Abstract
Mechanisms by which cancer cells communicate with the host organism to regulate lung colonization/metastasis are unclear. We show that this communication occurs via sphingosine 1-phosphate (S1P) generated systemically by sphingosine kinase 1 (SK1), rather than via tumour-derived S1P. Modulation of systemic, but not tumour SK1, prevented S1P elevation, and inhibited TRAMP-induced prostate cancer growth in TRAMP+/+SK1−/− mice, or lung metastasis of multiple cancer cells in SK1−/− animals. Genetic loss of SK1 activated a master metastasis suppressor, Brms1 (breast carcinoma metastasis suppressor 1), via modulation of S1P receptor 2 (S1PR2) in cancer cells. Alterations of S1PR2 using pharmacologic and genetic tools enhanced Brms1. Moreover, Brms1 in S1PR2−/− MEFs was modulated by serum S1P alterations. Accordingly, ectopic Brms1 in MB49 bladder cancer cells suppressed lung metastasis, and stable knockdown of Brms1 prevented this process. Importantly, inhibition of systemic S1P signalling using a novel anti-S1P monoclonal antibody (mAb), Sphingomab, attenuated lung metastasis, which was prevented by Brms1 knockdown in MB49 cells. Thus, these data suggest that systemic SK1/S1P regulates metastatic potential via regulation of tumour S1PR2/Brms1 axis.
Keywords: lung metastasis, sphingolipids, sphingomab, sphingosine kinase 1, sphingosine 1-phosphate
INTRODUCTION
It is known that cancer cells communicate with the host organism to regulate their metastasis. However, mechanisms of this communication between cancer cells and the host remain unknown.
Sphingolipids, especially ceramide and sphingosine 1-phosphate (S1P) are biologically active molecules, which regulate cellular processes such as survival, proliferation, migration, growth and/or cell death (Ogretmen & Hannun, 2004; Deng et al, 2008). Ceramide is generally a potent inducer of cell death, growth inhibition and senescence. (Hannun & Obeid, 2008). Ceramide is hydrolysed to yield sphingosine through the action of ceramidases, which liberate sphingosine and fatty acids (Spiegel & Milstien, 2011). Sphingosine is then phosphorylated by sphingosine kinase 1 or 2 (SK1 or SK2) to generate S1P, which signals through a family of G-protein coupled receptors, GPCRs (S1P receptors 1–5, S1PR1-5) via paracrine or autocrine manner to mediate cell growth, proliferation and/or survival (Spiegel & Milstien, 2011; Thangada et al, 2010).
Elevated S1P is found in the serum of ovarian cancer patients (Sutphen et al, 2004), and S1P receptor 1 (S1PR1) is required for tumour angiogenesis in vivo (Yonesu et al, 2009). Induction of SK1/S1P signalling results in malignant transformation and tumour formation (Pitson et al, 2005). Increased S1P promote proliferation and survival in human glioma, breast and ovarian cancer cells (Ruckhäberle et al, 2008; Wang et al, 2008; Young et al, 2009). SK1/S1P/S1PR2 signalling was shown to regulate Bcr-Abl stability and resistance to tyrosine kinase inhibitors (TKIs), such as imatinib and nilotinib in chronic myeloid leukemia models (Baran et al, 2007; Bonhoure et al, 2008; Li et al, 2007; Salas et al, 2011). In contrast, inhibition of SK1 results in cell death in human breast cancer cells (Sarkar et al, 2005), indicating that tumour SK1/S1P signalling plays important roles in growth/proliferation. Interestingly, the anti-cancer activity of an anti-S1P monoclonal antibody Sphingomab, which neutralizes S1P and inhibits extracellular signalling, provides evidence of the importance of systemic S1P in inducing tumour growth and/or progression (Visentin et al, 2006). However, roles and mechanisms of action of tumour versus systemic SK1/S1P signalling in the regulation of local tumour growth and/or metastasis are unclear. To this end, human BRMS1 (Seraj et al, 2000), and its murine homologue Brms1 (Samant et al, 2002) was initially discovered as a suppressor of metastasis in breast cancer models. Recently, roles of BRMS1 in controlling lung cancer metastasis were also reported (Nagji et al, 2010). However, whether systemic and/or tumour SK1/S1P signalling is involved in the regulation of Brms1 expression and/or metastasis remain unknown.
Therefore, the goal of this study was to define the roles and mechanisms of action of tumour versus systemic SK1/S1P signalling in the regulation of local tumour growth versus lung colonization/metastasis. Thus, with pharmacological, molecular and genetic tools, we obtained evidence that both cancer cells and systemic SK1/S1P regulate local tumour growth, whereas systemic SK1/S1P signalling is key for controlling lung metastasis. Mechanistically, our data suggest that systemic SK1/S1P regulates lung metastasis of cancer cells via down-regulation of a master suppressor of metastasis, Brms1, through S1PR2 signalling. Thus, these data suggest that systemic S1P, and not tumour-derived S1P, provides communication between cancer cells and host organism, promoting lung metastasis. Mechanistically, our data suggest that systemic S1P-mediated lung colonization/metastasis is controlled selectively by tumour Brms1 expression via S1PR2 signalling. In addition, these data also indicate that genetic and/or pharmacologic targeting of systemic SK1/S1P to interfere with the communication between cancer cells and host organism provides a mechanism-based strategy to inhibit tumour colonization/metastasis to the lungs.
RESULTS
Roles of SK1/S1P signalling in the regulation of tumour growth and/or lung colonization/metastasis
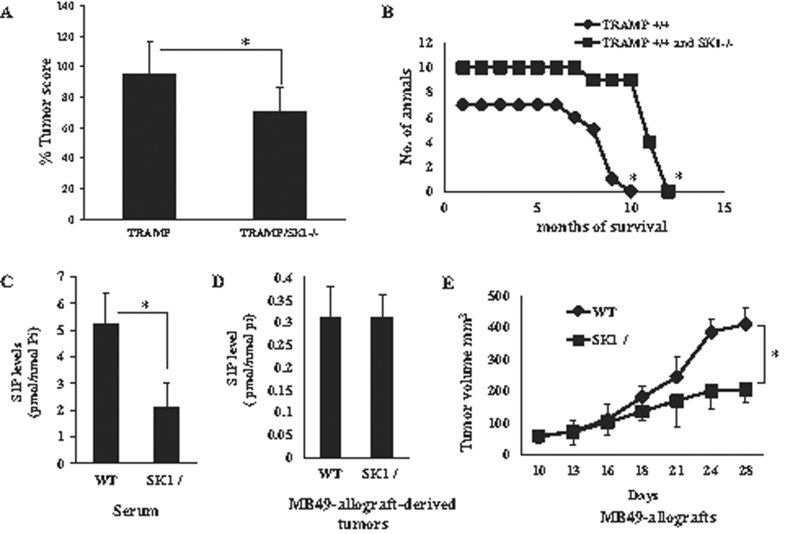
To examine the roles of SK1/S1P signalling in the regulation of tumour growth, first, we determined the effects of genetic loss of SK1 in the progression of TRAMP-induced prostate tumours (Foster et al, 1997) in mice. To achieve this, global SK1−/− knockout (ko) mice (Mizugishi et al, 2005) were crossbred with the TRAMP+/+ transgenic mice, and measured prostate tumour number and size (tumour score) and survival rates of mice with prostate tumours in TRAMP+/+/SK1+/+ compared to TRAMP+/+/SK1−/− mice. TRAMP−/−/SK1−/− mice had no spontaneous prostate tumours, but TRAMP+/+/SK1+/+ mice developed large prostate tumours, and within 10 months, all mice died (Fig 1A and B). Interestingly, genetic loss of SK1 slightly, but significantly (p < 0.05) decreased prostate tumour scores, and partially increased overall survival in TRAMP+/+/SK1−/−, which was extended to 12.5 months compared to 10 months in TRAMP+/+/SK1+/+ controls (Fig 1A and B, n = 10, p < 0.05). Thus, these data suggest that the genetic loss of SK1 is partially protective against TRAMP-induced prostate tumour development and/or progression, a finding consistent with the pro-survival roles of SK1/S1P (Pyne & Pyne, 2010; Spiegel & Milstien, 2007).
Figure 1. Genetic loss of systemic SK1 inhibits tumour growth and/or progression.
