Abstract
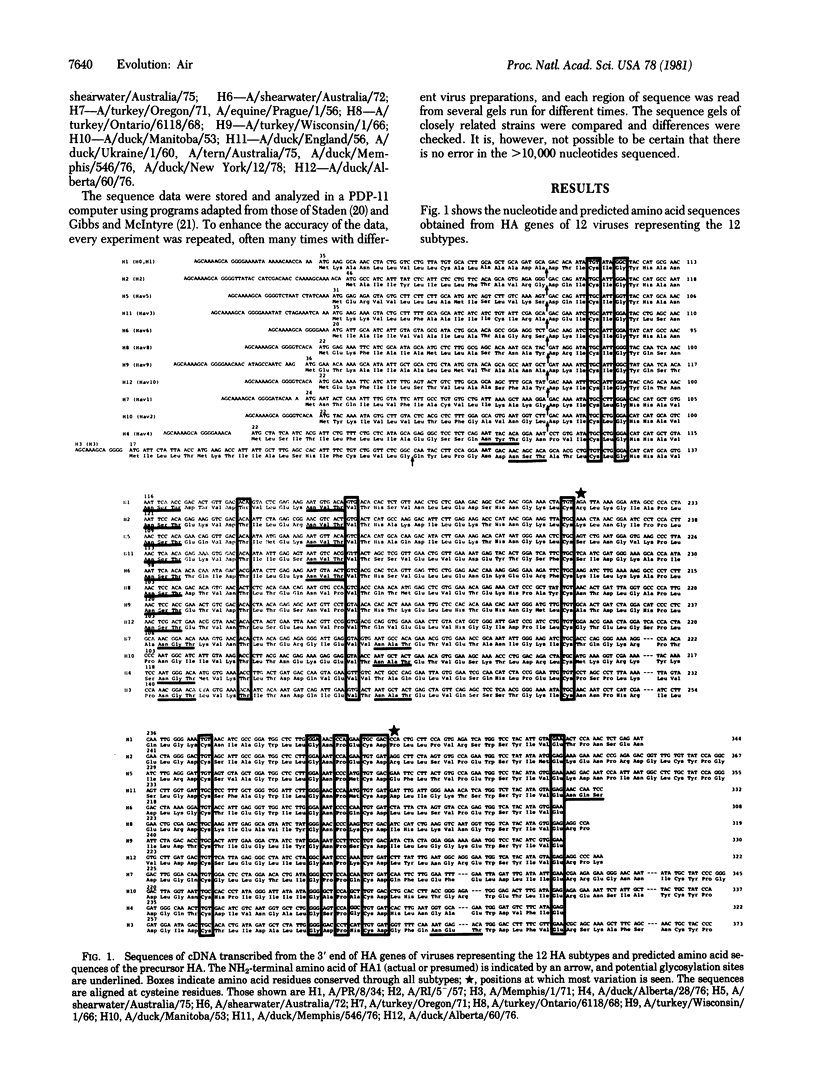
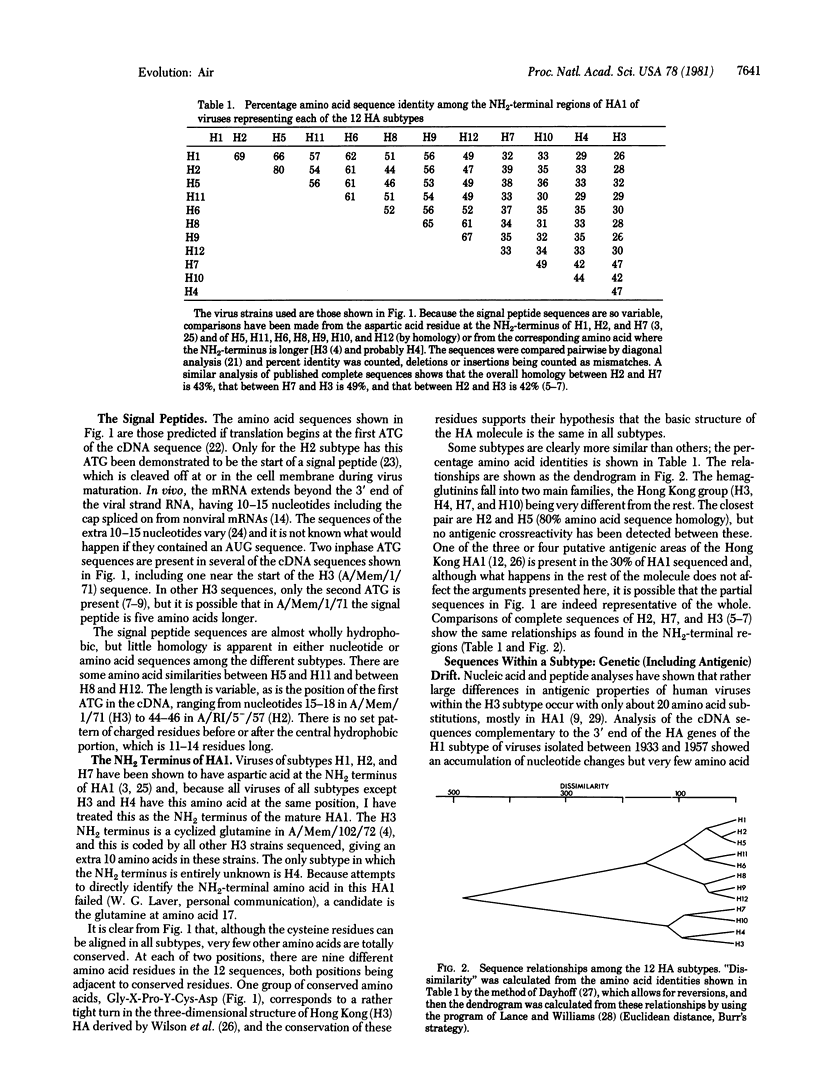
Nucleotide sequences of the 3' 20% of the hemagglutinin gene of 32 influenza A virus strains from the 12 known hemagglutinin subtypes have been determined. Although the sequences of hemagglutinin genes and proteins of different subtypes differ greatly, cysteine and some other amino acid residues are totally conserved, presumably reflecting evolution of the 12 different hemagglutinins from a single gene. When viruses of one subtype, isolated over a period of time, are compared, the hemagglutinin gene and protein sequences show a slow accumulation of nucleotide changes and some amino acid changes. Since sequence data from the genes coding for the matrix and nonstructural proteins also show an accumulation of changes with time, it seems that antigenic selection (of the surface antigens) does not contribute significantly to the rate of change on influenza gene sequences. Although the rate of nucleotide change during drift is more than sufficient to account for the amino acid sequence differences observed in the 12 subtypes, there is a clear distinction, by antigenic as well as sequence analyses, between viruses of one subtype (0-9% amino acid variation) and viruses of other subtypes (20-74% amino acid variation). No virus has yet been found that is intermediate between subtypes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M. Nucleotide sequence coding for the "signal peptide" and N terminus of the hemagglutinin from an asian (H2N2) strain of influenza virus. Virology. 1979 Sep;97(2):468–472. doi: 10.1016/0042-6822(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Blok J., Air G. M. Comparative nucleotide sequences at the 3' end of the neuraminidase gene from eleven influenza type A viruses. Virology. 1980 Nov;107(1):50–60. doi: 10.1016/0042-6822(80)90271-8. [DOI] [PubMed] [Google Scholar]
