Abstract
Background
Perivascular adipose tissue (PVAT) surrounds most vessels and shares common features with brown adipose tissue (BAT). Whereas adaptive thermogenesis in BAT increases energy expenditure and is beneficial for metabolic diseases, little is known on the role of PVAT in vascular diseases such as atherosclerosis. We hypothesize that the thermogenic function of PVAT regulates intravascular temperature and reduces atherosclerosis.
Methods and Results
PVAT shares similar structural and proteomics with BAT. We demonstrate that PVAT has thermogenic properties similar to BAT in response to cold stimuli in vivo. Proteomics analysis of the PVAT from mice housed in a cold environment identified differential expression in proteins highly related with cellular metabolic processes. In a mouse model deficient in PPARγ in smooth muscle cells (SMPG KO mice), we uncovered a complete absence of PVAT surrounding the vasculature likely due to PPARγ deletion also in the perivascular adipocyte precursor cells. Lack of PVAT, resulting in loss of its thermogenic activity, impairs vascular homeostasis causing temperature loss and endothelial dysfunction. We further show that cold exposure inhibits atherosclerosis and improves endothelial function in mice with intact PVAT, but not in SMPG KO mice, as a result of impaired lipid clearance. Pro-inflammatory cytokine expression in PVAT is not altered upon cold exposure. Finally, prostacyclin released from PVAT contributes to the vascular protection against endothelial dysfunction.
Conclusions
PVAT is a vasoactive organ with functional characteristics similar to BAT and is essential for intravascular thermoregulation upon cold acclimation. This thermogenic capacity of PVAT plays an important protective role in the pathogenesis of atherosclerosis.
Keywords: cold exposure, intravascular thermoregulation, PPARγ, perivascular adipose tissue, brown adipose tissue
Introduction
Two major types of fat tissue exist throughout the body, white adipose tissue (WAT) and brown adipose tissue (BAT). In addition to energy storage, WAT has been recognized as a major endocrine organ that produces hormones and cytokines1. Conversely, the main function of BAT is to generate heat and is essential for adaptive thermogenesis and energy expenditure2, 3. Existence of a functional BAT in adult humans is now accepted and it is evidenced by the presence of active BAT in PET scanning, which localized high metabolic activity of BAT to areas close to the clavicular, periaortic, cervical, and suprarenal regions4,5. Growing evidence obtained from human studies indicates that cold-induced activation of BAT increases thermogenesis and energy expenditure6. Also, obese and overweight subjects have lower BAT activity7. Thus, BAT has emerged as an attractive target for the treatment of obesity and associated cardiovascular diseases. In fact, fat tissue surrounding the vessels, known as perivascular adipose tissue (PVAT), has long been considered a vessel-supporting connective tissue8. Recent studies suggest that PVAT has BAT-like characteristics9, which imply that PVAT itself may exert thermogenic activity upon cold acclimation, with immediate translation into vascular activity. However, whether cold-induced thermogenesis has a direct impact on vessel biology is not known.
The vasoactive properties of PVAT are under active investigation. Accumulating data indicate that in response to vascular injury, PVAT itself enhances pro-inflammatory responses within the vasculature accompanied by a significant downregulation of adiponectin within PVAT10, 11. Other studies suggest that PVAT might increase inflammatory responses in the blood vessel wall and promote atherosclerosis12. On the other hand, PVAT has anticontractile effects on the vasculature13. In the murine vasculature and also in human small arteries, the presence of PVAT is associated with a reduced response to vasoconstricting agents, and this effect is reduced in obese subjects with metabolic syndrome13.
These observations suggest that PVAT could regulate vessel physiology and pathophysiology. Since adipocytes in PVAT have characteristics resembling brown adipocytes, we hypothesize that activation of PVAT during cold acclimation will affect endothelial function and the development of atherosclerosis. In our study, we have used a wide-variety of approaches including histology, proteomics, and physiological readouts to evaluate the basic functions of PVAT on the vasculature, as an organ in vivo, and its effects on the development of atherosclerosis. Using a genetic mouse model in which PVAT fails to develop from all vessels, we provide evidence that PVAT has a similar thermoregulatory function than BAT. Furthermore, we show that activation of PVAT during cold acclimation improves endothelial dysfunction and protects against atherosclerosis.
Materials and Methods
An expanded Methods section is available in the Online Data Supplement at http://circ.ahajournals.org/.
Breeding of mice lacking of PVAT
Mice lacking of PVAT were achieved by breeding SM22α-Cre knock-in mice (SM22αCreKI/CreKI)14 with PPARγ-floxed mice (PPARγflox/flox), both on a C57BL/6J background, with a resulting genotype of SM22αCreKI/+/PPARγflox/flox (SMPG KO mice). Littermate control mice (LC) with genotypes of (1) SM22αCreKI/+/PPARγ+/+ and (2) SM22α+/+/PPARγflox/flox are summarized in Supplemental Figure 1. To study atherosclerosis and endothelial function, SMPG KO mice were crossed with ApoE knockout mice (ApoE−/−) and the resulting offspring, with a genotype of SM22αCreKI/+/PPARγflox/flox/ApoE−/−, also lack PVAT (shown as SMPG KO/ApoE−/−). Littermate control mice, with genotypes of (1) SM22αCreKI/+/PPARγ+/+/ApoE−/− and (2)SM22α+/+/PPARγflox/flox/ApoE−/−, were selected as controls (shown as LC/ApoE−/−).
Intravascular and BAT temperature measurement
Intravascular temperature was monitored in anesthetized mice using a needle thermocouple microprobe (Adinstruments, MLT1406) inserted into the carotid artery by placing the mice on a metal pad pre-warmed at a temperature of 35°C. To simultaneously monitor thermal stimuli, a thermometer probe was placed between the mouse back and the pad. BAT temperature was monitored in anesthetized mice using the thermocouple microprobe inserted through a punctured hole generated by a 26-gauge needle in the interscapular area. Temporal control of BAT temperature was determined by immersing the hind limbs and tail in either cold (4°C) or warm (50°C) water.