- A,B. Prostate tumour scores (A) and survival (B) of TRAMP+/+ (n = 10) versus TRAMP+/+/SK1−/− (n = 7) mice were measured for 12 months. Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- C,D. S1P was measured using LC/MS/MS in serum obtained from WT and SK1−/− mice (C), or in tissues obtained from MB49-allograft-derived tumours (D), and normalized to Pi levels. Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- E. Volumes of tumours derived from MB49 allografts grown in WT and SK1−/− ko mice were measured for 28 days. Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
To further examine the roles of SK1/S1P on local tumour growth, MB49 murine bladder cancer cells (Varela et al, 2008) were implanted in the flanks of WT and SK1−/− animals, and tumour volumes were measured every 4 days for 28 days. Tumours and the animal sera were collected after the completion of tumour growth assessments for S1P measurements using LC/MS/MS. Loss of SK1 in SK1−/− mice significantly decreased (∼60%, p < 0.05) serum (systemic) S1P (Fig 1C) compared to wild type (WT) mice, but no significant changes were observed in intrinsic S1P measured in tumour tissues grown in WT and SK1−/− mice (Fig 1D). Importantly, genetic loss of SK1 suppressed (∼70%) local tumour growth (Fig 1E) when compared to WT controls.
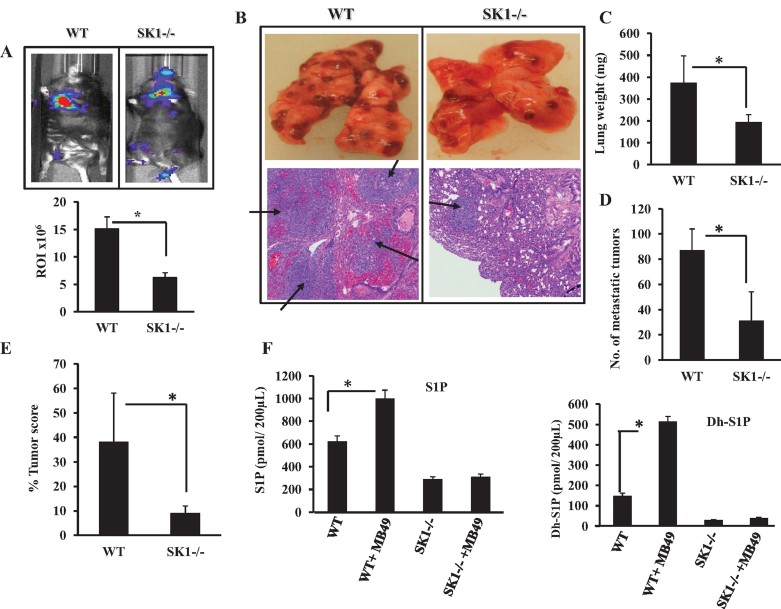
In addition to the involvement of intrinsic genetic alterations and tumour microenvironment factors, tumour-induced systemic host changes, such as serum S1P elevation, might contribute to tumour metastasis regulation. Therefore, to evaluate the role of genetic loss of SK1 and decreased systemic (serum) S1P in cancer metastasis, we injected MB49 cells into the tail vein of WT and SK1−/− mice, and examined lung colonization, which usually forms within 19–22 days of injection in WT mice. The global loss of SK1 significantly (∼60%, n = 6, p < 0.05) inhibited lung colonization of luciferase-labelled MB49 cells compared to WT controls as visualized by tomography (Fig 2A, upper and lower panels). These data were also consistent with decreased number of MB49-derived lung tumour nodules (Fig 2B, upper panel). In fact, histopathologic examination of lung tissues after H&E staining revealed an impressive tumour invasion (metastatic lesions of SCC) with significant bleeding in WT animals, whereas those few tumours detected in the lungs of SK1−/− mice were contained within the blood vessels, and did not invade lung tissues (Fig 2B, lower panel). Moreover, the genetic loss of SK1 resulted in ∼50% reduction of lung weight, ∼75% decrease in number of lung tumour nodules and ∼93% reduction of lung tumour scores compared to WT mice after injections of MB49 cells via tail vein (Fig 2C–E).
Figure 2. Effects of genetic loss of SK1 on the lung colonization/metastasis of MB49 murine bladder cancer cells.
- A,B. Effects of the genetic loss of SK1 on lung colonization/metastasis of MB49/luciferase (A) or MB49 (B) cells were detected in WT and SK1−/− mice via tail vein injections (n = 6/group). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- C-E. Tumour burden in the lungs of WT and SK1−/− mice (n = 10/group) after tail vein injections of MB49 cells were measured by lung weight (C), number of MB49-derived lung tumour colonies (D), and lung tumour scores (E). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- F. Effects of tail vein injections of MB49 cells into WT and SK1−/− mice on systemic S1P (left panel) or dhS1P (right panel) levels were measured by LC/MS/MS. Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
We next evaluated how the source of SK1 could influence serum S1P. For these experiments, we used either WT or global SK1−/− mice, and injected them with MB49 cells that expressed SK1. As expected, analysis of systemic S1P/dihydro (dh)S1P in serum showed a significant (p < 0.05) increase in serum S1P and dhS1P in tumour bearing WT mice as measured by LC/MS/MS (Fig 2F, left and right panels). A comparison of WT and SK1−/− mice showed that global knockout of SK1 resulted in ∼50% decrease in systemic S1P/dhS1P compared to WT mice (Fig 2F). Sphingosine (Sph) and dhSph were higher in the serum of SK1−/− compared to WT mice with/without MB49 cell injections (Supporting Information Fig S1A and B). Importantly, MB49 cell injections in SK1−/− mice had no significant effect on serum S1P/dhS1P (Fig 2F). Thus, these data suggest that cancer cells induce systemic S1P elevation generated by host SK1, which then regulates both local tumour growth and lung colonization. Interestingly, as seen in SK1−/− animals, in which the global knockout of SK1 in the host prevented systemic S1P (and dhS1P) elevation and reduced local tumour growth and lung colonization, indicating a mechanism for host/tumour communication via systemic SK1/S1P.
Regulation of local tumour growth and lung colonization/metastasis by systemic and tumour SK1/S1P
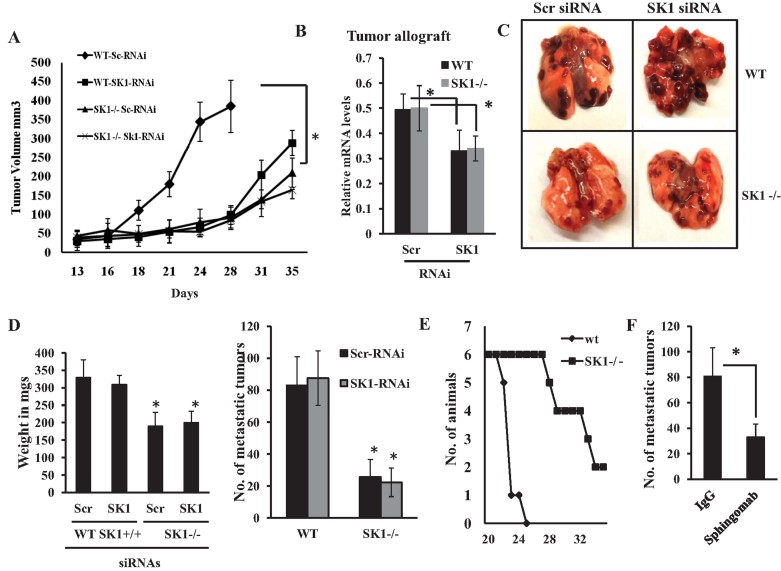
To determine whether alterations of systemic versus tumour SK1/S1P signalling are key in the regulation of local tumour growth and/or lung colonization/metastasis, we down-regulated SK1 expression using small interfering RNAs (siRNAs) in MB49 cells before implantation in the flanks or tail vein injections in WT or SK1−/− animals, and examined tumour volumes or number of tumour nodules in lungs, respectively. As shown in Fig 3A, knockdown of SK1 (∼60%, as determined using Q-PCR, see Fig 3B) in MB49 cells before implantation in WT-mice significantly reduced tumour growth (∼75%, n = 12, p < 0.05), compared to controls, allograft-derived tumours in WT mice obtained using MB49 cells transfected with non-targeting scrambled (SCR) siRNA. Also, knockdown of tumour SK1 in MB49 cells before transplantation in SK1−/− mice decreased tumour growth when compared to controls. Thus, these data suggest that systemic down-regulation of SK1 (in the host, using SK1−/− ko mice) or its knockdown locally in tumours (using siRNAs) play similar roles, each equally effective in suppressing local tumour growth in the flanks of WT or SK1−/− mice.
Figure 3. Roles of systemic versus tumour SK1 in the regulation of local tumour growth and lung colonization/metastasis.
