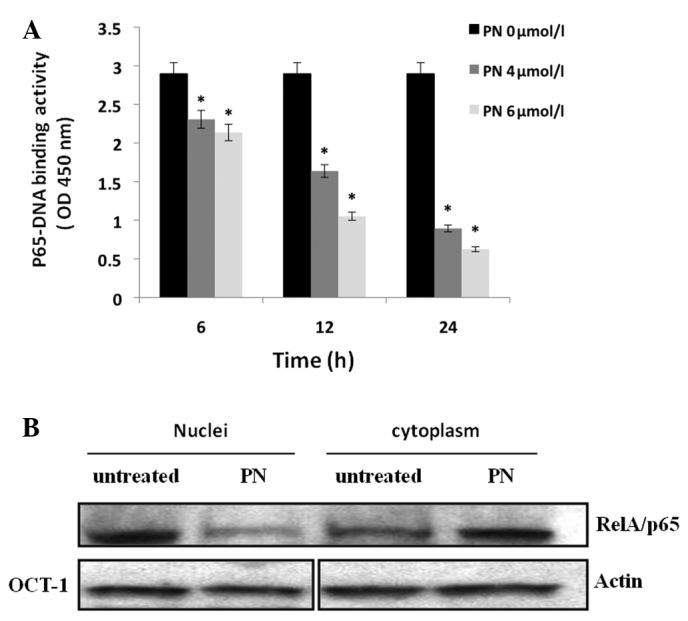
NF-κB activity was inhibited by PN in Raji cells. (A) Raji cells were treated with PN at different concentrations for 6, 12 and 24 h; nuclear extracts were prepared and subjected to a RelA/p65-specific NF-κB DNA binding assay (*P<0.05 vs. control group). (B) Raji cells were incubated with 4 μmol/l PN for 24 h. Intracellular localization of RelA/p65 was detected using western blotting. β-actin and Oct-1 served as the loading control for cytoplasmic and nuclear extracts, respectively. PN, parthenolide.
PN reactivated EBV in Raji cells
Expression of BZLF1 and BRLF1 mRNA was increased in Raji cells treated with 4 or 6 μmol/l of PN (P<0.05 compared with the control group), indicating that PN induced EBV lytic replication in the Raji cells (Fig. 4).
Figure 4.
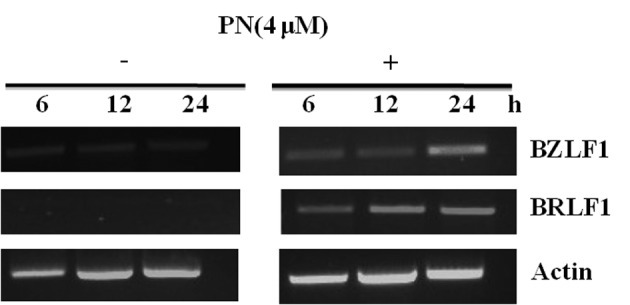
PN increased the mRNA levels of BZLF1 and BRLF1 in a time-dependent manner. PN, parthenolide.
GCV amplified cytotoxicity induced by PN in Raji cells
Raji cells were incubated with the indicated concentrations of PN, in the presence or absence of GCV for 48 h. Findings of the MTT assay showed that Raji cell viability was further diminished by GCV combined with PN in comparison to PN alone (P<0.05; Fig. 5). These findings suggest that the NF-κB inhibitor PN reactivated EBV lytic replication, allowing GCV to produce its active cytotoxic form, thereby inducing the cytotoxic activity of GCV.
Figure 5.
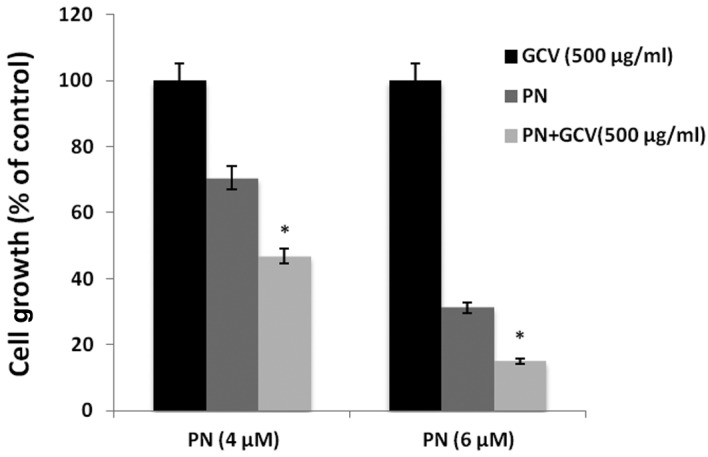
GCV amplified the cytotoxity of PN in Raji cells. *P<0.05 for treatments with PN alone vs. treatment with PN and GCV. GCV, ganciclovir; PN, parthenolide.