Results
Proteomics similarities between PVAT and BAT
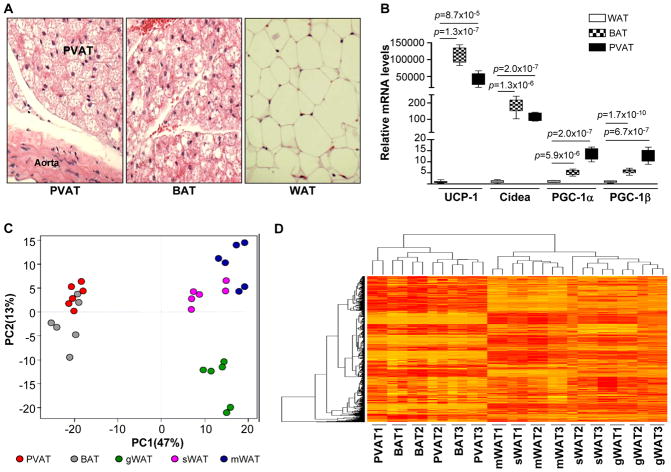
PVAT displays BAT-like morphology with a smaller adipocyte size than WAT (Figure 1A) and shares a similar gene expression pattern with BAT in rodents, which includes high expression of BAT marker genes including uncoupling protein-1 (UCP-1), Cidea, PGC-1α, and PGC-1β (Figure 1B)9, 15 To further reinforce the functional similarities between PVAT and BAT, we performed a comparative proteomics analysis of PVAT, BAT, and WAT in ApoE−/− mice. Principal component analysis (PCA) of the proteome profile identifies common protein expression patterns between PVAT and BAT that are distinct from WAT. The matrix profile of the PVAT proteome is similar to that of BAT, but clearly distinct from WAT obtained from gonadal (gWAT), mesenteric (mWAT), and subcutaneous (sWAT) adipose tissue (Figure 1C, Supplemental Excel file 1). In addition, HCA analysis demonstrates overlapping protein expression patterns of PVAT and BAT (Figure 1D). These data demonstrate that PVAT has similar structural, gene and proteomics features to those of BAT, but are clearly different from those in WAT, suggesting that PVAT might have a similar function to BAT, but not WAT.
Figure 1.
Proteomic similarities between PVAT and BAT. (A) H&E staining of sections obtained from 8 week-old ApoE−/− mouse PVAT, BAT, and WAT. (B) Real-time PCR of UCP-1, Cidea, PGC-1α, and PGC-1β mRNA in PVAT, BAT, and WAT. Data shown as box plots (Whiskers: min to max). n=6. (C) Principal component analysis (PCA) of protein distribution based on the proteome identified in PVAT, BAT, gonadal WAT (gWAT), mesenteric WAT (mWAT), and subcutaneous WAT (sWAT). Data points represent duplicated individual samples obtained from each tissue. n=3. (D) Hierarchical cluster analysis (HCA) was applied to the data in order to detect similarities among PVAT, BAT, gWAT, mWAT, and sWAT.
Loss of PVAT in an SM22α-driven PPARγ deletion mouse model
We previously developed a mouse model using a SM22α-Cre knock-in strategy14 to delete the adipogenic transcription factor PPARγ in the smooth muscle cells (VSMCs) (SMPG KO mice)16. SM22α promoter was transiently activated in the perivascular adipocytes during development as evidenced using X-gal staining of the aorta section obtained from the offspring of SM22αCreKI/CreKI mice crossed with GtRosa-lacZ reporter mice. Positive β-galactosidase activity was readily detected in the aorta and PVAT (Figure 2A). Thus, failure of PVAT to develop around the vasculature, as shown at the level of the thoracic artery, abdominal artery and also the aortic arch (Figure 2B), in the SMPG KO mice is due to deletion of PPARγ in perivascular adipocytes during development. PPARγ expression in WAT and BAT remains unaltered in the SMPG KO mice (Figure 2C). Furthermore, PPARγ deletion is essential to cause the loss of PVAT. For instance, PVAT and BAT tissues from the conventional PPARγ2 knockout mice (PPARγ2 KO) develop normally despite an extremely small mass of epididymal WAT17, and PVAT is also present in mice with a PPARδ deletion in perivascular adipocytes obtained by crossbreeding SM22αCreKI/CreKI mice and PPARδ flox/flox mice18 (Supplemental Figure 2).
Figure 2.
Perivascular phenotype of SMPG KO mice. (A) Representative X-gal staining of the aortic PVAT from the offspring resulting from the crossing of SM22αCreKI/CreKI mice with GtRosa reporter mice. (B) Representative photographs of the vasculature at the level of thoracic and abdominal aorta and the aortic arch, exposing PVAT from LC control and SMPG KO mice. (C) PPARγ mRNA levels from WAT and BAT in LC and SMPG KO mice. Data were normalized to β-actin and shown as box plots (Whiskers: min to max). n=6.
PVAT has BAT-like heat generating properties
We first determined whether BAT produced heat after the mice were subjected to environmental changes in temperature. BAT temperature was monitored in anesthetized mice by placing a thermoprobe directly into the interscapular BAT. Both the hind limbs and tail were subjected to cold-warm stimuli and BAT temperature was recorded in a time-dependent manner. Exposing the hind limbs to cold stimuli (4°C) promoted BAT-dependent heat generation as the temperature measured in the BAT depot increased along with the duration of cold exposure (Figure 3A). Removal of hind limbs from 4°C conditions and subsequent exposure to warm stimuli (50°C) simultaneously decreased BAT temperature (Figure 3A).
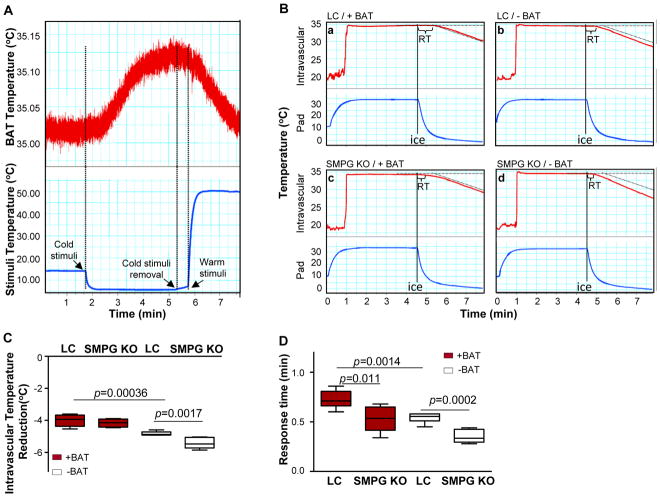
Figure 3.