- A. Effects of siRNA-mediated SK1 knockdown on MB49-derived allograft tumour growth in the flanks of WT versus SK1−/− mice compared to non-targeting Scr siRNA-transfected controls were measured for 35 days (n = 6/group). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- B. Efficacy of SK1 knockdown in response to siRNAs in MB49-derived allografts was measured by RT-PCR after surgical removal of tumours from the flanks of WT and SK1−/− mice. Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- C,D. Effects of siRNA-mediated knockdown of SK1 in MB49 cells on lung colonization/metastasis (C) and lung weight or metastatic lung tumour nodules (D, left and right panels, respectively) were measured after tail vein injections for 19–21 days (n = 8/group). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- E. Effects of the genetic loss of systemic SK1 on overall survival of WT and SK1−/− mice after tail vein injections of MB49 cells were measured for 35 days (n = 8/group).
- F. Effects of the pharmacologic inhibition of systemic S1P signalling using Sphingomab on lung colonization/metastasis of MB49 cells were measured for 16 days after tail vein injections compared to controls (n = 8/group). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
Then, we examined whether siRNA-mediated down-regulation of SK1 in MB49 cells before injections into the tail veins of WT versus SK1−/− ko mice alters lung colonization/metastasis of these cells. SiRNA-mediated knockdown of SK1 in MB49-cells locally had no significant effect on lung colonization in WT animals (Fig 3C–D). In contrast, systemic loss of SK1, which reduced serum S1P ∼50%, decreased MB49-derived lung tumour colonization and lung weight in SK1−/− mice. Knockdown of SK1 in MB49 cells before injection to SK1−/− mice via tail had no further effect on the reduction of lung colonization/metastasis (Fig 3C and D, left and right panels, respectively). These data were consistent with increased overall survival of SK1−/− over WT mice, which received tail vein injections of MB49 cells (Fig 3E). Moreover, pharmacologic inhibition of systemic S1P using Sphingomab [7.5 mg/kg/day for 16 days] (O'Brien et al, 2009; Visentin et al, 2006; Wojciak et al, 2009) decreased lung metastasis of MB49 cells in WT mice about 60% compared to controls (Fig 3F). Thus, these data suggest that systemic, but not tumour SK1/S1P is involved in the regulation of lung colonization/metastasis of MB49-derived tumours.
To determine whether systemic SK1/S1P in the regulation of lung metastasis is cell line or tissue-of-origin dependent, we examined lung colonization of murine B16 melanoma cells after tail vein injection in WT versus SK1−/− mice. Loss of SK1 significantly decreased the colonization of B16 (p < 0.05, n = 8) cells in the lungs of SK1−/− compared to control (WT) mice (Supporting Information Fig S2).
Overall, these results suggest that genetic loss in SK1−/− mice, and pharmacologic inhibition/targeting of systemic SK1 or S1P in WT mice using Sphingomab, inhibit lung colonization/metastasis of MB49 and B16 cells in vivo, supporting a role for systemic SK1/S1P in the regulation of lung colonization/metastasis.
Regulation of Brms1 in tumours by systemic SK1/S1P
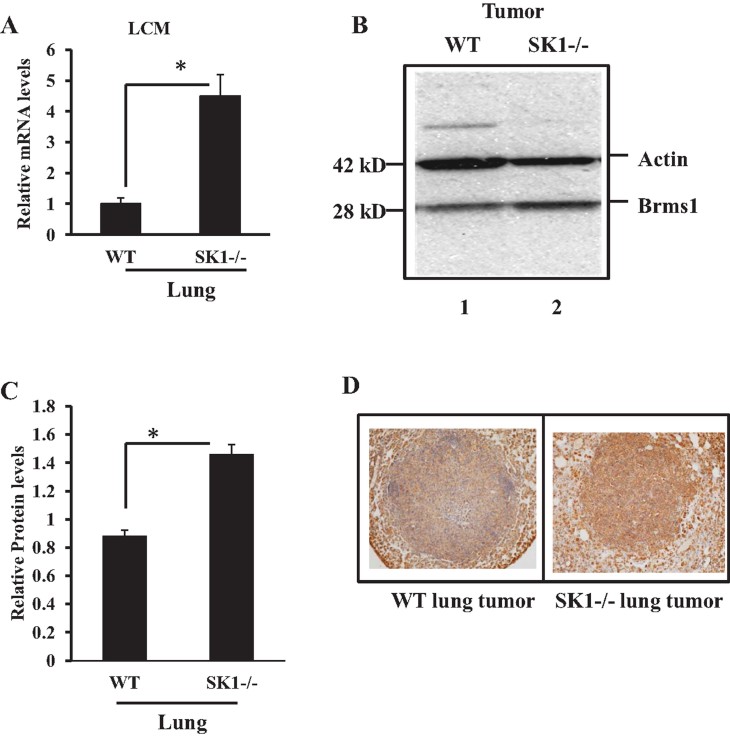
To determine the mechanisms by which systemic SK1/S1P regulates lung colonization, we removed the lungs from WT and SK1(−/−) animals 22 days after injection of MB49 cells, and then measured expression of 84 genes (Supporting Information Fig S3), involved in metastasis, using a Super Array Kit (SA Biosciences, MD) by Q-PCR. Interestingly, in these metastasis-focused array studies, expression of one gene, Brms1 (Samant et al, 2002), was significantly repressed in the lung tumours of WT compared to SK1(−/−) animals (Supporting Information Fig S3). In addition to breast cancer, BRMS1 is known to regulate metastasis of various other solid tumours, including lung tumours (Nagji et al, 2010), but it does not play a role in the regulation of local tumour growth (Hurst & Welch, 2011). These data were verified in independent Q-PCR analyses, which supported the repression of Brms1 expression about 80% in WT animal lung tumours compared to SK1(−/−) animals 22 days after injection of MB49 cells (Fig 4A). Reduced expression of Brms1 in lung tumours grown in WT compared to SK1−/− mice was confirmed using laser capture microdissection (LCM) followed by mRNA isolation and Q-PCR in lung tumour tissues after tail vein injections of MB49 cells (Fig 4A). These data were also consistent with decreased Brms1 protein in lung tumours grown in WT compared to SK1−/− mice, measured by Western blotting (Fig 4B, lanes 1 and 2, respectively, quantified using the ImageJ software as shown in Fig 4C), and IHC staining (Fig 4D). Thus, these data suggest a role for Brms1 for the modulation of tumour metastasis in response to systemic S1P signalling.
Figure 4. Detection of Brms1 expression in MB49-derived lung tumours of WT versus SK1−/− mice.