Discussion
Our findings have shown that PN inhibited Raji cell growth in a dose-dependent manner and increased the cell population in the G0/G1 phase while decreasing the cell population in the S phase. These results suggest that PN inhibits Raji cell proliferation by inducing cell cycle arrest at the G0/G1 phase. We also observed that PN induced Raji cell growth arrest by inducing Raji cell apoptosis via the mitochondrial pathway.
BL development has been reported to be markedly associated with EBV. The fact that EBV is generally present in cancer cells but rarely found in healthy cells represents an opportunity for targeted cancer therapy (22). One approach is to activate the lytic replication cycle of latent EBV, when viral proteins are expressed at a high level and progeny viruses are produced. Lytic EBV replication damages the cancer cells and triggers host immune responses against EBV and the infected cells (23–26). Entry into the viral lytic cycle is initiated by the expression of two IE EBV proteins, Z Epstein-Barr virus replication activator, encoded by BZLF1 (27), and Rta, encoded by BRLF1 (28). The two IE proteins activate the viral early genes, resulting in a cascade of events that lead to progeny virion. Activation of NF-κB is a feature of numerous viral infections (29). NF-κB activation during viral infection is believed to be a protective response of the host to the viral pathogen. Therefore, a number of viruses have evolved distinct strategies to control NF-κB activity to evade the immune response (30,31). Additionally, viruses may modulate the NF-κB pathway to enhance viral replication or prevent virus-induced apoptosis. Overexpression of NF-κB inhibits the activation of lytic promoters from EBV, suggesting that NF-κB is a novel target for the disruption of virus latency and therefore for the treatment of EBV-related malignancies (32).
Although the mechanisms mediating the various effects of PN in different diseases are not entirely clear, several studies have shown that a significant aspect of the antitumor activity of this compound appears to be associated with its activity in inhibiting the NF-κB signal pathway by preventing the degradation of IκBα (33). Our results showed that PN suppressed NF-κB activity in Raji cells by restricting the location of NF-κB from the cytoplasm to the nucleus, which is in agreement with previous reports that showed that PN is an inhibitor of NF-κB activity (34–36), and acted as one mechanism for killing EBV-positive Raji cells. However, our finding that PN induced a fully lytic form of EBV suggests that PN has a second mechanism for killing tumor cells. We found that treatment with PN increased BZLF1 and BRLF1 mRNA expression, indicating that PN was capable of inducing EBV lytic replication in Raji cells. In this study, GCV amplified the cytotoxicity induced by PN, indicating a synergistic cytotoxicity. Our results also suggest lytic virus replication in PN-treated Raji cells, as GCV functions only in the lytic phase of EBV infection and our findings showed that GCV treatment alone had no cytotoxic effect on Raji cells. Moreover, the inhibition of NF-κB activity specifically causes lytic cytotoxicity in EBV-positive Raji cells.
In conclusion, findings of this study have shown the ability of PN to induce EBV lytic replication and produce apparent anticancer effects in EBV-positive carcinoma cells through the inhibition of NF-κB activity. When GCV was added to PN, the cytotoxic effect was even more potent. However, more studies are required to test the effect of PN in EBV-positive malignancies in animal models.
Acknowledgements
This study was supported by grants from the Fujian Provincial Department of Science and Technology (2009-CXB-57 and 2011J01252) and Bureau of Science and Technology of Xiamen, China (3502Z20094012). We thank Dr Lan V Pham from MD Anderson Cancer Center for valuable comments. We appreciate Dr Markeda Wade from MD Anderson Cancer Center for revising our manuscript.