- Bucher D. J., Li S. S., Kehoe J. M., Kilbourne E. D. Chromatographic isolation of the hemagglutinin polypeptides from influenza virus vaccine and determination of their amino-terminal sequences. Proc Natl Acad Sci U S A. 1976 Jan;73(1):238–242. doi: 10.1073/pnas.73.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder K. T., Bye J. M., Skehel J. J., Waterfield M. D., Smith A. E. In vitro synthesis, glycosylation, and membrane insertion of influenza virus haemagglutinin. Virology. 1979 Jun;95(2):343–350. doi: 10.1016/0042-6822(79)90489-6. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Bye J., Skehel J., Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980 Sep 25;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- Gibbs A. J., McIntyre G. A. The diagram, a method for comparing sequences. Its use with amino acid and nucleotide sequences. Eur J Biochem. 1970 Sep;16(1):1–11. doi: 10.1111/j.1432-1033.1970.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Hall R. M., Air G. M. Variation in nucleotide sequences coding for the N-terminal regions of the matrix and nonstructural proteins of influenza A viruses. J Virol. 1981 Apr;38(1):1–7. doi: 10.1128/jvi.38.1.1-7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw V. S., Webster R. G., Bean W. J., Sriram G. The ecology of influenza viruses in ducks and analysis of influenza viruses with monoclonal antibodies. Comp Immunol Microbiol Infect Dis. 1980;3(1-2):155–164. doi: 10.1016/0147-9571(80)90051-x. [DOI] [PubMed] [Google Scholar]
- Jou W. M., Verhoeyen M., Devos R., Saman E., Fang R., Huylebroeck D., Fiers W., Threlfall G., Barber C., Carey N. Complete structure of the hemagglutinin gene from the human influenza A/Victoria/3/75 (H3N2) strain as determined from cloned DNA. Cell. 1980 Mar;19(3):683–696. doi: 10.1016/s0092-8674(80)80045-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Dopheide T. A., Ward C. W. Amino acid sequence changes in the haemagglutinin of A/Hong Kong (H3N2) influenza virus during the period 1968--77. Nature. 1980 Jan 31;283(5746):454–457. doi: 10.1038/283454a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Gerhard W., Ward C. W., Dopheide T. A. Antigenic drift in type A influenza virus: sequence differences in the hemagglutinin of Hong Kong (H3N2) variants selected with monoclonal hybridoma antibodies. Virology. 1979 Oct 15;98(1):226–237. doi: 10.1016/0042-6822(79)90540-3. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Ecology of influenza viruses in lower mammals and birds. Br Med Bull. 1979 Jan;35(1):29–33. doi: 10.1093/oxfordjournals.bmb.a071537. [DOI] [PubMed] [Google Scholar]