- A. Brms1 mRNA was measured using Q-PCR in lung tumours grown via tail vein injections of MB49 cells in WT or SK1−/− mice after LCM of lung tumour tissues. Total RNA was isolated from lung tumours of WT or SK1−/− mice after LCM, and Brms1 mRNA was measured in lung tumours after LCM using Q-PCR. Levels of rRNA were used for normalization. Data, obtained from duplicates at least in two independent trials, are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- B-D. Brms1 protein was detected in lung tumours of WT and SK1−/− mice after tail vein injections of MB49 cells either by Western blotting, lines 1 and 2, respectively (B), which were quantified using ImageJ (C) or by IHC (D) using the anti-Brms1 antibody. Actin levels were measured as loading controls in Western blots (B). Data, obtained from duplicates at least in two independent trials, are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
Systemic SK1/S1P regulates tumour Brms1 via S1PR2 signalling
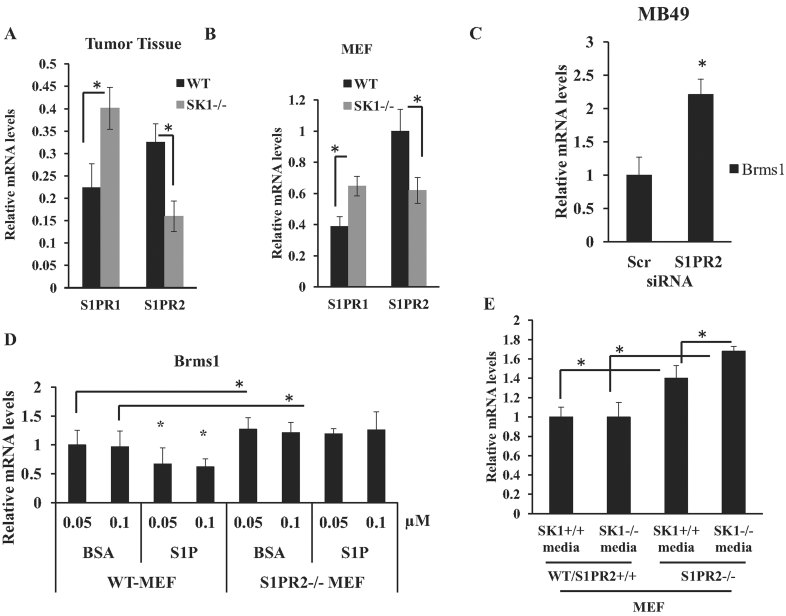
It then became important to define the mechanisms involved in the regulation of tumour Brms1 expression via systemic SK1/S1P signalling. It is well established that S1P engages with G-protein coupled S1P receptors (S1PR1-5) to transduce various biological events, including cancer proliferation and/or progression (Hla & Brinkmann, 2010; Marsolais & Rosen, 2009). Therefore, we first examined whether alterations of systemic S1P in response to systemic loss of SK1 is involved in the regulation of S1PR expression in tumours, which might then controls Brms1 expression. Accordingly, we assessed expression of S1PRs in lung tumours in WT and SK1−/− mice after injection of MB49 cells into the tail vein using Q-PCR. Interestingly, loss of systemic SK1 increased S1PR1 and decreased S1PR2 in lung tumours obtained from SK1−/− mice compared to WT mice (Fig 5A). There were no significant changes observed in the expression of S1PR3-5 in these tumours (data not shown). These data were also confirmed in SK1+/+ (WT) and SK1−/− MEFs, in which the expression of S1PR1 and S1PR2 were up and down-regulated, respectively (Fig 5B). To determine if S1PR1 and/or S1PR2 is involved in the regulation of BRMS1 expression, we treated A549 human lung cancer cells with pharmacologic antagonists for S1PR2 and S1PR1 using JTE013 and FTY720, respectively (Arikawa et al, 2003; Brinkmann, 2009; Valentine et al, 2011), and assessed their effects on BRMS1 mRNA. Inhibition of S1PR2 signalling using JTE013 (at 10–100 nM) induced BRMS1 expression about 1.5–2.0-fold, whereas S1PR1 regulation by FTY720 (20 or 100 nM) had no effect on BRMS1 mRNA (Supporting Information Fig S4A). Inhibition of S1PR2 signalling using JTE013 (20 nM) also increased Brms1 expression around 2–3-fold also in MB49 cells (Supporting Information Fig S4B). In addition, these data were consistent in studies, in which siRNA-mediated knockdown of S1PR2 (∼60% compared to Scr-siRNA-transfected controls, measured by Q-PCR, see Supporting Information Fig S4C) significantly (p < 0.05) increased (∼2.2-fold) Brms1 in MB49 cells (Fig 5C).
Figure 5. Regulation of tumour Brms1 by S1PR2 signalling.
- S1PR1 and S1PR2 mRNAs were measured by RT-PCR in lung tumours after tail vein injections of MB49 cells in WT or SK1−/− mice (normalized to beta-actin mRNA). Data, obtained from duplicates at least in two independent trials, are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- Brms1 mRNA in MEFs isolated from WT or SK1−/− mice was measured by RT-PCR (normalized to beta-actin). Data, obtained from duplicates at least in two independent trials, are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- Levels of Brms1 mRNA were measured in MB49 cells after transfections with S1PR2 compared to Scr siRNAs (100 nM). Data, obtained from duplicates at least in two independent trials, are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- Role of BSA-conjugated S1P (0.05–0.1 µM) in the modulation of Brms1 was measured in WT and S1PR2−/− MEFs compared to BSA-treated controls. Data, obtained from duplicates at least in two independent trials, are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- Effects of genetic loss of S1PR2 on the regulation of Brms1 expression were determined using Q-PCR in MEFs isolated from WT versus S1PR2−/− ko mice, and grown in conditioned control media obtained from WT (SK1+/+) or SK1−/− mice with reduced S1P (normalized to rRNA). Data, obtained from duplicates at least in two independent trials, are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
In addition, Brms1 was found increased ∼40% in S1PR2−/− compared to WT MEFs (Fig 5D). Importantly, treatment of WT-MEFs with BSA-conjugated S1P (0.05–0.1 µM, 24 h) reduced Brms1 expression ∼40–50% compared to controls (Fig 5D). However, exogenous S1P treatment had no significant effect on Brms1 in S1PR2−/− MEFs (Fig 5D). Then, to define the role of altered systemic SK1/S1P in cellular expression of Brms1 via S1PR2 signalling, S1PR2−/− MEFs were grown in conditioned media obtained from SK1−/− MEFs, which contain reduced serum S1P (as confirmed by LC/MS/MS, data not shown) compared to WT controls, and Brms1 expression was measured using Q-PCR. Critically, loss of S1PR2 increased Brms1 expression in S1PR2−/− MEFs compared to WT controls (Fig 5E), and exposure of S1PR2−/− MEFs to reduced serum S1P, obtained from SK1−/− conditioned media, further increased Brms1 expression in these cells compared to controls (Fig 5E). Moreover, neutralizing S1P using Sphingomab (0.2–1 µM) in control media increased Brms1 mRNA (∼60–70%, respectively) compared to IgG-treated controls in WT, but not in S1PR2−/− MEFs (Supporting Information Fig S4D). Thus, these data suggest a key role for S1P/S1PR2 signalling in the regulation of Brms1 expression.
Overall, these data also suggest that systemic/serum SK1/S1P mediates down-regulation of Brms1 via S1PR2 signalling in cancer cells. In contrast, alterations of systemic/serum S1P via knockdown of SK1 modulate tumour S1PR2 signalling, resulting in the up-regulation of Brms1, which then possibly leads to suppression of lung metastasis.
Regulation of tumour Brms1 via systemic SK1/S1P is sufficient and/or necessary for controlling lung metastasis
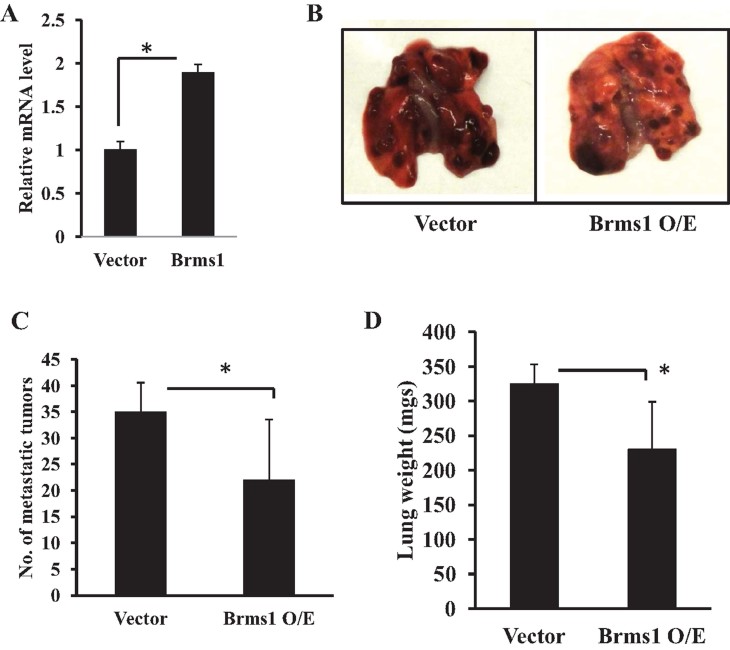
To test whether regulation of tumour Brms1 plays a functional role in the modulation of tumour metastasis in response to systemic SK1/S1P signalling, we first examined the consequences of the overexpression of Brms1 in MB49 cells for lung metastasis in WT mice. Stable expression of Brms1 in MB49 cells (over-expressed around twofold, as determined by Q-PCR, see Fig 6A), resulted in the inhibition of lung metastasis compared to controls (Fig 6B–D), consistent with its roles in the suppression of metastasis.
Figure 6. Roles of ectopic Brms1 expression on the suppression of lung colonization/metastasis in WT mice.