PVAT has BAT-like heat generating properties. (A) Evaluation of thermogenic activation within BAT as described in Methods. (B) Intravascular temperature measurement in LC and SMPG KO mice subjected to surgical removal of BAT (−BAT) or sham-operated (+BAT) and exposed to cold stimuli. Each tracing is representative of six recordings. (C) Quantification of the intravascular temperature reduction after 5 min of cold exposure for each experimental group in B. (D) Quantification of the time difference between the thresholds of the onset of intravascular temperature loss and pad temperature drop defined as “response time” (shown as “RT” in upper panels) for each experimental group. Data are shown in C and D as box plots (Whiskers: min to max). n=6.
Next, we developed optimal experimental conditions in which both thermal stimuli and intravascular temperature can be simultaneously monitored. The intravascular temperature was monitored using a thermometer sensor inserted into the carotid artery in anesthetized mice placed onto a 35°C metal pad to maintain the normal body temperature. To simultaneously monitor thermal stimuli, a thermometer probe was placed between the mouse back and the pad (Figure 3B). Intravascular temperature was determined in mice in which interscapular BAT was surgically removed (−BAT) compared to sham-controls (+BAT) (Supplemental Figure 3). Exposing the mice to a cold stimulus by rapidly reducing the pad temperature to 4°C (blue tracings) reduced intravascular temperature in all mice (red tracings) even though BAT was generating heat (Figure 3A) during these experimental conditions. However, the intravascular temperature dropped faster in −BAT compared to +BAT mice, indicating that BAT generates heat to compensate for the reduction in body temperature (Figure 3B-panels a and b, and Figure 3C). Next, we investigated the potential heat-producing properties of PVAT by using SMPG KO mice. Exposure of SMPG KO mice to a 4°C pad results in a further reduction of intravascular temperature compared to LC mice under conditions where BAT is surgically removed (−BAT) (Figure 3B-panels b and d, and Figure 3C). We defined “response time” as the time difference between the initial fall in pad temperature and the time when intravascular temperature begins to drop after the mice were transferred from warm to cold conditions, which indicates blood temperature changes in response to the cold stimuli. As so defined, the response times of SMPG KO mice were shorter compared with LC mice regardless of whether BAT was surgically removed or not (Figure 3D), indicating that PVAT per se has thermogenic properties and generates heat for the maintenance of intravascular temperature.
Thermogenic activation of PVAT in response to long-term mild cold exposure
In accordance with the thermogenic properties of PVAT during acute cold stimulation, we also demonstrate that PVAT is activated when housing the animals in a mild cold environment. ApoE−/− mice were housed for 2 months at 60°F (mice housed at 72°F served as controls), a suitable temperature for long-term cold exposure in mice. Additionally, under these specific temperature conditions, brown fat is active in humans19. Protein expression patterns in PVAT from mice housed either at 72°F or 60°F were analyzed using hierarchical clustering analysis and identified 341 known proteins significantly increased in PVAT from mice housed at 60°F versus 72°F (Figure 4A). Importantly, Gene Ontology analysis classified 54.6% of those as proteins related to cellular metabolic processes, and 48.6% are related to primary metabolic processes (Supplemental Table 1). Enzymes known to be involved in glycolysis, fatty acid metabolism, the tricarboxylic acid cycle, and the electron transport chain are increased in PVAT from mice housed at 60°F versus 72°F (Supplemental Figure 4 and Excel File 2). In addition, mRNA expression of UCP-1, PGC-1α and PGC-1β are significantly increased in PVAT from mice housed at 60°F (Figure 4B). These changes in the protein and mRNA expression patterns in response to cold conditions strongly indicate that the metabolic activity of cold-housed mice is enhanced, and these metabolic changes in PVAT might translate into biological changes in the contiguous vessels.
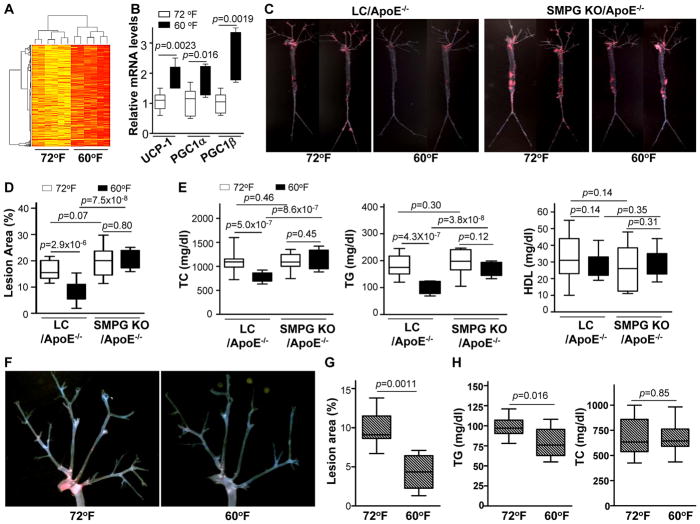
Figure 4.
PVAT activation by housing mice at 60°F reduces atherosclerosis. (A) Hierarchical cluster analysis (HCA) for proteomic clusters in the PVAT of ApoE−/− mice housed at 60°F or 72°F for 2 months. Red color clusters represent enriched proteins compared to yellow clusters. n=6. (B) Real-time PCR mRNA expression levels of UCP-1, PGC-1α, and PGC-1β in PVAT isolated from 8-weeks-old ApoE−/− mice fed a HFD and housed at 60°F or 72°F for 2 months. Data shown as box plots (Whiskers: min to max). n=6. (C) Representative images corresponding to en face Oil Red O staining of the atherosclerotic lesion of whole-aortic trees from 8-week old LC/ApoE−/− and SMPG KO/ApoE−/− mice fed for 4 months with HFD housed at either 60°F or 72°F. (D) Quantification of the ratio (%) of atherosclerotic lesion area to total aortic area for each experimental group in C. Data are shown as box plots (Whiskers: min to max). n=10–15. (E) Total plasma cholesterol (TC), triglycerides (TG), and HDL were analyzed in blood samples collected from mice in each experimental group in C. Data are shown as box plots (Whiskers: min to max). n=10–15. (F) Representative images of en face Oil Red O stained aortic arches from HFD-fed ApoE−/− mice housed at 72°F or 60°F for 2 months following surgical removal of BAT. (G) Quantification of the ratio (%) of atherosclerotic lesion area to total aortic arch area for each experimental condition in F. Data are shown as box plots (Whiskers: min to max). n=10. (H) Total plasma TC and TG were analyzed in blood samples collected from each experimental group in F. Data are shown as box plots (Whiskers: min to max). n=10.
