Abstract
Background
In our previous study, about 75% of cow’s milk-allergic children tolerated baked-milk products, which improved their prognosis and quality of life.
Objective
We sought to identify biomarkers of varying degrees of clinical tolerance among a cohort of cow’s milk-allergic children.
Methods
132 subjects were initially classified as baked-milk-reactive, baked-milk-tolerant or “outgrown milk allergy” based on oral food challenges. The baked-milk tolerant group was then divided into 3 groups based upon the amount and degree of heat-denatured milk protein that they could tolerate. Serum was analyzed for allergen-specific IgE and IgG4, basophil reactivity was assessed in whole blood stimulated with serial 10-fold dilutions of milk protein, and prick skin tests were performed to commercial milk extract. Activated basophils were defined using flow cytometry as CD63brightCD203c+CD123+HLA-DRdim/−CD41a− lineage−. Data were analyzed using the Jonckheere-Terpstra test.
Results
Significant differences across the five clinical groups were seen for median casein- and milk-specific IgE, casein-specific IgG4 and casein IgE/IgG4; milk-specific to non-specific basophil activation ratio, median basophil reactivity, and spontaneous basophil activation (CD203c expression following stimulation with RPMI); and milk PST wheal diameters. Casein- and milk-specific IgE, milk-specific basophil reactivity and milk prick skin test wheal diameter are all significantly greater among milk-allergic patients who react to baked-milk than among those who tolerate it.
Conclusions
The majority of milk-allergic patients are able to tolerate some forms of baked-milk in their diets. Different phenotypes of cow’s milk-allergic children can be distinguished by casein- and milk-specific IgE, milk-specific basophil reactivity, and milk prick skin test mean wheal diameters. Spontaneous basophil activation is greater among patients with more severe clinical milk reactivity.
Keywords: Cow’s milk allergy, tolerance, extensively heated, baked, immunotherapy, immunomodulation, biomarker, basophil activation
Introduction
Cow’s milk allergy is the most common food allergy among young children. The majority of children develop tolerance to milk by school age, and this proportion continues to rise through adolescence 1. Although the proportion that ultimately develops clinical tolerance has remained steady over the years, in recent decades the timing of this event has grown later; in 1990 Host and Halken showed that 75% outgrew IgE-mediated cow’s milk allergy by age 3 years 2. However in 2007 when Skripak and colleagues analyzed the natural history of milk allergy in a referral population 1, they found that it took until the age of 16 years for 79% to reach this outcome. So while strict milk avoidance has been recommended in recent decades in the belief that it expedites the development of natural tolerance, in retrospect this common management practice has in fact coincided with delayed resolution of allergy. Not only has strict milk avoidance failed to yield improved long-term outcomes, it also has a major impact on the quality of life of patients, many of whom believe from experience that they can tolerate some milk-containing products. In 2008 our group showed that 75% of milk-allergic children tolerated extensively-heated (baked) milk products 3. After three months of ingesting baked-milk products, subjects’ growth and intestinal permeability were not adversely affected, and immunological parameters showed changes consistent with desensitization. Longer-term follow-up of these same patients suggests that the ingestion of baked-milk products accelerates and increases the overall likelihood of these patients completely outgrowing their milk allergy, with no significant adverse effects 4.
A randomized, controlled trial is currently underway at our center to more rigorously address the question of whether aggressive inclusion of baked-milk in the diets of those milk-allergic patients who tolerate it does improve the rate and likelihood of total resolution of milk allergy. Based on our previous study 4, we hypothesized that this dietary manipulation is a form of natural immunotherapy, and to further study this, we are following several immunological parameters over time. Additionally, we hope to identify biomarkers that will help predict the likelihood of milk-allergic patients tolerating extensively heat-denatured milk products.
Here we summarize several clinical and immunological characteristics of our interventional subjects at entry into the study (baseline), and examine how immunological characteristics vary among the groups of subjects with different levels of milk tolerance.
