Abstract
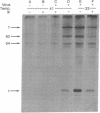
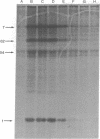
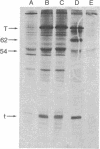
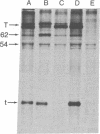
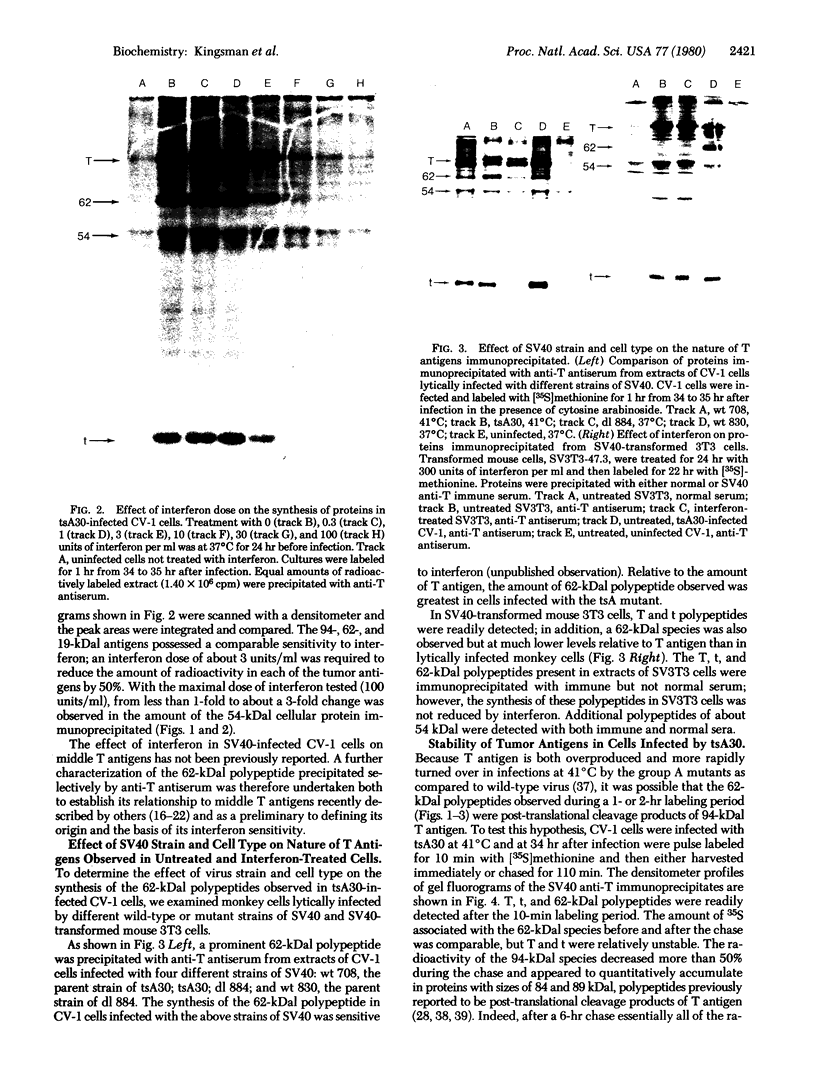
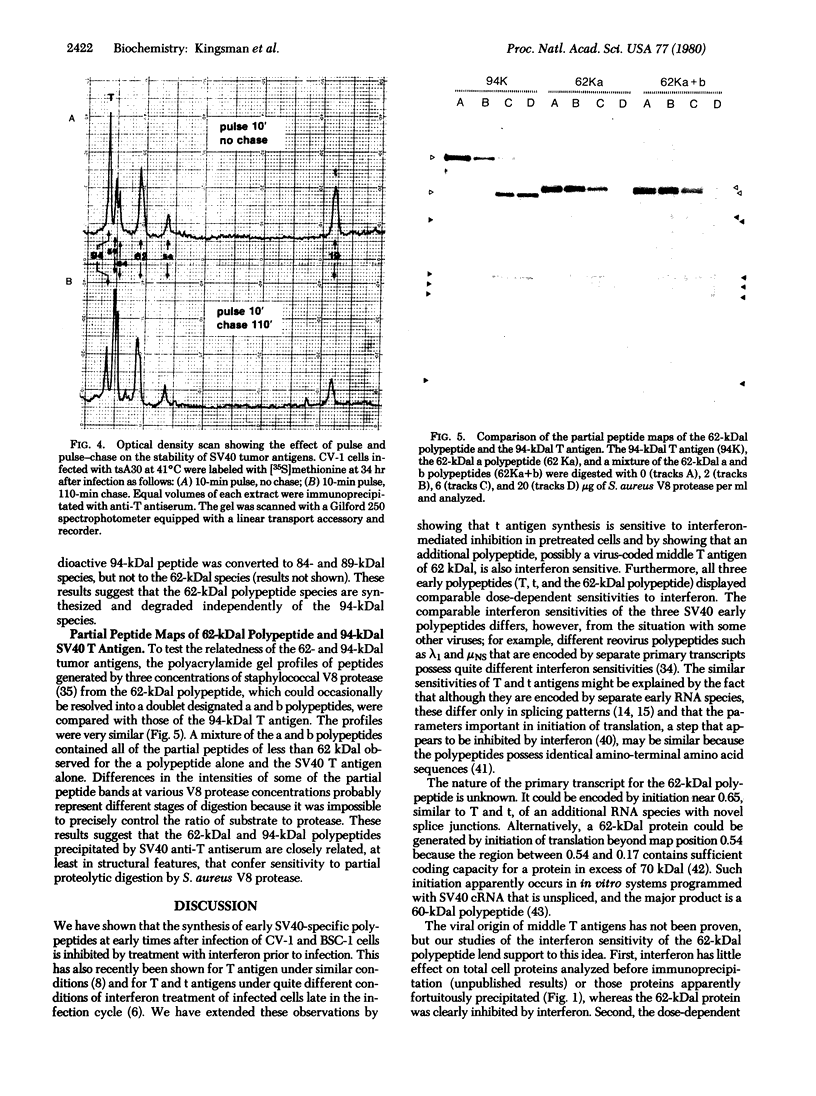
The effect of interferon treatment on proteins synthesized in simian virus 40 (SV40)-infected cells in the presence of cytosine arabinoside was investigated. The following results were obtained: (i) In addition to previously described large tumor (T) antigen (94 kilodaltons) and small tumor (t) antigen (19 kilodaltons), a 62-kilodalton polypeptide was immunoprecipitated by SV40 anti-T antiserum from extracts of infected CV-1 and BSC-1 monkey kidney cells and transformed SV3T3 mouse cells. The 94-, 62-, and 19-kilodalton polypeptides were not precipitated with normal serum from extracts of infected cells, and they were not present in extracts of uninfected cells. (ii) The de novo synthesis of the 94-, 62-, and 19-kilodalton tumor antigens was inhibited in CV-1 and BSC-1 cells treated with interferon before infection; total cellular protein synthesis was not significantly affected by interferon treatment. The relative interferon sensitivity of the three polypeptides in lytically infected monkey cells was comparable; by contrast, interferon did not affect their synthesis in transformed mouse cells. (iii) The 62-kilodalton polypeptide was detected in monkey cells infected with the following strains of SV40: tsA30 at both 33°C and 41°C; wt 708, the parent of tsA30; dI 884; and wt 830, the parent of dI 884. The amount of the 62-kilodalton species relative to T antigen was significantly greater in tsA30-infected cells as compared to cells infected with other SV40 strains. (iv) T, t, and 62-kilodalton polypeptides were readily labeled with [35S]methionine during a 10-min pulse; in a subsequent chase, the 35S-labeled 94-kilodalton T antigen was apparently converted to 89- and 84-kilodalton polypeptides but not to either the 62-kilodalton polypeptide species or t antigen. (v) Partial peptide maps suggest that the 62-kilodalton polypeptide and T antigen are closely related. (vi) In addition to the above described 62-kilodalton polypeptide, a 54-kilodalton polypeptide was also detected. However, the 54-kilodalton species appears to be of cellular origin because it was immunoprecipitated with both normal and anti-T antiserum from uninfected and lytically infected cells and from virally transformed cells.