Cold-mediated PVAT activation reduces atherosclerosis
Next, we hypothesized that cold-dependent PVAT activation may directly attenuate the development of atherosclerosis. To this aim, LC/ApoE−/− and SMPG KO/ApoE−/− mice were fed with a HFD and housed either at 60°F or 72°F for 4 months. In LC/ApoE−/− mice, total aortic lesion area was significantly lower at 60°F versus 72°F as shown by en face staining of lipid-rich lesions (Figure 4C and 4D), indicating that cold conditions inhibits atherosclerosis. Reduced atherosclerosis at 60°F was associated with a significant reduction of the total cholesterol (TC) and triglyceride (TG) levels compared to 72°F, whereas HDL remained unaltered (Figure 4E). In SMPG KO/ApoE−/− mice housed at 72°F, atherosclerosis was greater compared to LC/ApoE−/− mice (Figure 4C and 4D), despite no significant changes in total lipid profiles between SMPG KO/ApoE−/− and LC/ApoE−/− mice at 72°F (Figure 4E). These results from SMPG KO/ApoE−/− at 72°F are in agreement with the anti-inflammatory role of PPARγ in VSMCs20, which causes a direct protection against atherosclerosis, rather than a change in lipid metabolism. However, body weights of SMPG KO mice are significantly lower than LC mice due to lack of overall PVAT throughout the vasculature, as shown in the aortic tree and the abdominal region (Figure 2) but more significantly in the mesenteric arteries (Supplemental Figure 5). Of importance, housing SMPG KO/ApoE−/− mice at 60°F did not result in reduced atherosclerosis (Figure 4C and 4D) and plasma lipid profiles of SMPG KO/ApoE−/− mice are not reduced at 60°F as occurs in LC/ApoE−/− mice (Figure 4E) despite reduced body weight. Glucose- and insulin-tolerance tests were not different under any experimental conditions (Supplemental Figure 6). This indicates that lack of cold-mediated PVAT activation in SMPG KO/ApoE−/− mice fails to reduce TG levels and accordingly atherosclerosis is not efficiently reduced under cold conditions. In order to distinguish the contribution of either PVAT or BAT, we next determined whether cold-dependent atheroprotection is still observed after surgical removal of interscapular BAT in ApoE−/− (−BAT/ApoE−/−) mice. Atherosclerosis (Figure 4F and G) and plasma TG levels (Figure 4H) in −BAT/ApoE−/− mice with intact PVAT were still reduced at 60°F versus 72°F, indicating that the cold environment protected against atherosclerosis even under conditions in which BAT is surgically removed. Taken together, these data indicate that PVAT is centrally involved in the control of vascular homeostasis during atherogenesis.
Cold-induced PVAT activation attenuates age-dependent and HFD-induced endothelial dysfunction
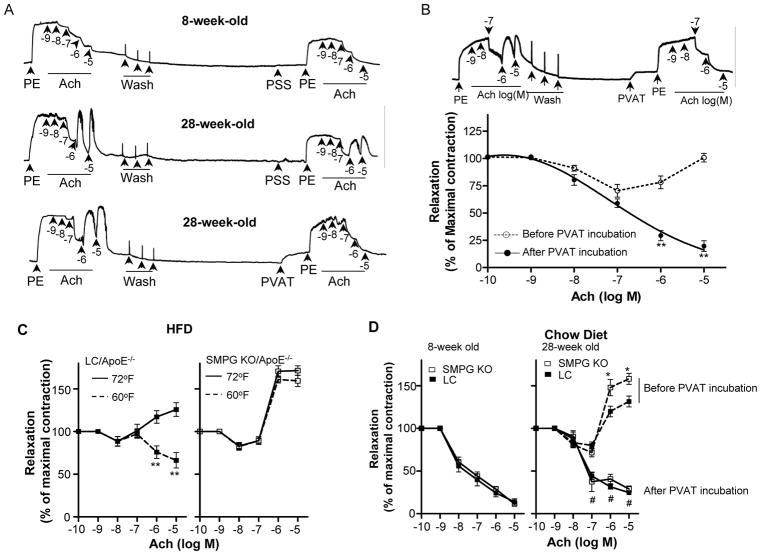
We then investigated whether PVAT protects against atherosclerosis via improvement of endothelial function. Acetylcholine (Ach)-induced endothelial-dependent vasodilation is the gold standard to evaluate endothelial function. In the presence of pathophysiological conditions, Ach constricts the vessels in an endothelium-dependent manner21, 22, which has long been recognized as a bona fide indication of endothelial dysfunction23. We used this paradoxical vasoconstriction in response to Ach to test endothelial function and determine the vascular responses to PVAT. Carotid artery rings from 8-week-old wild-type mice fed a normal chow diet show normal vasodilation following Ach administration (Figure 5A, upper panel). However, on segments from 28-weeks-old mice, serial additions of increasing concentrations of Ach caused an initial relaxation and subsequent contraction, evidencing endothelial dysfunction (middle panel). Of interest, minced PVAT from donor mice (8-week-old C57BL/6J mice) added into the myograph chamber completely reversed Ach-induced vasoconstriction (lower panel). Under more severe disease stages (e.g., in the carotid arteries of 1 month HFD-fed 8-weeks-old ApoE−/− mice), the addition of minced donor PVAT to the bath reversed Ach-induced constriction as well (Figure 5B).
Figure 5.