Methods
Participants
Milk-allergic subjects were recruited from pediatric allergy clinics at Mount Sinai and referring allergists from August 2008 to June 2011. The study was approved by the Mount Sinai Institutional Review Board and informed consent was obtained prior to enrollment. Eligible individuals were between the ages of 4 and 10 years, and had a positive prick skin test (PST) to milk or detectable serum milk-specific IgE and a history of an allergic reaction to milk within two years prior to study entry; or milk-specific IgE levels 14–35 kUA/L or PST wheal diameter >10 mm regardless of reaction history. Patients were excluded if they had milk-specific IgE >35 kUA/L (in the original study levels >35 kUA/L were associated with low probability (14%) of tolerating baked milk; subjects with such levels comprised 7% (7/99) subjects 3) or a history of a life-threatening anaphylactic reaction to milk within the two years prior to study entry.
Design
Each patient commenced a series of challenges to muffin, pizza, rice pudding or similar dish and unheated milk; and stopped when the first positive challenge occurred. The challenge foods were chosen with input from our patients, as foods whose inclusion in their diets would be of clinical relevance by improving their quality of life. The foods were placed in an ordered ranking for challenge progression by increasing amount of milk protein and decreasing level of heat- denaturation. Subjects were instructed to continue only those foods that they had tolerated during a challenge in their diet on a regular basis at home. In addition to improving quality of life, advancing the amount of milk protein and decreasing the degree of denaturation with these advancing products is intended to accelerate the development of tolerance, similar to the manner in which subcutaneous immunotherapy is increased to the maximum tolerated dose. Patients were classified into five levels of milk tolerance based on challenge outcome. The groups were labeled “baked-milk (BM)-reactive”, “BM-tolerant” (muffin, pizza or rice pudding), and “outgrown milk allergy”. On the first challenge day, prior to starting challenges, a PST to milk was performed, whole blood was collected for basophil activation studies, and a serum sample was collected for the measurement of specific IgE to milk, casein and β-lactoglobulin, and IgG4 to casein and β-lactoglobulin using the UniCAP system (Thermo Fisher Scientific; Portage, Michigan). Table 1e in the online repository records the sources of all reagents and supplies.
PST procedure
PSTs were performed with a sterile bifurcated needle, commercial milk extract, and a negative saline and positive histamine control. The size of the skin test response was calculated as a mean of the longest diameter and its longest orthogonal measured at 10 to 15 minutes5.
Challenge procedure
Food challenges were performed openly under physician supervision in the Mount Sinai Clinical Research Center. Each muffin contained 1.5 grams of milk protein and was baked at 350°F for 30 minutes. A serving of pizza contained 4 grams of milk protein, and was baked at 425°F for at least 13 minutes. A serving of rice pudding (or equivalent, for subjects who refused rice pudding) contained 7.7 grams of milk protein baked at 325°F for 90 minutes. A serving of unheated milk contained 10 grams of milk protein, and had undergone no heating besides standard pasteurization. The milk protein in the muffin and rice pudding consisted of the usual 80% casein and 20% whey proteins found in milk, and the cheese on the pizza consisted of 94.1% casein and 5.9% whey proteins. Each challenge was administered in 4–6 progressively larger portions over one hour. Up to two challenges could take place on the same day, separated by at least two hours. If further challenges were required, a second challenge day was scheduled within two weeks of the first. Subjects were monitored throughout the challenges and for 2–4 hours following the final challenge of each day. Challenges were discontinued at the first objective sign of reaction and appropriate treatment was initiated immediately. Subjects who failed the muffin challenge continued on a strict milk-avoidance diet and returned one year later for repeat baseline challenge. Subjects who reported subsequent symptoms at home to foods that they had apparently tolerated during the initial challenge returned for repeat baseline challenge at the first opportunity. For each subject, only the most recent baseline visit was included in the analysis.