- A. Brms1 mRNA was measured by Q-PCR after transfection of MB49 cells with the expression of vectors containing the full-length Brms1 cDNA compared to vector-transfected controls. Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- B–D. Effects ectopic expression of Brms1 in MB49 cells on suppression of lung metastasis (B), as confirmed by decreased lung tumour colonization (C) and lung weight (D) compared to controls (n = 8/group). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
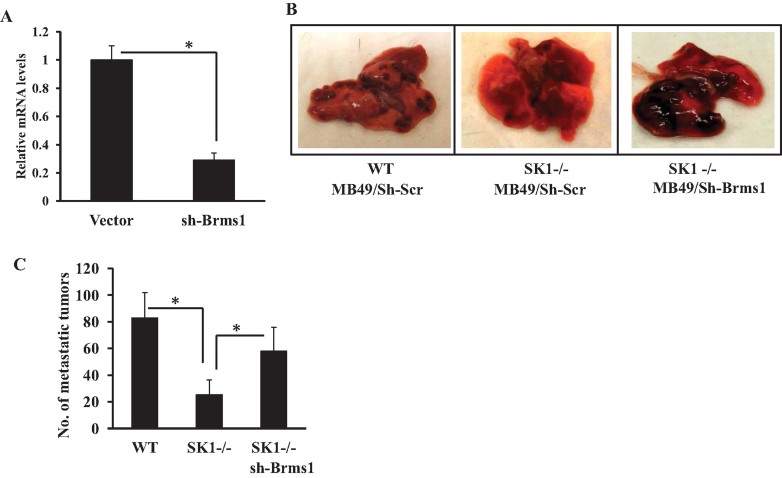
In reciprocal experiments, we determined the effects of stable knockdown of Brms1 using lentiviral targeting and non-targeting shRNA constructs in MB49 cells on the regulation of lung metastasis in SK1−/− mice. We reasoned that if Brms1 plays key roles for the suppression of lung metastasis of MB49 cells, then, its knockdown should prevent the inhibition of lung metastasis in SK1−/− mice. First, we examined the effective knockdown of Brms1 after stable transfections using the lenti-viral-dependent shRNA against Brms1 compared to controls by Q-PCR, which knocked down Brms1 approximately 75% compared to controls in MB49 cells (Fig 7A). Then, we assessed the role of shRNA-mediated knockdown of Brms1 in the regulation of lung metastasis in SK1−/− mice after tail vein injection of MB49 cells. The data showed that Brms1 knockdown in MB49 cells significantly abrogated the inhibition of lung metastasis in SK1−/− mice when compared to the lung metastasis control MB49 cells (Fig 7B and C). Overall, these data suggest that Brms1 plays important roles in the inhibition of lung metastasis, and knockdown of Brms1 expression in MB49 cells prevents the modulation of lung metastasis by systemic loss of SK1 and reduced systemic S1P generation in vivo. These data also suggest a novel view that systemic SK1/S1P provides a signalling function for the communication between the host and tumour cells via regulation of Brms1 expression in tumours for the regulation of lung metastasis.
Figure 7. Brms1 expression plays important roles in the suppression of MB49-derived lung colonization/metastasis in SK1−/− mice.
- A. Stable knockdown of Brms1 in response to lentiviral vector based expression of shRNAs (against Brms1 compared to non-targeting Scr shRNAs) in MB49 cells was confirmed using Q-PCR (normalized using rRNA levels). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
- B,C. Effects of shRNA-mediated knockdown of Brms1 in MB49 cells on lung metastasis (B) and colonization (C) after tail vein injections in WT and SK1−/− mice (n = 8) were measured compared to controls (sh-Scr). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
Inhibition of systemic S1P signalling using Sphingomab suppresses lung colonization/metastasis
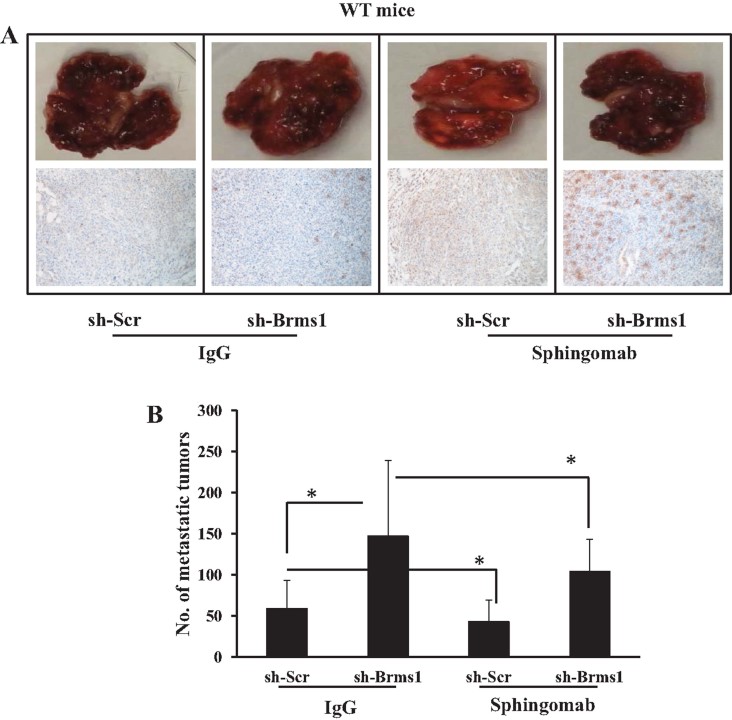
To determine whether inhibition of systemic S1P signalling provides a therapeutic intervention to alter tumour S1PR2 signalling to regulate lung metastasis via activation of BRMS1, we took advantage of Sphingomab, a monoclonal antibody, which binds and neutralizes systemic S1P, inhibiting S1P signalling. MB49/Scr-shRNA and MB49/BRMS1-shRNA cells were injected into SK1+/+ mice via tail vein, animals were treated with control IgG versus Sphingomab (20 mg/kg, at every 3 days for 22 days), and effects of Sphingomab (Visentin et al, 2006) on lung metastasis were examined. Treatment of SK1+/+ mice that contain MB49/Scr-shRNA-induced lung tumours with Sphingomab significantly (∼40%) inhibited lung metastasis compared to controls treated with IgG (Fig 8A–B). Stable knockdown of Brms1 slightly increased lung metastasis in response to MB49/BRMS1-shRNA cell injections compared to MB49/Scr-shRNA-induced lung metastasis (Fig 8A and B). Remarkably, knockdown of Brms1 partially but significantly (p < 0.05) reversed the suppression of MB49/Brms1-shRNA-induced lung metastasis by Sphingomab compared to controls (MB49/Scr-shRNA-derived lung metastasis) (Fig 8A and B). These data were also consistent with decreased S1PR2 expression in MB49/Scr-shRNA-induced lung tumours in response to Sphingomab treatment compared to IgG-treated controls (Fig 8A and B). Thus, these data suggest that inhibition of systemic S1P using Sphingomab suppresses lung metastasis via, at least in part, regulation of tumour Brms1, indicating a mechanism-based therapeutic strategy to inhibit lung metastasis by Sphingomab.
Figure 8. Suppression of MB49-derived lung tumour colonization/metastasis by inhibition of systemic S1P signalling using Sphingomab in WT mice.
- A,B. Effects of systemic S1P inhibition by Sphingomab compared to IgG control treatment on lung tumour metastasis (A) or colonization (B) were measured in response to tail vein injection of MB49/sh-Scr versus MB49/sh-Brms1 into WT mice (n = 8/group). Expression of Brms1 in lung tumours after tail vein injections of MB49/sh-Scr and MB49/sh-Brms1 cells into WT mice in response to Sphingomab compared to IgG control treatment was detected by IHC (A, lower panel). Data are represented as mean ± SD. Error bars represent standard deviations. p < 0.05 (*) was considered significant.
DISCUSSION
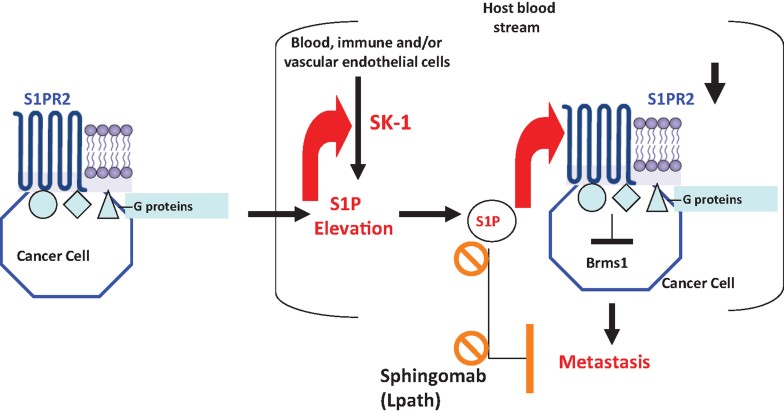
Tumour metastasis is regulated by cancer cell-induced systemic changes in the host organism. However, how cancer cells communicate with the host to induce systemic changes, and how these systemic changes regulate tumour metastasis are largely unknown. Here, we showed that dissemination of cancer cells into the blood stream induce SK1-dependent systemic S1P elevation in the serum, leading to S1PR2-mediated down-regulation of Brms1, a master suppressor of metastasis, in cancer cells, and enhancing lung metastasis (Fig 9). Genetic loss of SK1, or neutralizing/inhibiting systemic S1P signalling with the anti-S1P monoclonal antibody (mAb), Sphingomab, suppressed lung colonization/metastasis of MB49 cells (Fig 9). Overall, these data suggest a mechanism by which cancer cells communicate with the host through alterations of systemic S1P signalling to regulate lung colonization/metastasis via controlling tumour S1PR2/Brms1 axis (Fig 9). Importantly, we also showed here that genetic and/or pharmacologic intervention of systemic S1P inhibits lung metastasis via regulation of tumour S1PR2/Brms1 signalling, providing a mechanism-based therapeutic strategy to suppress lung metastasis (Fig 9).