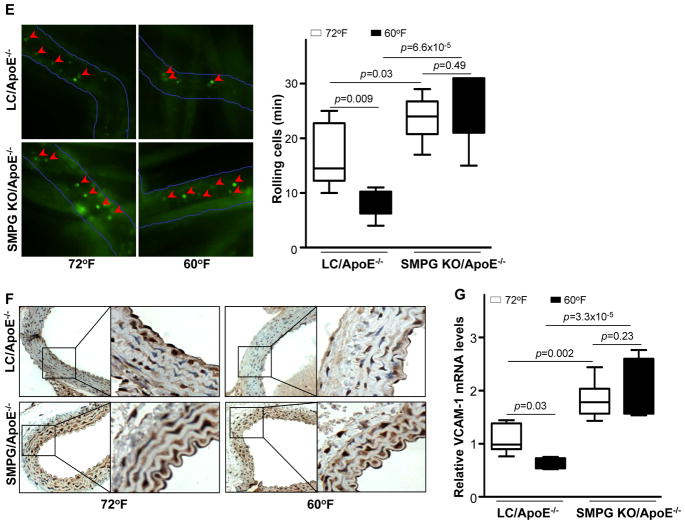
PVAT attenuates age-dependent and HFD-induced endothelial dysfunction in response to cold conditions. (A) Carotid artery rings were pre-constricted with 10−6M phenylephrine (PE) and endothelial function evaluated following serial additions of acetylcholine (Ach). Upper panel: carotid artery rings isolated from 8-weeks-old C57BL/6J mice. Middle and lower panels: carotid artery rings isolated from 28-weeks-old C57BL/6J mice. PSS: physiological saline solution; PVAT: 15mg of minced PVAT particles (~1mm3) dissected from 8-weeks-old C57BL/6J donor mice. (B) Carotid artery rings from ApoE−/− mice fed a HFD for 1 month were pre-constricted with 10−6M PE and endothelial function was evaluated following serial additions of Ach. **p<0.01 vs. before PVAT incubation. (C) Response to Ach of carotid artery rings harvested from 8-weeks-old LC/ApoE−/− and SMPG KO/ApoE−/− mice housed at 72°F or 60°F and fed 1 month with a HFD. Quantification is shown as % of maximal response set as 10−6M PE. n=12. **p<0.01 vs. 72°F.(D) Response to Ach of carotid artery rings harvested from 8-weeks-old (left panel) or 28-weeks-old (right panel) LC or SMPG KO mice fed with a chow diet before and after addition of donor PVAT (from 8-weeks-old C57BL/6J mice). #p<0.05 after vs. before PVAT incubation. *p<0.05 vs. LC group. n=12. (E) Leukocyte rolling after challenge with HFD for 2 months in LC/ApoE−/− vs. SMPG KO/ApoE−/− mice housed at 72°F or 60°F. Data were collected as the number of rolling cells per minute (see accompanying Movies in the Online Data Supplement). (F) Representative immunohistochemical staining of VCAM-1 on thoracic aortic sections from LC/ApoE−/− and SMPG KO/ApoE−/− mice challenged with HFD while housed at 72°F or 60°F for 2 months. (G) Relative VCAM-1 mRNA in the PVAT of LC/ApoE−/− and SMPG KO/ApoE−/− mice housed for 2 months at 72°F or 60°F. Data are shown as box plots (Whiskers: min to max) in (F) and (G). n=6.
Next, we determined whether PVAT activation in response to cold conditions attenuates endothelial dysfunction. In vessels from HFD-challenged 8-week-old LC/ApoE−/− mice housed at 60°F, Ach-induced vasoconstriction was significantly reduced compared to mice at 72°F (Figure 5C, left panel). However, vessels from HFD-challenged age-matched SMPG KO/ApoE−/− mice displayed further increased vasoconstriction irrespective of whether mice were housed at 72°F or 60°F (Figure 5C, right panel). Thus, cold stimuli improved endothelial function in LC/ApoE−/− mice with intact PVAT, but not in SMPG KO/ApoE−/− mice, which lack PVAT. We further demonstrated that improvement of endothelial function by PVAT is independent of PPARγ in VSMCs. Carotid arteries isolated from 8-week-old SMPG KO or LC mice did not evidenced an impaired vasorelaxation in response to Ach (Figure 5D, left panel) but 28-week-old SMPG KO mice showed increased Ach-induced constriction compared to age-matched LC mice (Figure 5D, right panel). However, donor PVAT (8-week-old C57BL/6J donor mice) still reverses Ach-induced vasoconstriction of either LC or SMPG KO mice (Figure 5D, right panel). Thus, PVAT promotes Ach-dependent vasorelaxation despite enhanced vascular contraction due to PPARγ deletion.
In addition, we tested whether decreased atherosclerosis and improvement of endothelial dysfunction observed at 60°F are due to reduced inflammation upon cold-induced PVAT activation. In HFD-fed ApoE−/− mice, mRNA expression levels of the pro-inflammatory cytokines IL-6, TNF-α, and MCP-1 were significantly up-regulated in the PVAT compared to mice fed with regular chow (Supplemental Figure 7). However, cytokine expression in the PVAT of HFD-fed ApoE−/− mice at 60°F was not different than at 72°F. Thus, cold-dependent inhibition of atherosclerosis is not the result of a reduction in the PVAT inflammatory state per se. Next, we determined whether leukocyte adhesion to endothelial cells was inhibited in the cold condition. The number of rolling leukocytes in the circulation was significantly reduced in LC/ApoE−/− mice at 60°F compared to 72°F (Figure 5E and Supplemental Movies), and molecular markers of leukocyte adhesion (e.g., VCAM-1) were also reduced (Figure 5F and 5G). On the other hand, both the number of rolling leukocytes (Figure 5E and Supplemental Movies) and VCAM-1 mRNA expression levels were similar in SMPG KO/ApoE−/− mice housed at 72°F and 60°F (Figure 5G). In SMPG KO mice, the number of rolling leukocytes and VCAM-1mRNA were increased compared to those in LC mice (Figure 5E and 5G).