Basophil activation test
Whole blood aliquots (250 μL) were incubated with equal volumes of basophil stimulation buffer (RPMI + IL-3 at 2 ng/mL) alone, or with the addition of milk powder in phosphate-buffered saline (PBS) at serial 10-fold dilutions (from 1 × 103 to 1 × 10−1 μg/mL total protein), polyclonal anti-IgE antibody (1 μg/mL; positive control), PMA/CaI (Phorbol myristate acetate (PMA) 0.25 μg/mL/calcium ionophore (CaI) 1 μg/mL; positive control) or N-formyl-methionyl-leucyl-phenylalanine (fMLP) (1 μM; IgE-independent positive control); or RPMI alone (negative control) at 37°C for 30 minutes. The reaction was stopped with 50 μL of cold PBS + ethylenediaminetetraacetic acid (EDTA) 20 mM. Cells were then stained for expression of CD63, CD123, CD203c, CD41a, CD3, CD14, CD19 and HLA-DR at 4°C in the dark for 30 minutes. Following incubation, cells were washed with PBS + 0.5% bovine serum albumin + 2 mM EDTA. Red cells were then lysed by adding 4 mL of FACS Lysing Solution to each sample for 15 minutes.
Flow cytometry
Basophil activation was assessed by flow cytometry 6. Samples were analyzed on a BD LSRII flow cytometer. Single-color compensation samples were prepared using anti-mouse Ig beads. Fluorescence data were acquired and auto-compensated on a modified LSR-II configured for seven-color parameters using FACSDiva version 6.0 (BD Biosciences; San Jose, CA). Basophils were identified as CD123+ HLA-DRdim/− CD41a− CD3− CD14− CD19− and activated basophils additionally as CD63+ CD203c+, as shown in figure 1e in the online repository. A minimum of 50 CD123+ HLA-DRdim/− CD41a− CD3− CD14− CD19− events (i.e. basophils) were recorded for each condition or the sample was excluded. Non-responders were defined as individuals with less than 5% CD63/CD203c up-regulation (basophil activation) in response to all milk concentrations and the anti-IgE control condition, and were also excluded. Milk-specific basophil reactivity was defined as an individual’s maximum basophil activation in response to any milk concentration 7. Analysis of cytometry data was performed using FlowJo version 8.8.6 (Tree Star, Inc.).
Statistics
Graphical display and statistical analyses were performed using R analysis 2.12.1 8, 9, 10. The frequencies of missing data and basophil non-responders were compared between groups using the Fisher exact t-test. For determining the significance of differences in each measure across clinical groups (with the three baked-milk-tolerant groups considered both together and separately), the Jonckheere-Terpstra test for ordered alternatives was used. This is a “between groups trend” test, where in order to reject the null hypothesis, the median level of a measure must decrease in an orderly fashion (i.e. demonstrate a monotonic trend). Post-hoc tests for pair-wise differences between adjacent clinical groups were performed using Wilcoxon signed-rank tests, and a Bonferroni correction for multiple comparisons was performed.
Results
In total, 147 subjects were evaluated. Fifteen subjects were not challenged and assumed reactive to baked milk because their milk-specific IgE exceeded 35kUA/L3. Of the 132 subjects challenged, 37 reacted to baked-milk (muffin), 31 tolerated muffin, 12 tolerated pizza, 44 tolerated rice pudding or equivalent, and 8 had outgrown their milk allergy (tolerated unheated milk). Thus, 65% of those studied in this cohort tolerated baked-milk, compared with only 6% who tolerated whole milk. Eight subjects who reacted to muffin one year previously returned for a repeat baseline visit. Of these, 5 again reacted to muffin, 2 tolerated muffin, and 1 tolerated rice pudding. One subject who tolerated muffin at the original baseline visit developed symptoms on repeated ingestion at home and then reacted to muffin during a re-challenge 3 months later. Overall the subjects’ median age was 7.6 years (range 4.0–11.0 years), and 92 (70%) were male. Age and gender distribution by clinical outcome are shown in table 1. Twelve (9.1%) of the subjects exhibited the basophil non-responder phenotype, and this proportion was consistent with the rate of non-responders previously reported in the normal population 11,12. In 12 (9.1%) of the samples, insufficient basophils were acquired. The non-responders and subjects for whom insufficient basophils were acquired were eliminated from subsequent analyses of basophil activation. There was no serum available for immunoglobulin measurement in 2 samples (1.5%). There was no significant difference between groups in the frequency of non-responders, or samples otherwise excluded from basophil or immunoglobulin analysis (data not shown).