Keywords: tumor antigens, antiviral agents, translational control
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad-Zadeh C., Allet B., Greenblatt J., Weil R. Two forms of simian-virus-40-specific T-antigen in abortive and lytic infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1097–1101. doi: 10.1073/pnas.73.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner G., Mueller N. Cytosine arabinoside- and interferon-mediated control of polyoma and SV40 genome expression. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):305–308. doi: 10.1101/sqb.1974.039.01.040. [DOI] [PubMed] [Google Scholar]
- Brandner G., Mueller N., Graessmann A., Graessmann M., Niebel J., Hoffmann H. Inhibition by interferon of SV40 tumor antigen formation in cells injected with SV40 cRNA transcribed in vitro. FEBS Lett. 1974 Mar 1;39(3):249–251. doi: 10.1016/0014-5793(74)80122-5. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Smith A. E. Monomer molecular weight of T antigen from simian virus 40-infected and transformed cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2254–2258. doi: 10.1073/pnas.73.7.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Simmons D. T., Martin M. A., Mora P. T. Identification and partial characterization of new antigens from simian virus 40-transformed mouse cells. J Virol. 1979 Aug;31(2):463–471. doi: 10.1128/jvi.31.2.463-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A., Khoury G., Martin R. G. Phosphorylation of T-antigen and control T-antigen expression in cells transformed by wild-type and tsA mutants of simian virus 40. J Virol. 1979 Feb;29(2):753–762. doi: 10.1128/jvi.29.2.753-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Rassoulzadegan M., Cuzin F. Expression of simian virus 40 early genes in transformed rat cells is correlated with maintenance of the transformed phenotype. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4987–4991. doi: 10.1073/pnas.75.10.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Nathans D. The genome of simian virus 40. Adv Virus Res. 1977;21:85–173. doi: 10.1016/s0065-3527(08)60762-9. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kress M., May E., Cassingena R., May P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J Virol. 1979 Aug;31(2):472–483. doi: 10.1128/jvi.31.2.472-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Melero J. A., Stitt D. T., Mangel W. F., Carroll R. B. Identification of new polypeptide species (48-55K) immunoprecipitable by antiserum to purified large T antigen and present in SV40-infected and -transformed cells. Virology. 1979 Mar;93(2):466–480. doi: 10.1016/0042-6822(79)90250-2. [DOI] [PubMed] [Google Scholar]
- Metz D. H., Levin M. J., Oxman M. N. Mechanism of interferon action: further evidence for transcription as the primary site of action in simian virus 40 infection. J Gen Virol. 1976 Aug;32(2):227–240. doi: 10.1099/0022-1317-32-2-227. [DOI] [PubMed] [Google Scholar]
- Mozes L. W., Defendi V. The differential effect of interferon on T antigen production in simian virus 40-infected or transformed cells. Virology. 1979 Mar;93(2):558–568. doi: 10.1016/0042-6822(79)90258-7. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Black P. H. Inhibition of SV40 T antigen formation by interferon. Proc Natl Acad Sci U S A. 1966 May;55(5):1133–1140. doi: 10.1073/pnas.55.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman M. N., Levin M. J. Interferon and transcription of early virus-specific RNA in cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1971 Feb;68(2):299–302. doi: 10.1073/pnas.68.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Harvey R., Smith A. E. Cell-free synthesis of simian virus 40 T-antigens. J Virol. 1978 Oct;28(1):154–170. doi: 10.1128/jvi.28.1.154-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Mellor A., Harvey R., Smith A. E., Hewick R. M., Waterfield M. D. Large and small tumor antigens from simian virus 40 have identical amino termini mapping at 0.65 map units. Proc Natl Acad Sci U S A. 1978 May;75(5):2165–2169. doi: 10.1073/pnas.75.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E., Farris D. A. Mechanism of interferon action. Species specificity of interferon and of the interferon-mediated inhibitor of translation from mouse, monkey, and human cells. Virology. 1977 Apr;77(2):556–565. doi: 10.1016/0042-6822(77)90481-0. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Takemoto K. K., Martin M. A. Properties of simian virus 40 and BK virus tumor antigens form productively infected and transformed cells. Virology. 1978 Mar;85(1):137–145. doi: 10.1016/0042-6822(78)90418-x. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Ito Y. Three species of polyoma virus tumor antigens share common peptides probably near the amino termini of the proteins. Cell. 1978 Dec;15(4):1427–1437. doi: 10.1016/0092-8674(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe M. E., Joklik T. W. The mechanism of inhibition of reovirus replication by interferon. Virology. 1975 Jul;66(1):229–240. doi: 10.1016/0042-6822(75)90193-2. [DOI] [PubMed] [Google Scholar]
- Yakobson E., Prives C., Hartman J. R., Winocour E., Revel M. Inhibition of viral protein synthesis in monkey cells treated with interferon late in simian virus 40 lytic cycle. Cell. 1977 Sep;12(1):73–81. doi: 10.1016/0092-8674(77)90186-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Yamaguchi N., Oda K. Mechanism of interferon-induced inhibition of early simian virus 40(SV40) functions. Virology. 1975 Nov;68(1):58–70. doi: 10.1016/0042-6822(75)90147-6. [DOI] [PubMed] [Google Scholar]