Prostacyclin released by PVAT improves HFD-induced endothelial dysfunction
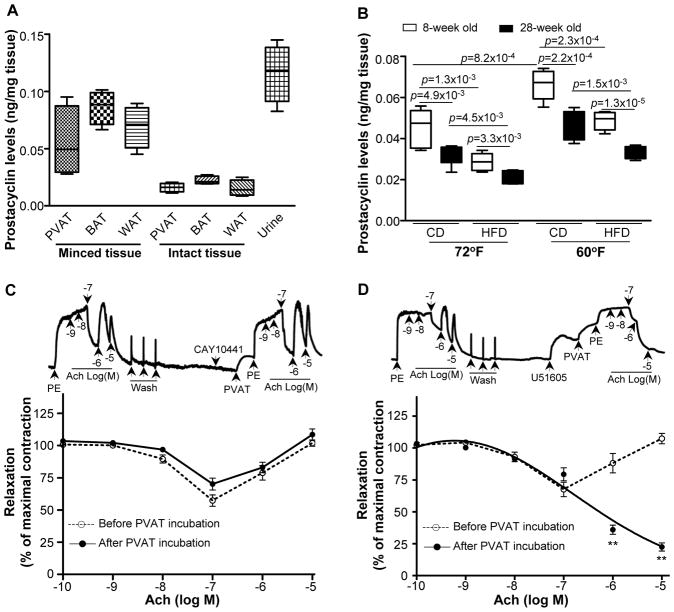
Prostacyclin is a potent vasodilator that targets prostacyclin receptors on the vasculature conferring atheroprotection24. Evidence of reduced prostacyclin release has been shown in age-related studies and hypertensive conditions25. Whereas the endothelium is a major source of prostacyclin, it is also readily detected in PVAT (Figure 6A). Consistent with these findings, we show that 1) prostacyclin released by PVAT declines with age, as levels obtained from the conditioned media of older mice are significantly reduced compared to levels from younger mice; 2) HFD feeding also results in a decline of PVAT-derived prostacyclin; and 3) most importantly, prostacyclin released from PVAT is enhanced in mice housed at 60°F compared to 72°F (Figure 6B). Therefore, we hypothesized that prostacyclin released by PVAT may contribute to the inhibition of Ach-induced vasoconstriction. Pre-incubation of vessel rings isolated from HFD-fed ApoE−/− mice with the prostacyclin receptor antagonist, CAY10441, prevented PVAT from reversing Ach-induced vasoconstriction (Figure 6C). However, PVAT was still able to reverse Ach-induced vasoconstriction after the addition of the prostacyclin synthesis inhibitor, U51605 (Figure 6D), indicating that prostacyclin released by PVAT, rather than endothelial-derived prostacyclin, reverses Ach-induced vasoconstriction in our experimental conditions. These data demonstrate that prostacyclin released from PVAT is protective on the endothelium.
Figure 6.
PVAT-released prostacyclin improves HFD-induced endothelial dysfunction. (A) The production of prostacyclin was monitored by measurement of 6-keto-PGF1α from PVAT, BAT, and WAT in either minced tissue or collected from the conditioned media obtained from intact tissue isolated from C57BL/6J mice. Urine served as a positive control. Data are shown as box plots. n=5. (B) Prostacyclin release was measured in PVAT of 8-week old ApoE−/− mice on a regular chow diet (CD) or HFD and housed at 72°F or 60°F for 2 months. Data are shown as box plots (Whiskers: min to max). n=7. (C) Pre-treatment of carotid artery rings isolated from HFD-fed mice with CAY10441 (10−7M), a prostacyclin receptor antagonist, prevents PVAT-dependent inhibition of acetylcholine-induced vasoconstriction. (D) Pre-treatment of carotid artery rings isolated from HFD-fed mice with U51605 (10−7M), a prostacyclin synthase inhibitor, fails to block PVAT-dependent inhibition of Ach-induced vessel constriction. **p<0.01 after vs. before PVAT incubation. Quantitative data shown are mean±S.E. n=10.
Discussion
BAT is essential for adaptive thermogenesis and energy expenditure in human infants26. It was previously thought that BAT is converted into WAT following infancy, and that a functional BAT did not exist in adults2, 3. However, there is accumulating evidence demonstrating that BAT, present in areas close to the clavicular, periaortic, cervical, and suprarenal regions, is activated in response to cold exposure4,5. In addition, recent studies show that cold-induced activation of BAT increases energy expenditure6 and that in obese and overweight subjects BAT activity is significantly impaired7. Collectively, these studies imply that BAT is an attractive target for the treatment of obesity and associated disorders. PVAT is defined as adipose tissue that surrounds vessels. However, PVAT displays BAT-like morphology, implying that PVAT has BAT-like function9,15. Investigating the function of PVAT in the vasculature is important to understand and treat cardiovascular diseases27. However, the biology, physiology, and pathology of PVAT are unclear. Published data showing a proinflammatory phenotype in PVAT following vascular injury28, 29, along with reported secretion of several adipokines/cytokines from PVAT15, led to the hypothesis that PVAT may contribute to the development of atherosclerosis30, 31. However, there is no experimental evidence to support that PVAT contributes to atherosclerosis. We are the first to report that PVAT, similar to BAT, is a heat-generating organ, and is critical for the maintenance of intravascular temperature. Applying an external cold stimulus to mice causes a progressive loss of intravascular temperature. When BAT is surgically removed, the temperature reduction is enhanced, consistent with its thermogenic capacity. Lack of PVAT in SMPG KO mice causes a further loss of intravascular temperature. In mammals, changes in the environmental temperature induce vascular reactions, which involve endothelial and smooth muscle cell functions. This may occur in humans as well, where an increasing intravascular temperature gradient is formed in large veins as blood approaches the heart32. Therefore, PVAT is crucial to regulate vascular homeostasis. Atherosclerosis develops generally associated with impaired energy metabolism and endothelial dysfunction. The former is highly related to body temperature. For instance, basal metabolic rates are lower in individuals living in a tropical environment compared with residents of a temperate zone32. Cold conditions also stimulate glucose uptake33 and triglyceride clearance34. Our study demonstrated that a mild cold environment activates PVAT, as evidenced by increases in enzymes related to metabolic processes when animals are housed at 60°F. As a result of cold-dependent activation of PVAT, the development of atherosclerosis is attenuated. Plasma triglyceride and cholesterol levels are reduced in mice at 60°F. Therefore, the reduced atherosclerotic lesions in mice housed in a cold environment might be a consequence of enhancing total lipid clearance by thermogenic activation of both BAT and PVAT. However, acclimation at 60°F of mice in which BAT was surgically removed still results in reduced plasma triglyceride levels and protects against atherosclerosis, suggesting that activation of PVAT alone may be responsible for these effects. In support of this conclusion we uncovered an overall increased atherosclerotic burden in SMPG KO/ApoE−/− mice housed at room temperature, which is not reduced in cold conditions.