Table 1.
Age and gender distribution by clinical outcome
| Total | Median Age in years | Males (%) | |
|---|---|---|---|
| Baked-milk Reactive | 37 | 8.1 | 29 (78%) |
| Tolerated Muffin | 31 | 7.4 | 23 (74%) |
| Tolerated Pizza | 12 | 6.5 | 9 (75%) |
| Tolerated Rice Pudding | 44 | 7.6 | 27 (61%) |
| Outgrown | 8 | 6.6 | 4 (50%) |
Immunoglobulins
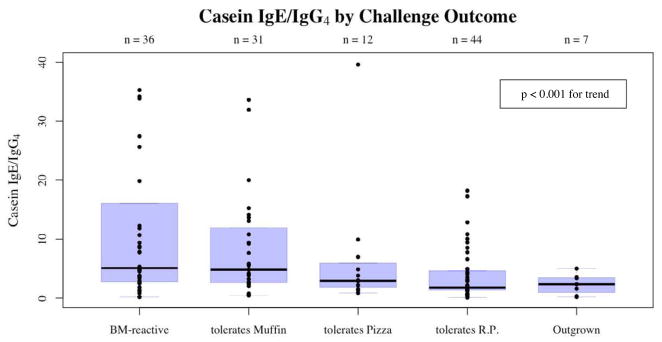
Median casein-specific IgE (p<0.001), casein-specific IgG4 (p<0.05) and casein-specific IgE/IgG4 ratio (p<0.001) all differed significantly across the five clinical groups (figures 1, 2 and 3). Casein-specific IgE varied from 13.75 kUA/L (range 0.36–49.9 kUA/L) among baked-milk-reactive subjects to 0.44 kUA/L (range <0.35–1.79 kUA/L) among subjects who had outgrown their allergy. Casein-specific IgG4 varied from 1.87 mgA/L (range 0.15–10.3 mgA/L) among baked-milk-reactive subjects to 0.28 mgA/L (range <0.10–2.32 mgA/L) among subjects who had outgrown their allergy. Casein-specific IgE to IgG4 ratios varied from 5.08 (range 0.18–202.7) among baked-milk-reactive subjects to 2.3 (range 0.18–5.0) among subjects who had outgrown their milk allergy. Post-hoc analysis shows that casein-specific IgE differed significantly between those who reacted to baked-milk and those who tolerated it (p<0.001), and between those who tolerated baked-milk and those who had fully outgrown their milk allergy (p<0.005), but not among different gradations of heated-milk tolerance. Casein IgE to IgG4 ratios effectively discriminated between those who reacted to baked-milk and those who tolerated it (p<0.01), but not between those who tolerate baked-milk and those who had fully outgrown their milk allergy (p=0.25).
Figure 1.
Casein-specific IgE by five levels of challenge outcome. With the three central groups pooled, post-hoc analysis showed a significant difference between those who reacted to baked-milk and those who tolerated it (p<0.001), and between those who tolerated baked-milk and those who had fully outgrown their milk allergy (p<0.005).
Figure 2.
Casein-specific IgG4 by five levels of challenge outcome.
Figure 3.
Ratio of casein-specific IgE to IgG4 ratio by five levels of challenge outcome. With the three central groups pooled, post-hoc analysis showed a significant difference between those who reacted to baked-milk and those who tolerated it (p<0.01), but not between those who tolerate baked-milk and those who had fully outgrown their milk allergy (p=0.25).