Figure 9. Mechanism by which systemic SK1/S1P control tumour S1PR2/Brms1 axis to regulate tumour lung colonization/metastasis.
Our data suggest that cancer cells communicate with the host organism via systemic SK1/S1P to regulate lung metastasis/colonization. Cancer cells induce systemic S1P elevation, which then induces tumour S1PR2 expression, leading to modulation tumour Brms1 expression, and enhanced lung metastasis. Genetic and pharmacologic inhibition of systemic SK1 and/or S1P signalling induces tumour Brms1 and suppresses lung metastasis.
The role of SK1/S1P in the regulation of cancer cell proliferation and/or progression has been well documented previously. Overexpression of SK1 was detected in lung tumour compared to pathologically non-cancerous adjacent lung tissues (Johnson et al, 2005), and ectopic expression of SK1 induces tumourigenesis (Xia et al, 2000). In contrast, genetic loss of SK1 prevented colon carcinogenesis (Kawamori et al, 2009), and head and neck cancer (Shirai et al, 2011), and inhibition of S1P signalling using Sphingomab, attenuated tumour growth (Sabbadini, 2006; Sabbadini, 2011). However, roles of SK1/S1P in the regulation of tumour metastasis have been unclear.
It was generally assumed that the tumour itself is the major source of S1P for the regulation of tumourigenesis and metastatic potential, and that S1P in the tumour microenvironment might feedback in an autocrine or paracrine fashion to promote tumour growth and metastasis (Sabbadini, 2011). For the first time, we report here that these two important characteristics of tumour biology, tumour growth and metastasis, depend on different pools of S1P. Our data show that while both systemic and tumour SK1 are important for tumour growth/proliferation, tumour-derived S1P does not determine lung colonization/metastatic potential. Interestingly, systemic, but not tumour SK1/S1P, seemed to regulate lung metastasis. This was demonstrated in experiments where global loss of host SK1 or systemic neutralization of extracellular S1P with the anti-S1P mAbs substantially inhibited lung colonization/metastasis of MB49 and B16 cells. On the other hand, knockdown of SK1 selectively in cancer cells had no effect on metastasis if systemic S1P was maintained intact in animals not deficient in SK1.
Mechanistically, we determined that systemic S1P promotes metastasis by downregulation of Brms1 expression in murine bladder cancer and melanoma cells, and BRMS1 in human A549 lung adenocarcinoma cells. These data are in agreement with recent studies, which showed that BRMS1 expression correlates with improved survival in non-small cell lung cancer (NSCLC) patients (Smith et al, 2009), and ectopic expression of BRMS1 inhibited H1299 NSCLC cell-derived pulmonary and hepatic metastasis (Phadke et al, 2008). The mechanism by which systemic SK1/S1P signalling modulates Brms1 expression remains unknown. Repression of BRMS1 transcription via promoter methylation by the phospho-RelA/p65 complex suggested the involvement of NF-κB signalling in the regulation of metastatic disease (Liu et al, 2006). Recently, it was shown that (Liu et al, 2012) phosphorylation of p65/RelA subunit of NF-κB mediates the repression of Brms1 transcription via recruitment of DNMT-1 (DNA [cytosine 5]-methyltransferase-1) in response to tumour necrosis factor (TNF). It was also known that SK-1/S1P signalling is associated with NF-κB activation (Billich et al, 2005). Therefore, it is possible that SK1/S1P/S1PR2 signalling might inhibit Brms1 expression through activation of NF-κB, and conversely, inhibition of SK1/S1P induces Brms1 via alterations of p65/RelA phosphorylation. This possible mechanism, however, needs to be further evaluated in future experiments. As a down-stream mechanism, BRMS1 is known to inhibit metastasis in multiple organs by blocking several steps of metastatic cascade. For example, fewer breast cancer cells that stably express BRMS1 reached lungs or bone compared to controls, suggesting BRMS1-mediated cell death during transit most likely via anoikis (Hedley et al, 2008). Moreover, after reaching secondary sites, most of BRMS1-expressing cells did not proliferate, indicating a role for BRMS1 in the inhibition of colonization (Phadke et al, 2008). However, how induced Brms1 in cancer cells via alterations of systemic SK1/S1P modulates their lung colonization/metastasis remains unknown, and need to be determined.
We showed here also that genetic loss or pharmacologic inhibition of systemic S1P using Sphingomab, induced Brms1 expression in tumours via silencing of S1PR2 signalling. In addition to its involvement in drug resistance in CML (Baran et al, 2007; Salas et al, 2011), S1PR2 also plays an essential role in hypoxia-triggered pathological angiogenesis of the mouse retina, which was suppressed in S1P2(−/−) knockout mice (Skoura et al, 2007). Moreover, TGF-beta-induced migration and invasion of esophageal cancer cells was shown to involve SK1-generated S1P via S1PR2 signalling (Miller et al, 2008). Interestingly, disruption of the S1P2 gene led to the development of diffuse large B-cell lymphoma (DLBCL) in some of the S1P2−/− ko mice (Cattoretti et al, 2009). Indeed, in our studies ectopic expression of S1PR2 reduced Brms1 expression, whereas genetic loss, siRNA-mediated knock-down, or pharmacologic inhibition (using JTE-013) of S1PR2 enhanced human and murine Brms1 expression in multiple cancer cell lines. Thus, these data suggest that systemic SK1/S1P regulate lung metastasis via tumour S1PR2/Brms1 axis, providing a mechanism for communication between cancer cells and the host organism for controlling metastasis. These data are in agreement with studies in which carcinogenesis-induced factors mediated metastasis via TLR2 in myeloid cells (Kim et al, 2009).
Importantly, inhibition of systemic S1P signalling using Sphingomab (Visentin et al, 2006) inhibited lung metastasis of MB49 cells, however, shRNA-mediated knockdown of Brms1 prevented Sphingomab-mediated suppression of metastasis, providing a mechanism-based therapeutic approach to inhibit metastasis by Sphingomab.
Overall, our data suggest that while both systemic and tumour SK1/S1P are involved in the regulation of local tumour growth (Heffernan-Stroud et al, 2012), cancer cells communicate with the host to regulate metastasis via alterations of systemic SK1/S1P, which in turn repress tumour Brms1 expression by S1PR2 signalling, enhancing tumour metastasis. These studies have important clinical implications for the development of new strategies to inhibit lung metastasis via targeting systemic SK1/S1P signalling using pharmacologic inhibitors of SK1 and/or systemic inhibition of S1P using Sphingomab. In fact, our data suggest that monitoring elevation of serum S1P might provide a biomarker for detection of tumour metastasis. To this end, inhibition of systemic S1P using Sphingomab suppresses lung metastasis via, at least in part, activation of tumour Brms1, indicating a mechanism-based therapeutic strategy to inhibit lung metastasis. The humanized version of Sphingomab, sonepcizumab/ASONEP (Sabbadini, 2011), has recently completed Phase I clinical trials in cancer, and will be advanced into Phase II safety and efficacy trials.
The paper explained
PROBLEM:
It is well established that tumour metastasis is associated with cancer cell-induced systemic changes in the host organism, which in turn, regulate tumour metastasis. However, how cancer cells induce systemic changes, and how these systemic changes regulate metastatic potential of cancer cells remain enigmatic. Bioactive lipid sphingosine 1-phosphate (S1P) is known to regulate tumour growth and/or proliferation generally via signalling through S1P receptors (S1PR1-5). However, roles and mechanisms of action of S1P signalling in the regulation of metastasis have not been described previously. In addition, whether S1P generated in tumours or host organism regulate metastasis is unknown. Therefore, we investigated the mechanisms by which communication between cancer cells and host organism is regulated via systemic S1P signalling to regulate tumour lung metastasis.