References
- 1.Yang L, Parkin DM, Whelan S, Zhang S, Chen Y, Lu F, Li L. Statistics on cancer in China: cancer registration in 2002. Eur J Cancer Prev. 2005;14:329–335. doi: 10.1097/00008469-200508000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol. 2009;20:1472–1482. doi: 10.1093/annonc/mdp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 5.Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt’s lymphoma. Nat Rev Microbiol. 2008;6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JI, Bollard CM, Khanna R, Pittaluga S. Current understanding of the role of Epstein-Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leuk Lymphoma. 2008;49(Suppl 1):27–34. doi: 10.1080/10428190802311417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood. 2007;109:2571–2578. doi: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu DX, Tanhehco Y, Chen J, et al. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat Med. 2008;14:1118–1122. doi: 10.1038/nm.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landais E, Saulquin X, Scotet E, et al. Direct killing of Epstein-Barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood. 2004;103:1408–1416. doi: 10.1182/blood-2003-03-0930. [DOI] [PubMed] [Google Scholar]
- 10.Feng WH, Israel B, Raab-Traub N, Busson P, Kenney SC. Chemotherapy induces lytic EBV replication and confers ganciclovir susceptibility to EBV-positive epithelial cell tumors. Cancer Res. 2002;62:1920–1926. [PubMed] [Google Scholar]
- 11.Hailfinger S, Nogai H, Pelzer C, et al. Malt1-dependent RelB cleavage promotes canonical NF-kappaB activation in lymphocytes and lymphoma cell lines. Proc Natl Acad Sci USA. 2011;108:14596–14601. doi: 10.1073/pnas.1105020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song YJ, Kang MS. Roles of TRAF2 and TRAF3 in Epstein-Barr virus latent membrane protein 1-induced alternative NF-kappaB activation. Virus Genes. 2010;41:174–180. doi: 10.1007/s11262-010-0505-4. [DOI] [PubMed] [Google Scholar]
- 13.Cahir-McFarland ED, Davidson DM, Schauer SL, Duong J, Kieff E. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc Natl Acad Sci USA. 2000;97:6055–6060. doi: 10.1073/pnas.100119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18:5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 15.Izumi KM, Kieff ED. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller SA, Schattner EJ, Cesarman E. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537–2542. [PubMed] [Google Scholar]
- 17.Smolinski AT, Pestka JJ. Comparative effects of the herbal constituent parthenolide (Feverfew) on lipopolysaccharide-induced inflammatory gene expression in murine spleen and liver. J Inflamm (Lond) 2005;2:6. doi: 10.1186/1476-9255-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pareek A, Suthar M, Rathore GS, Bansal V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn Rev. 2011;5:103–110. doi: 10.4103/0973-7847.79105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.deGraffenried LA, Chandrasekar B, Friedrichs WE, et al. NF-kappa B inhibition markedly enhances sensitivity of resistant breast cancer tumor cells to tamoxifen. Ann Oncol. 2004;15:885–890. doi: 10.1093/annonc/mdh232. [DOI] [PubMed] [Google Scholar]
- 20.Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004;23:7330–7344. doi: 10.1038/sj.onc.1207995. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y, Guzman ML, Chen S, et al. The NF (Nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol. 2010;151:70–83. doi: 10.1111/j.1365-2141.2010.08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israel BF, Kenney SC. Virally targeted therapies for EBV-associated malignancies. Oncogene. 2003;22:5122–5130. doi: 10.1038/sj.onc.1206548. [DOI] [PubMed] [Google Scholar]
- 23.Flemington EK. Herpesvirus lytic replication and the cell cycle: arresting new developments. J Virol. 2001;75:4475–4481. doi: 10.1128/JVI.75.10.4475-4481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Zhao Y, Zeng L, Tang M, El-Deeb A, Li JJ, Cao Y. BZLF1 controlled by family repeat domain induces lytic cytotoxicity in Epstein-Barr virus-positive tumor cells. Anticancer Res. 2004;24:67–74. [PubMed] [Google Scholar]
- 25.Chen YL, Law PY, Loh HH. Inhibition of PI3K/Akt signaling: an emerging paradigm for targeted cancer therapy. Curr Med Chem Anticancer Agents. 2005;5:575–589. doi: 10.2174/156801105774574649. [DOI] [PubMed] [Google Scholar]
- 26.Feng WH, Kenney SC. Valproic acid enhances the efficacy of chemotherapy in EBV-positive tumors by increasing lytic viral gene expression. Cancer Res. 2006;66:8762–8769. doi: 10.1158/0008-5472.CAN-06-1006. [DOI] [PubMed] [Google Scholar]
- 27.Urier G, Buisson M, Chambard P, Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 1989;8:1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mogensen TH, Paludan SR. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001;65:131–150. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiscott J, Kwon H, Genin P. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J Clin Invest. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro MG, Rossi A, Amici C. NF-kappaB and virus infection: who controls whom. EMBO J. 2003;22:2552–2560. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown HJ, Song MJ, Deng H, Wu TT, Cheng G, Sun R. NF-kappaB inhibits gammaherpesvirus lytic replication. J Virol. 2003;77:8532–8540. doi: 10.1128/JVI.77.15.8532-8540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi S, Koshiba K, Hatashita M, et al. Thermosensitization and induction of apoptosis or cell-cycle arrest human prostate cancer androgen-independent cell lines. Int J Mol Med. 2011;28:1033–1042. doi: 10.3892/ijmm.2011.760. [DOI] [PubMed] [Google Scholar]
- 35.Gunn EJ, Williams JT, Huynh DT, et al. The natural products parthenolide and andrographolide exhibit anti-cancer stem cell activity in multiple myeloma. Leuk Lymphoma. 2011;52:1085–1097. doi: 10.3109/10428194.2011.555891. [DOI] [PubMed] [Google Scholar]
- 36.Ghashghaeinia M, Toulany M, Saki M, et al. The NFkB pathway inhibitors Bay 11-7082 and parthenolide induce programmed cell death in anucleated Erythrocytes. Cell Physiol Biochem. 2011;27:45–54. doi: 10.1159/000325204. [DOI] [PubMed] [Google Scholar]