Cow’s milk IgE and β-lactoglobulin IgE also decreased significantly in order of the five clinical groups (p<0.001 for both trends). Cow’s milk IgE varied from 12.4 kUA/L (range 0.6 – 43.6 kUA/L) among baked-milk-reactive subjects to 0.7 kUA/L (range <0.35–4.3 kUA/L) among subjects who had outgrown their milk allergy. β-lactoglobulin IgE levels varied from 2.1 kUA/L (range <0.35–15.4 kUA/L) among baked-milk-reactive subjects to <0.35 kUA/L (range <0.35–0.46 kUA/L) among subjects who had outgrown their milk allergy.
Basophil reactivity
The ratio of milk-specific basophil reactivity to non-specific (anti-IgE) basophil activation showed a significant trend (p<0.005) across the five levels of baked-milk-tolerant subjects, with those reacting to all forms of baked-milk exhibiting the highest ratios (median 2.4, range 0.4–15.5), and subjects who tolerated unheated milk exhibiting the lowest (median 0.6, range 0.3–11.0) (figure 4).
Figure 4.
Ratio of milk-specific to Anti-IgE (non-specific) basophil activation by five levels of challenge outcome. With the three central groups pooled, post-hoc analysis showed a significant difference between those who reacted to baked-milk and those who tolerated it (p<0.05), but not between those who tolerate baked-milk and those who had fully outgrown their milk allergy (p=0.11).
Post-hoc analyses of pair-wise differences between adjacent groups showed a significant difference between those who reacted to baked-milk and those who tolerated it (p<0.05), but not between those who tolerated baked-milk and those who had fully outgrown their milk allergy (p=0.11).
Spontaneous basophil activation, as measured by both piecemeal (CD203c+) and anaphylactic (CD63+) degranulation 13 under negative control conditions, was also greater among more milk-allergic individuals. This was evident not only when the basophils were incubated with IL-3, a known priming agent, but also when subjected to RPMI alone. Figure 5 shows the significant (p<0.05) reduction in CD203c mean fluorescent intensity with stimulation by RPMI alone with increasing milk tolerance measured across five clinical groups.
Figure 5.
Mean fluorescent intensity of CD203c following stimulation by RPMI (negative control) by five levels of challenge outcome. This measure forms an indication of spontaneous activation.
The basophil reactivity taken alone, and not as a ratio of anti-IgE basophil activation, also showed a significant trend across the five levels of milk tolerance (p<0.001), with subjects who were reactive to all forms of baked-milk exhibiting the highest levels of basophil reactivity (median 50.7%, range 7.3–95.1%), and subjects who tolerated unheated milk exhibiting the lowest (median 9.165%, range 1.9–31.8%) (See figure 2e in the online repository). In this case, post-hoc analysis after pooling all subjects who tolerated some form of baked milk, basophil reactivity was significantly greater in baked-milk-reactive subjects than in baked-milk-tolerant subjects (p<0.01), and significantly greater in baked-milk-tolerant subjects than in those who have outgrown their allergy (p<0.05).
Mast cell activity
The decrease in the size of the mean wheal diameter following prick skin testing with commercial milk extract is significant (p<0.001) in order of the five levels of milk reactivity, extending from a median of 10 mm among baked-milk-reactive subjects (range 5.5–20 mm) to a median of 6 mm (range 1–9 mm) among subjects who had outgrown their milk allergy (figure 6). Post-hoc analysis shows a significant decrease in wheal size in those who tolerate baked-milk compared with those who react to it (p<0.005), and in those who have fully outgrown their milk allergy compared with those who tolerate baked-milk (p<0.005), but no differences among different gradations of baked-milk-tolerance.
Figure 6.
Wheal upon PST with commercial milk extract by three levels of challenge outcome (baked-milk-tolerant subjects have been pooled). Post-hoc analysis shows a significant decrease in wheal size in those who tolerate baked-milk compared with those who react to it (p<0.005), and in those who have fully outgrown their milk allergy compared with those who tolerate baked-milk (p<0.005).