RESULTS:
We show here that both systemic and tumour S1P regulate local tumour growth, whereas lung colonization/metastasis is controlled selectively via systemic S1P, generated by sphingosine kinase 1 (SK1). Modulation of systemic, but not tumour SK1, prevented S1P elevation, and inhibited TRAMP-induced prostate cancer growth in TRAMP+/+SK1−/− mice, or lung metastasis of multiple cancer cells in SK1−/− animals. Genetic loss of SK1 activated a master metastasis suppressor, Brms1 (breast carcinoma metastasis suppressor 1), via modulation of S1P receptor 2 (S1PR2) in cancer cells. Alterations of S1PR2 using pharmacologic and genetic tools enhanced Brms1. Moreover, Brms1 in S1PR2−/− MEFs was modulated by serum S1P alterations. Accordingly, ectopic Brms1 in MB49 bladder cancer cells suppressed lung metastasis, and stable knockdown of Brms1 prevented suppression of tumour lung metastasis in SK1−/− mice. Importantly, inhibition of systemic S1P signalling using a novel anti-S1P monoclonal antibody (mAb), Sphingomab, attenuated lung metastasis. Moreover, knockdown of Brms1 in MB49 cells prevented Sphingomab-mediated suppression of lung colonization/metastasis.
IMPACT:
These novel data suggest that cancer cells and host organism communicate via systemic S1P for the regulation of tumour lung metastasis via modulation of tumour S1PR2/Brms1 axis. These studies have important clinical implications for the development of novel strategies to inhibit lung metastasis via targeting systemic SK1/S1P signalling using pharmacologic inhibitors of SK1 and/or systemic inhibition of S1P using Sphingomab. In fact, our data suggest that monitoring serum S1P elevation might provide a novel marker for detection of tumour metastasis. Accordingly, inhibition of systemic S1P using Sphingomab suppresses lung metastasis via, at least in part, activation of tumour Brms1, indicating a novel and mechanism-based therapeutic strategy to suppress lung metastasis. The humanized version of Sphingomab, sonepcizumab/ASONEP (Lpath), has recently completed Phase I clinical trials in cancer, and will be advanced into Phase II safety and efficacy trials, which might be efficacious for controlling tumour growth and metastasis.
MATERIALS AND METHODS
Reagents
FTY720 and JTE-013 were purchased from Cayman Chemical Company. Sphingomab (LT1002) is a murine mAb directed against S1P that has been recently described (O'Brien et al, 2009).
TRAMP+/+ and TRAMP+/+/SK1−/− mice
Experimental protocols involving vertebrate animals were approved by IACUC at MUSC. A transgenic mouse model for prostate cancer (TRAMP) was purchased from Dr. Greenberg's laboratory (Fred Hutchinson Cancer Research Center), and utilized to develop TRAMP+/+/SK1−/− mice model by crossbreeding with SK1−/− mice. Genotyping was performed by using the following sets of primers: For TRAMP, MßCx forward 5′-GATGTGCTCCAGGCTAAAGTT-3′ and MßCx reverse 5′-AGAAACGGA-ATGTTGTGGAGT-3′ primers with a product size of 500 bp, which is specific for MßCasein control, and pb-1 forward 5′-CCGGTCGACCGGAAGCTTCCACAAGTG-CATTTA-3′ and SV40Tag reverse 5′-CTCCTTTCAAGACCTAGAAG-GTCCA-3′ with a product size of 600 bp, which is specific for the TRAMP transgene were used. For SK1, SK1-1 (5′-TGTCACCCATGAAC-CTGCTGTCCCTGCAC-3′), SK1-2 (5′-AGAAGG-CACTGGCTCCTCCAGAGGAACAAG-3′) and SK1-3 (5′-TCGTGCTT-TACGGTA-TCGCCGCTCCCGATT-3′) primers were used. The primer set SK1-1 and SK1-2 specific for the WT allele yields a 300 bp product, and the primer set SK1-3 and SK1-2, specific for the SK1 knockout mutant allele, yields a 340 bp product. Prostate tumour development and the survival of TRAMP+/+/SK1−/− were compared with TRAMP+/+ male mice.
Cell lines and culture conditions
The MB49 bladder cancer and B16 melanoma cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and 1% penicillin and streptomycin.
Induction of tumour allografts in mice
The roles of SK1/S1P signalling in the regulation of local tumour growth were examined using MB49 cells to obtain a tumour allograft in WT and SK1−/− mice models. Briefly, 1–5 × 105 MB49 cells were injected (sc) in the flanks, and tumour volumes were measured (length × width2 × 0.52) over 10–28 days, as described (Salas et al, 2011).
Tail vein injection of cancer cells in mice
To analyse lung colonization/metastasis in WT and SK1−/− mice, murine cancer cells were injected into tail veins (1 × 105 MB49, LLC1 or B16 in 100 µl PBS). Then, mice were sacrificed after 16–22 days of injection. Serum samples were collected for sphingolipid analysis, and lung tissues were analysed for tumour metastasis and proliferation.
Plasmids, siRNAs and transfections
Scr control and siRNAs against SK1 and S1PR2 were purchased from Thermo Scientific Dharmacon and cell transfection was performed with Oligofectamine transfection agent (Invitrogen) as described by the manufacturer. Sh-Brms1 (cat# RMM4532-NM_134155; Open Biosystem) was utilized to knockdown Brms1 in cell culture with Effectine (Qiagen) transfection reagent selected with puromycin. To express Brms1 in MB49 cells, Brms1/ORF (cat#: EX-Mm13102-Lv23), control vector with GFP (cat#: EX-EGFP-Lv23) and a lenti-pac FIV (cat#: FPK-LvTR-20) expression packaging kit were purchased from GeneCopeia to construct viral particles (Meyers-Needham et al, 2012; Salas et al, 2011).
Pathway-specific Q-PCR-based metastasis gene array
A Super Array Kit for mouse tumour metastasis (cat# PAMM-28, SA Biosciences) was utilized to identify the gene expression profile of a gene panel relevant to the tumour metastasis pathway. The array represented 84 genes known to be involved in metastasis. Tumour metastasis-specific gene expression profiles of lung tissues from WT and SK1−/− mice, which were injected with MB49 cells were compared with the Q-PCR based super array.
Q-PCR and Western blotting
One microgram of total RNA isolated with the Qiagen RNA isolation kit was used to obtain cDNA by reverse transcription, and this cDNA was used to perform RT-PCR and Q-PCR. From total cell lysates, 20 µg total protein was used for Western blotting (Salas et al, 2011). Quantification of Western blots was performed using the ImageJ software as described (Meyers-Needham et al, 2012).
Laser capture microdissection (LCM)
The lung tumours of WT and SK1−/− mice were localized and RNA was isolated via LCM and the quality of isolated RNA was analysed and confirmed with the Agilent Bioanalyzer, as described by the manufacturer. Relative Brms1 mRNA from WT and SK1−/− mice lung tumours were compared with real time probes (Applied Biosystems).
Small animal imaging
Longitudinal images depicting bioluminescent (firefly luciferase) signals in WT and SK1−/− lung tissues were analysed and compared after MB49 cell injection.
Measurement of sphingolipids
High performance liquid chromatography coupled to atmospheric pressure chemical ionization-mass spectrometry (LC/MS/MS) was used to measure S1P and dhS1P in mice serum as described (Salas et al, 2011).
Immunohistochemistry (IHC)
Buffered formalin (10%) fixed lung tissues embedded and sectioned were immunostained with Brms1 antibody (Santa Cruz, Cat# sc-49390) and tumour expression of Brms1 was analysed from mice injected with MB49 cells (tail vein) and treated with Sphingomab (Johnson et al, 2005). Tumour scores were obtained after H&E staining of tissues by an independent pathologist at the Hollings Cancer Center Tumour Repository.
Statistical analysis
An unpaired Student's t-test was performed using Prism/GraphPad software; p < 0.05 was considered significant (Salas et al, 2011).
Acknowledgments
We thank Dr. Jennifer G. Schnellmann (Medical University of South Carolina, MUSC, Charleston, SC) for her editorial review. We also thank Dr. Yusuf A. Hannun (MUSC) for helpful discussions. SK1−/− and S1PR2−/− mice were kindly provided to us by Dr. Richard L. Proia (National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health, Bethesda, MD). This work was supported by research grants obtained from the National Institutes of Health (CA088932, DE016572 and CA097165 to BO), Biogen/IDEC, Inc. (San Diego, CA) and CTSA/SCTR (MUSC). The core facilities utilized for animal studies, lipidomics and imaging were constructed using support from NIH (C06 RR015455, or P30 CA138313).
Supporting Information for additional data is available at EMBO Molecular Medicine online.
Conflict of interest statement: Authors would like to disclose that Dr. Roger Sabbadini is a founder of Lpath, and a member of its scientific advisory board.