This SMPG KO mouse model lacking PVAT was genetically achieved by breeding SM22α-Cre knock-in mice (SM22αCreKI/CreKI)14 with PPARγflox/flox mice16, and those animals were subsequently bred with ApoE−/− mice to obtain SMPG KO/ApoE−/− mice with a genotype of SM22αCreKI/+/PPARγflox/flox/ApoE−/−. These animals lack PVAT due to following reasons. First, although SM22α is one of the earliest markers of differentiated smooth muscle cells, it is also expressed in mesenchymal populations surrounding the aorta as early as E9.035. Our data showed that SM22α was transiently expressed in perivascular adipocyte precursor cells as well, although there is no SM22α expression in mature adipose tissue. This is consistent with reports showing that perivascular precursor cells might share the same developmental origin with VSMCs36–38. Therefore, when SM22αCreKI/CreKI mice are crossed with PPARγflox/flox mice, PPARγ is deleted in VSMCs as well as in perivascular precursor cells. As a consequence, no adipose tissue is visible around the major arteries in our model. Second, PPARγ, but not PPARδ, is a key regulator of white and brown adipocyte differentiation, and even when tissue specific PPARδ knock-out is generated with the same SM22α-Cre knock-in strategy18, PVAT is present in those animals. In this regard, it was reported that SM22α deficiency increased atherosclerosis in mice39, and our SM22α-Cre knock-in strategy to obtain SMPG KO mice leads to SM22α knockdown as well. However, as we and others have previously reported, heterozygous SM22αCreKI/+ mice, in which one allele is left intact to allow endogenous SM22α expression, did not show any VSMC-related phenotype, including atherosclerosis or blood pressure regulation16, 39, 40. Therefore, the use of heterozygous SM22αCre/+ mice in this study excludes the potential contribution of SM22α deficiency on atherosclerosis.
Concurrent PPARγ deletion in VSMCs, heart and skeletal muscle, as in our SMPG KO/ApoE−/− mice, while indispensable to achieve a phenotype of total absence of PVAT surrounding the vasculature, constitutes the shortcoming of the present study. In addition, HFD-fed SMPG KO/ApoE−/− mice are leaner due to lack of PVAT throughout the vasculature including the aortic arch, thoracic and abdominal aorta, and a significant reduction of mesenteric fat. However, PPARγ deletion in VSMCs, heart and skeletal muscle, and body weight reduction do not result in impaired energy balance, as the plasma lipid profile in SMPG KO/ApoE−/− mice was not different from that in LC/ApoE−/− mice at room temperature. Indeed, prior work20, including ours16 has demonstrated that PPARγ deletion in VSMCs does not affect plasma lipid profiles and whole body metabolism. PPARγ deletion in VSMCs significantly contributed to atherosclerosis20, as we confirmed in SMPG KO/ApoE−/− mice at 72°F. Chronic deficiency of PPARγ in VSMCs cannot be excluded as a contributor to atherosclerosis in SMPG KO/ApoE−/− mice. We also cannot exclude the possibility that ensuing PPARγ deletion in the heart and skeletal muscle might contribute to atherosclerosis in these studies since heart and skeletal muscle are major organs that account for a large extent of energy expenditure. However, our data showing that total plasma cholesterol and triglyceride levels are not reduced in SMPG KO/ApoE−/− mice housed at 60°F, but are reduced in LC/ApoE−/− mice, argues for a prevalent role of thermogenic activation of PVAT rather than PPARγ deletion in VSMC, heart and skeletal muscle. In SMPG KO mice, cold-dependent PVAT activation does not occur and thus, failure to clear plasma lipids contributes to atherosclerosis in the 60°F environment. Also, PVAT thermogenic activation reduces atherosclerosis even under conditions in which BAT was surgically removed. Furthermore, donor PVAT efficiently reverse endothelial dysfunction of vessels isolated from SMPG KO mice. As a whole, these data demonstrate a significant contribution of the thermogenic activation of PVAT to inhibition of atherosclerosis resulting in improvement of endothelial function in a cold environment.
Our study further demonstrated that prostacyclin derived from PVAT declines with age and HFD feeding and increases in mice during cold acclimation, suggesting that activation of PVAT leads to secretion of vasoactive agents, and that prostacyclin is one of the major factors contributing to vasculoprotection against HFD-induced endothelial dysfunction in these conditions. It was reported that PVAT releases other specific factors, named PVAT-derived relaxing factors (PVATRFs), that attenuate agonist-induced (e.g., phenylephrine, serotonin, angiotensin II, and U46619) vasoconstriction41. Such PVATRFs relax the vasculature through direct effects on the VSMCs layers42. However, at this stage we have no evidence to address whether prostacyclin derived from PVAT directly relaxes VSMCs layers in vivo. It is not likely that prostacyclin from PVAT exerts effects directly on VSMCs to relax vessels in our in vitro experimental conditions. Ach-induced vasoconstriction, an indication of endothelial dysfunction, is endothelial-dependent43. Additionally, following removal of the endothelium, prostacyclin released by PVAT did not further dilate vessels (data not shown), thus arguing that the effects observed are circumscribed to endothelial cells.
In summary, our study demonstrates for the first time that PVAT, while sharing similar structural characteristics and proteomics profile to BAT, plays a key role in intravascular thermoregulation. Cold-induced PVAT activation prevents temperature loss in the vasculature and controls vascular tone under disease conditions. Of significance, we show that lack of PVAT resulting in impaired thermogenic capacity increases atherosclerosis, which reinforces a direct beneficial impact of PVAT adaptive thermogenesis in protection against cardiovascular diseases.
Supplementary Material
Clinical Perspective.