ROC curves
The receiver operating characteristics shown in figure 7 describe the performance of casein- and milk-specific IgE levels, milk-specific basophil reactivity and mean milk prick skin test wheal diameter in differentiating baked-milk-tolerant from baked-milk-reactive subjects. The AUCs of these measures and their respective confidence intervals (detailed in the legend of figure 7) show that each measure has significant effectiveness in predicting baked-milk reactivity.
Figure 7.
Receiver operating characteristics showing performance of various tests in predicting clinical reactivity to baked milk. Casein IgE has Area Under the Curve (AUC) 0.78 (95% CI 0.69–0.88); Cow’s Milk IgE has AUC 0.73 (95% CI 0.63–0.83); Milk-specific Basophil Reactivity has AUC 0.69 (95% CI 0.59–0.80); and Milk PST wheal diameter has AUC 0.68 (95% CI 0.58–0.78).
Discussion
Our group was the first to show significant differences in easily measurable parameters between milk-allergic patients who react to baked-milk and those who tolerate it. IgE levels and their corresponding likelihoods of clinical milk reactivity were established in 1997 14 and the ability of mean wheal diameter to predict milk reactivity has been studied since at least 1977 15, 16. However all previous studies of IgE levels and mean wheal diameters seek to differentiate patients with any reactivity to milk from those with no clinical reactivity, and have not addressed differences between those patients who do or do not react to heat-denatured milk.
Our data show that IgE levels both to whole milk and to caseins, the most abundant proteins in milk, differ significantly between baked-milk tolerant and reactive patients.
We examined IgG4 levels to determine whether they could improve on the accuracy of IgE alone in predicting our patients’ level of clinical reactivity, and also to elucidate the role of IgG4 in the development of tolerance (figure 3). In patients undergoing immunotherapy with increasing doses of environmental allergens, IgG4 typically increases over the course of treatment, and then decreases following termination of immunotherapy 17. Higher IgG4 levels do not seem to reflect the absolute dose of allergen tolerated, but rather are more likely a reflection of increasing allergen exposure over the recent past. Hence in this cross-sectional view of our subjects, who all have a recent history of total milk avoidance, it is not surprising that no correlation between higher IgG4 levels and greater milk tolerance is seen. If, as our subjects go on to include more and more milk in their diets, a pattern of short-term increase in IgG4 followed by a tapering off is borne out for individual subjects, it will support the theory that the careful introduction of baked-milk-containing products as tolerated acts as a form of “natural immunotherapy”.
IgE receptor density on basophils is closely related to serum IgE concentration 18. Therefore, since we know IgE levels are related to clinical milk reactivity, it is not surprising to see that measures of basophil activation are also related 19. Milk-specific basophil reactivity was examined as a ratio with non-specific (anti-IgE mediated) basophil activation (figure 4) to account for the higher IgE receptor density seen in atopic individuals compared to healthy controls 7. Studies showing this were done in patients with seasonal and other environmental allergies 20 who are unable to strictly avoid the relevant allergen. Given that up-regulation of FcεRI, the high affinity IgE receptor, is mediated by its interaction with IgE 21, and spontaneous histamine release is much lower in patients with atopic dermatitis and food allergy who are avoiding their allergens compared to those who are not 22, it is possible that the higher receptor density is not seen in patients following strict allergen avoidance. On the other hand, subjects with food allergy and atopic dermatitis who are practicing strict avoidance appear to have greater histamine release than atopic dermatitis patients with no food allergies. This would be consistent with the hypothesis that more milk-allergic subjects have greater IgE receptor density regardless of allergen exposure, but could also be explained by other factors, such as histamine-releasing factor present in the blood 22. Our finding of greater spontaneous activation with increasing clinical milk reactivity at oral challenge is consistent with either possibility.