- Moore B. W., Webster R. G., Bean W. J., van Wyke K. L., Laver W. G., Evered M. G., Downie J. C. Reappearance in 1979 of a 1968 Hong Kong-like influenza virus. Virology. 1981 Feb;109(1):219–222. doi: 10.1016/0042-6822(81)90492-x. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Desselberger U., Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978 Jul 27;274(5669):334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A strategy of DNA sequencing employing computer programs. Nucleic Acids Res. 1979 Jun 11;6(7):2601–2610. doi: 10.1093/nar/6.7.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeyen M., Fang R., Jou W. M., Devos R., Huylebroeck D., Saman E., Fiers W. Antigenic drift between the haemagglutinin of the Hong Kong influenza strains A/Aichi/2/68 and A/Victoria/3/75. Nature. 1980 Aug 21;286(5775):771–776. doi: 10.1038/286771a0. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Espelie K., Elder K., Skehel J. J. Structure of the haemagglutinin of influenza virus. Br Med Bull. 1979 Jan;35(1):57–63. doi: 10.1093/oxfordjournals.bmb.a071543. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Determination of the number of nonoverlapping antigenic areas on Hong Kong (H3N2) influenza virus hemagglutinin with monoclonal antibodies and the selection of variants with potential epidemiological significance. Virology. 1980 Jul 15;104(1):139–148. doi: 10.1016/0042-6822(80)90372-4. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Webster R. G., Gerhard W. U. Antigenic variation in three distinct determinants of an influenza type A haemagglutinin molecule. Nature. 1979 May 17;279(5710):246–248. doi: 10.1038/279246a0. [DOI] [PubMed] [Google Scholar]
- Young J. F., Desselberger U., Palese P. Evolution of human influenza A viruses in nature: sequential mutations in the genomes of new H1N1. Cell. 1979 Sep;18(1):73–83. doi: 10.1016/0092-8674(79)90355-6. [DOI] [PubMed] [Google Scholar]