Recent confirmation that, in addition to white adipose tissue, brown adipose tissue (BAT) is found in adult humans, playing a distinct role in adaptive thermoregulation and energy metabolism, has sparked active research on adipose tissues as bioactive organs mostly focusing on their pathophysiological aspects for treatment of metabolic disorders. This revival led to the recognition of perivascular adipose tissue (PVAT) not just as a support structure but rather a biologically active regulatory component of the vasculature. Despite increased understanding of PVAT autocrine and/or paracrine vascular roles, there is a paucity of knowledge regarding its involvement on atherosclerosis. PVAT shares some characteristics with BAT but, so far, its specific roles remain obscure due to limited animal models. We present experimental proteomics evidence that in vivo chronic cold stimulus results in activation of PVAT characterized by cellular metabolic pathways shared with BAT. A PPARγ-knockout strategy targeting smooth muscle cells results in loss of PVAT surrounding the vasculature. Use of this unique model in combination with our newly developed surgical removal of BAT reveals, for the first time, robust atheroprotection mediated by adaptive thermogenic activation of PVAT in chronic cold adaptation associated with a reduction of plasma lipid profiles and improvement of the endothelial dysfunction. Endothelial protective effects likely result from increased prostacyclin release by activated PVAT. Thus, we uncovered a new paradigm directly linking PVAT to the beneficial effects of cold exposure and thermogenesis in atherosclerosis likely to mimic those observed in humans.
Acknowledgments
Funding Sources: This work was partially funded by the National Institutes of Health (R01-HL105114, R01-HL089544 and R01-HL068878 to Y.E.C), the American Heart Association (National Scientist Development Grants 09SDG2230270 to L.C., 10SDG4150085 to L.V., and 0835237N to J.Z.), the Chinese Ministry of Science and Technology Grant (2011CB910200 to R.Z.) and the International Partnership Program for Creative Research Teams (CAS/SAFEA 20080491026 to J.W.). Y.E.C. is an Established Investigator of the American Heart Association (0840025N) and endowed Frederick G. L. Huetwell Collegiate Professor of Cardiovascular Medicine at the University of Michigan Medical Center (UMMC). This work utilized Core Services supported by the National Institutes of Health (DK089503 to the UMMC)
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Himms-Hagen J. Thermogenesis in brown adipose tissue as an energy buffer. Implications for obesity. N Engl J Med. 1984;311:1549–1558. doi: 10.1056/NEJM198412133112407. [DOI] [PubMed] [Google Scholar]
- 3.Seaton TB, Welle S, Campbell RG. Thermogenesis in brown adipose tissue. N Engl J Med. 1985;312:1062–1063. doi: 10.1056/NEJM198504183121617. [DOI] [PubMed] [Google Scholar]
- 4.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 5.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 7.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 8.Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75:690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–H1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 11.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, Sata M. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 12.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE. Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol. 2006;26:e23–e24. doi: 10.1161/01.ATV.0000202661.61837.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang L, Villacorta L, Zhang J, Garcia-Barrio MT, Yang K, Hamblin M, Whitesall SE, D’Alecy LG, Chen YE. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-gamma deletion leads to hypotension. Circulation. 2009;119:2161–2169. doi: 10.1161/CIRCULATIONAHA.108.815803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Fu M, Cui T, Xiong C, Xu K, Zhong W, Xiao Y, Floyd D, Liang J, Li E, Song Q, Chen YE. Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci U S A. 2004;101:10703–10708. doi: 10.1073/pnas.0403652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin KJ, Deng Z, Hamblin M, Zhang J, Chen YE. Vascular PPARδ protects against stroke-induced brain injury. Arterioscler Thromb Vasc Biol. 2011;31:574–581. doi: 10.1161/ATVBAHA.110.221267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian V, Golledge J, Ijaz T, Bruemmer D, Daugherty A. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPAR{gamma} Circ Res. 2010;107:953–958. doi: 10.1161/CIRCRESAHA.110.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 22.Bossaller C, Habib GB, Yamamoto H, Williams C, Wells S, Henry PD. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5′-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson JM, Marshall JJ. Direct vasoconstriction and endothelium-dependent vasodilation. Mechanisms of acetylcholine effects on coronary flow and arterial diameter in patients with nonstenotic coronary arteries. Circulation. 1989;79:1043–1051. doi: 10.1161/01.cir.79.5.1043. [DOI] [PubMed] [Google Scholar]
- 24.Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, Fitzgerald GA. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–1957. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension. 2009;53:973–978. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawkins MJ, Scopes JW. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature. 1965;206:201–202. doi: 10.1038/206201b0. [DOI] [PubMed] [Google Scholar]
- 27.Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol. 2012;165:659–669. doi: 10.1111/j.1476-5381.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagita S, Osaka M, Shimokado K, Yoshida M. Adipose inflammation initiates recruitment of leukocytes to mouse femoral artery: role of adipo-vascular axis in chronic inflammation. PLoS One. 2011;6:e19871. doi: 10.1371/journal.pone.0019871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, Sata M. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 30.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 31.Iacobellis G, Gao YJ, Sharma AM. Do cardiac and perivascular adipose tissue play a role in atherosclerosis? Curr Diab Rep. 2008;8:20–24. doi: 10.1007/s11892-008-0005-2. [DOI] [PubMed] [Google Scholar]
- 32.Robinson S. Physiological effects of heat and cold. Annu Rev Physiol. 1952;14:73–96. doi: 10.1146/annurev.ph.14.030152.000445. [DOI] [PubMed] [Google Scholar]
- 33.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 38.Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feil S, Hofmann F, Feil R. SM22alpha modulates vascular smooth muscle cell phenotype during atherogenesis. Circ Res. 2004;94:863–865. doi: 10.1161/01.RES.0000126417.38728.F6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JC, Kim S, Helmke BP, Yu WW, Du KL, Lu MM, Strobeck M, Yu Q, Parmacek MS. Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol Cell Biol. 2001;21:1336–1344. doi: 10.1128/MCB.2001.21.4.1336-1344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des. 2007;13:2185–2192. doi: 10.2174/138161207781039634. [DOI] [PubMed] [Google Scholar]
- 42.Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernandez Alfonso MS. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Varadharaj S, Zhao X, Parinandi N, Flavahan NA, Zweier JL. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1027–H1032. doi: 10.1152/ajpheart.00226.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.