Despite the rationale for examining the ratio of milk-specific to non-specific basophil activation, only the milk-specific basophil reactivity alone, without adjustment for non-specific activation, showed significant pair-wise differences both between baked-milk-reactive and -tolerant subjects, and between baked-milk-tolerant subjects and those who have outgrown their milk allergy (figure 2e in the online repository).
Indirect measurement of mast cell activity via skin testing is simple to perform and is relatively comparable to immunoglobulin measurements in predicting clinical reactivity 23. We have shown that mast cell mean wheal diameters (figure 6) also perform favorably in comparison to immunoglobulin levels and basophil activation in differentiating between baked-milk-reactive and baked-milk-tolerant subjects (figure 7). Mast cells are known to have “memory”, in that skin tests often remain positive for years after the development of clinical tolerance to allergen, and this may explain why skin testing does not differentiate well between different degrees of heat-denatured-milk tolerance, as this progressive tolerance likely evolves over much shorter periods.
Receiver operating characteristics (ROC curves) were generated to analyze the ability of these biomarkers to predict baked-milk tolerance (figure 7). Choosing optimal cutoffs for each test would take into account not only the maximum accuracy that can be simultaneously achieved for sensitivity and specificity, but also the relative “costs” of false negative and false positive results. As such an analysis is beyond the scope of this paper, no thresholds are suggested for clinical use. Like most forms of allergy testing, none of the tests evaluated provided simultaneously high sensitivity and specificity, reinforcing the need for physician-supervised food challenges to accurately diagnose food allergy.
This study again confirms the finding that the majority of milk-allergic patients are able to tolerate some forms of baked-milk in their diets. Casein- and milk-specific IgE and milk prick skin test mean wheal diameters, as well as milk-specific basophil reactivity, can differentiate among different phenotypes of cow’s milk-allergic patients. We have also demonstrated that spontaneous basophil activation is greater among patients with more severe clinical milk reactivity. These findings help to illuminate some of the immunologic mechanisms that underlie milk allergy.
Supplementary Material
Clinical Implications.
Most milk-allergic patients can tolerate some forms of baked milk. Biomarkers including IgE levels, prick skin tests and milk-specific basophil reactivity can differentiate among different phenotypes of cow’s milk-allergic patients.
Acknowledgments
Funding:
Hugh Sampson, MD is funded in part by grants from the National Institutes of Health/ National Institute of Allergy and Infectious Diseases, AI44236 and AI066738, and the National Center for Research Resources, RR026134.
Lara Ford MD, MPH is funded by the AAAAI/Elliot and Roslyn Jaffe Third-Year Fellowship Food Allergy Research Award at Mount Sinai School of Medicine
We thank Beth Strong and Sally Noone for research coordination; the nursing staff of the Clinical Research Unit; Luda Bardina, Ramon Bencharitiwong, Michelle Mishoe, Anita Saha, Alyssa Chase, Emily Hertzberg, Akshay Bhatt and Neisha Rivers for laboratory technical assistance; Scott Sicherer, Julie Wang, Jennifer Kim, Amanda Cox, Kirsi Jarvinen-Seppo, and Justin Skripak for patient referrals; and Dr Jim Godbold from Mount Sinai School of Medicine, Department of Biostatistics, for assistance with statistical analysis. Finally, we thank the participants and their families who have made this study possible.