Author contributions
SP, SPS, SM, TK, AJS and YS designed and/or performed experiments and analysed data; LMO and RS provided key materials for experiments, and analysed data; BO conceived and designed experiments, and wrote the manuscript.
Supplementaary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Arikawa K, Takuwa N, Yamaguchi H, Sugimoto N, Kitayama J, Nagawa H, Takehara K, Takuwa Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J Biol Chem. 2003;278:32841–32851. doi: 10.1074/jbc.M305024200. [DOI] [PubMed] [Google Scholar]
- Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, Ogretmen B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17:1203–1217. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Bonhoure E, Lauret A, Barnes DJ, Martin C, Malavaud B, Kohama T, Melo JV, Cuvillier O. Sphingosine kinase-1 is a downstream regulator of imatinib-induced apoptosis in chronic myeloid leukemia cells. Leukemia. 2008;22:971–979. doi: 10.1038/leu.2008.95. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G, Mandelbaum J, Lee N, Chaves AH, Mahler AM, Chadburn A, Dalla-Favera R, Pasqualucci L, MacLennan AJ. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer Res. 2009;69:8686–8692. doi: 10.1158/0008-5472.CAN-09-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–115. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hedley BD, Vaidya KS, Phadke P, MacKenzie L, Dales DW, Postenka CO, MacDonald IC, Chambers AF. BRMS1 suppresses breast cancer metastasis in multiple experimental models of metastasis by reducing solitary cell survival and inhibiting growth initiation. Clin Exp Metastasis. 2008;25:727–740. doi: 10.1007/s10585-008-9184-0. [DOI] [PubMed] [Google Scholar]
- Heffernan-Stroud LA, Helke KL, Jenkins RW, De Costa AM, Hannun YA, Obeid LM. Defining a role for sphingosine kinase 1 in p53-dependent tumors. Oncogene. 2012;31:1166–1175. doi: 10.1038/onc.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulation. Neurology. 2010;76:S3–S8. doi: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- Hurst DR, Welch DR. Unraveling the enigmatic complexities of BRMS1-mediated metastasis suppression. FEBS Lett. 2011;585:3185–3190. doi: 10.1016/j.febslet.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QF, Huang WR, Duan HF, Wang H, Wu CT, Wang LS. Sphingosine kinase-1 mediates BCR/ABL-induced upregulation of Mcl-1 in chronic myeloid leukemia cells. Oncogene. 2007;26:7904–7908. doi: 10.1038/sj.onc.1210587. [DOI] [PubMed] [Google Scholar]
- Liu Y, Smith PW, Jones DR. Breast cancer metastasis suppressor 1 functions as a corepressor by enhancing histone deacetylase 1-mediated deacetylation of RelA/p65 and promoting apoptosis. Mol Cell Biol. 2006;26:8683–8696. doi: 10.1128/MCB.00940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mayo MW, Nagji AS, Smith PW, Ramsey CS, Li D, Jones DR. Phosphorylation of RelA/p65 promotes DNMT-1 recruitment to chromatin and represses transcription of the tumor metastasis suppressor gene BRMS1. Oncogene. 2012;31:1143–1154. doi: 10.1038/onc.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Rosen H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nat Rev Drug Discov. 2009;8:297–307. doi: 10.1038/nrd2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers-Needham M, Ponnusamy S, Gencer S, Jiang W, Thomas RJ, Senkal CE, Ogretmen B. Concerted functions of HDAC1 and microRNA-574-5p repress alternatively spliced ceramide synthase 1 expression in human cancer cells. EMBO Mol Med. 2012;4:78–92. doi: 10.1002/emmm.201100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AV, Alvarez SE, Spiegel S, Lebman DA. Sphingosine kinases and sphingosine-1-phosphate are critical for transforming growth factor beta-induced extracellular signal-regulated kinase 1 and 2 activation and promotion of migration and invasion of esophageal cancer cells. Mol Cell Biol. 2008;28:4142–4151. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagji AS, Liu Y, Stelow EB, Stukenborg GJ, Jones DR. BRMS1 transcriptional repression correlates with CpG island methylation and advanced pathological stage in non-small cell lung cancer. J Pathol. 2010;221:229–237. doi: 10.1002/path.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien N, Jones ST, Williams DG, Cunningham HB, Moreno K, Visentin B, Gentile A, Vekich J, Shestowsky W, Hiraiwa M, et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res. 2009;50:2245–2257. doi: 10.1194/jlr.M900048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Phadke PA, Vaidya KS, Nash KT, Hurst DR, Welch DR. BRMS1 suppresses breast cancer experimental metastasis to multiple organs by inhibiting several steps of the metastatic process. Am J Pathol. 2008;172:809–817. doi: 10.2353/ajpath.2008.070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Ruckhäberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grösch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- Sabbadini RA. Targeting sphingosine-1-phosphate for cancer therapy. Br J Cancer. 2006;95:1131–1135. doi: 10.1038/sj.bjc.6603400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol. 2011;162:1225–1238. doi: 10.1111/j.1476-5381.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas A, Ponnusamy S, Senkal CE, Meyers-Needham M, Selvam SP, Saddoughi SA, Apohan E, Sentelle RD, Smith C, Gault CR, et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941–5952. doi: 10.1182/blood-2010-08-300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samant RS, Debies MT, Shevde LA, Verderame MF, Welch DR. Identification and characterization of the murine ortholog (brms1) of breast-cancer metastasis suppressor 1 (BRMS1) Int J Cancer. 2002;97:15–20. doi: 10.1002/ijc.1569. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Seraj MJ, Samant RS, Verderame MF, Welch DR. Functional evidence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer Res. 2000;60:2764–2769. [PubMed] [Google Scholar]
- Shirai K, Kaneshiro T, Wada M, Furuya H, Bielawski J, Hannun YA, Obeid LM, Ogretmen B, Kawamori T. A role of sphingosine kinase 1 in head and neck carcinogenesis. Cancer Prev Res (Phila) 2011;4:454–462. doi: 10.1158/1940-6207.CAPR-10-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PW, Liu Y, Siefert SA, Moskaluk CA, Petroni GR, Jones DR. Breast cancer metastasis suppressor 1 (BRMS1) suppresses metastasis and correlates with improved patient survival in non-small cell lung cancer. Cancer Lett. 2009;276:196–203. doi: 10.1016/j.canlet.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC, Jr, LaPolla JP, Arango H, Hoffman MS, Martino M, Wakeley K, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1185–1191. [PubMed] [Google Scholar]
- Thangada S, Khanna KM, Blaho VA, Oo ML, Im DS, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine WJ, Godwin VI, Osborne DA, Liu J, Fujiwara Y, Van Brocklyn J, Bittman R, Parrill AL, Tigyi G. FTY720 (Gilenya) phosphate selectivity of sphingosine 1-phosphate receptor subtype 1 (S1P1) G protein-coupled receptor requires motifs in intracellular loop 1 and transmembrane domain 2. J Biol Chem. 2011;286:30513–30525. doi: 10.1074/jbc.M111.263442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JC, Imai M, Atkinson C, Ohta R, Rapisardo M, Tomlinson S. Modulation of protective T cell immunity by complement inhibitor expression on tumor cells. Cancer Res. 2008;68:6734–6742. doi: 10.1158/0008-5472.CAN-08-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhao Z, Caperell-Grant A, Yang G, Mok SC, Liu J, Bigsby RM, Xu Y. S1P differentially regulates migration of human ovarian cancer and human ovarian surface epithelial cells. Mol Cancer Ther. 2008;7:1993–2002. doi: 10.1158/1535-7163.MCT-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak JM, Zhu N, Schuerenberg KT, Moreno K, Shestowsky WS, Hiraiwa M, Sabbadini R, Huxford T. The crystal structure of sphingosine-1-phosphate in complex with a Fab fragment reveals metal bridging of an antibody and its antigen. Proc Natl Acad Sci USA. 2009;106:17717–17722. doi: 10.1073/pnas.0906153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D'Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- Yonesu K, Kawase Y, Inoue T, Takagi N, Tsuchida J, Takuwa Y, Kumakura S, Nara F. Involvement of sphingosine-1-phosphate and S1P1 in angiogenesis: analyses using a new S1P1 antagonist of non-sphingosine-1-phosphate analog. Biochem Pharmacol. 2009;77:1011–1020. doi: 10.1016/j.bcp.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Young N, Pearl DK, Van Brocklyn JR. Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61. Mol Cancer Res. 2009;7:23–32. doi: 10.1158/1541-7786.MCR-08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.