Abbreviations
- CMA
Cow’s milk allergic
- PST
Prick skin test
- RP
Rice Pudding
- ROC
Receiver Operating Characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007 Nov;120(5):1172–7. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Host A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990 Nov;45(8):587–96. doi: 10.1111/j.1398-9995.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 3.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008 Aug;122(2):342–7. 7 e1–2. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Nowak-Węgrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. Journal of Allergy and Clinical Immunology. 2011;128(1):125–31.e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstege A, Mehl A, Rolinck-Werninghaus C, Staden U, Nocon M, Beyer K, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy. 2005 Sep;35(9):1220–6. doi: 10.1111/j.1365-2222.2005.2324.x. [DOI] [PubMed] [Google Scholar]
- 6.Shreffler WG. Evaluation of basophil activation in food allergy: present and future applications. Curr Opin Allergy Clin Immunol. 2006 Jun;6(3):226–33. doi: 10.1097/01.all.0000225165.83144.2f. [DOI] [PubMed] [Google Scholar]
- 7.Kleine-Tebbe J, Erdmann S, Knol EF, MacGlashan DW, Jr, Poulsen LK, Gibbs BF. Diagnostic tests based on human basophils: potentials, pitfalls and perspectives. Int Arch Allergy Immunol. 2006;141(1):79–90. doi: 10.1159/000094495. [DOI] [PubMed] [Google Scholar]
- 8.Team RDC; R Foundation for Statistical Computing. R: A language and environment for statistical computing. 2.12.1. Vienna, Austria: 2011. [Google Scholar]
- 9.Sing T, Sander O, Beerenwinkel N, Lengauer T. R package version 1.0-4 ed. 2009. ROCR: Visualizing the performance of scoring classifiers. [Google Scholar]
- 10.Bromberg P. R package version 1.24.0 ed. 2006. SAGx: Statistical Analysis of the GeneChip. [Google Scholar]
- 11.Nguyen KL, Gillis S, MacGlashan DW., Jr A comparative study of releasing and nonreleasing human basophils: nonreleasing basophils lack an early component of the signal transduction pathway that follows IgE cross-linking. J Allergy Clin Immunol. 1990 Jun;85(6):1020–9. doi: 10.1016/0091-6749(90)90046-7. [DOI] [PubMed] [Google Scholar]
- 12.Marone G, Poto S, Giugliano R, Bonini S. Studies on human basophil releasability. Int Arch Allergy Appl Immunol. 1985;77(1–2):103–6. doi: 10.1159/000233761. [DOI] [PubMed] [Google Scholar]
- 13.MacGlashan D. Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clinical & Experimental Allergy. 2010;40(9):1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997 Oct;100(4):444–51. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 15.Bock SA, Buckley J, Holst A, May CD. Proper use of skin tests with food extracts in diagnosis of hypersensitivity to food in children. Clin Allergy. 1977 Jul;7(4):375–83. doi: 10.1111/j.1365-2222.1977.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 16.Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy. 2000 Nov;30(11):1540–6. doi: 10.1046/j.1365-2222.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 17.Peng ZK, Naclerio RM, Norman PS, Adkinson NF., Jr Quantitative IgE- and IgG-subclass responses during and after long-term ragweed immunotherapy. J Allergy Clin Immunol. 1992 Feb;89(2):519–29. doi: 10.1016/0091-6749(92)90318-v. [DOI] [PubMed] [Google Scholar]
- 18.Malveaux FJ, Conroy MC, Adkinson NF, Jr, Lichtenstein LM. IgE receptors on human basophils. Relationship to serum IgE concentration. J Clin Invest. 1978 Jul;62(1):176–81. doi: 10.1172/JCI109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. Journal of Allergy and Clinical Immunology. 2009;123(4):789–94.e20. doi: 10.1016/j.jaci.2008.12.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conroy MC, Adkinson NF, Jr, lichtenstein LM. Measurement of IgE on human basophils: relation to serum IgE and anti-IgE-induced histamine release. J Immunol. 1977 Apr;118(4):1317–21. [PubMed] [Google Scholar]
- 21.MacGlashan D, Jr, Lichtenstein LM, McKenzie-White J, Chichester K, Henry AJ, Sutton BJ, et al. Upregulation of FcepsilonRI on human basophils by IgE antibody is mediated by interaction of IgE with FcepsilonRI. J Allergy Clin Immunol. 1999 Aug;104(2 Pt 1):492–8. doi: 10.1016/s0091-6749(99)70399-4. [DOI] [PubMed] [Google Scholar]
- 22.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989 Jul 27;321(4):228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 23.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010 Dec;126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.