Abstract
The adenosine monophosphate-activated protein kinase (AMPK) is a metabolic sensor of energy metabolism at the cellular as well as whole-body level. It is activated by low energy status that triggers a switch from ATP-consuming anabolic pathways to ATP-producing catabolic pathways. AMPK is involved in a wide range of biological activities that normalizes lipid, glucose, and energy imbalances. These pathways are dysregulated in patients with metabolic syndrome (MetS), which represents a clustering of major cardiovascular risk factors including diabetes, lipid abnormalities, and energy imbalances. Clearly, there is an unmet medical need to find a molecule to treat alarming number of patients with MetS. AMPK, with multifaceted activities in various tissues, has emerged as an attractive drug target to manage lipid and glucose abnormalities and maintain energy homeostasis. A number of AMPK activators have been tested in preclinical models, but many of them have yet to reach to the clinic. This review focuses on the structure-function and role of AMPK in lipid, carbohydrate, and energy metabolism. The mode of action of AMPK activators, mechanism of anti-inflammatory activities, and preclinical and clinical findings as well as future prospects of AMPK as a drug target in treating cardio-metabolic disease are discussed.
Keywords: atherosclerosis, cholesterol, diabetes, drug therapy, dyslipidemias, fatty acid, inflammation, obesity, hepatic steatosis
The pathophysiology of diabetes and obesity is a very complex process involving many pathways. Deregulation of these pathways gives rise to metabolic abnormalities termed as metabolic syndrome (MetS) (1), characterized by a clustering of major risk factors for developing cardiovascular disease. Obesity, representing derangement in energy balances, is tightly linked with the development of type-2 diabetes through its ability to engender insulin resistance, leading to glucose intolerance and development of type-2 diabetes and dyslipidemia. Thus, insulin resistance is associated with a wide array of the pathophysiological sequelae, including hyperlipidemia, hypertension, and atherosclerosis (1, 2). Because the number of patients with MetS is growing at an alarming rate worldwide and 25% of the developed world's population have pathological conditions of MetS, there is global unmet medical need to find a molecule for monotherapy. Currently, patients with MetS are on 3–5 concomitant medications to treat conditions of hyperglycemia, hypertension, dyslipidemia, and elevated levels of pro-inflammatory proteins. This requirement for concomitant treatment is associated with poor compliance and risk of drug-drug interactions (DDI) (3), leading to discontinuing therapy.
5′ adenosine monophosphate-activated protein kinase (AMPK) is an enzyme that controls key players of metabolic pathways, thus emerging as a major regulator of glucose and lipid metabolism through multiple beneficial roles in the target tissues, liver, adipose, and muscle (4). AMPK is a phylogenetically conserved serine/threonine kinase that mediates cellular energy homeostasis (5, 6) through the enzymatic activity stimulated by phosphorylation of threonine-172 (7, 8). AMPK was discovered as an enzyme whose activity catalyzes the phosphorylation and subsequent inhibition of acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase, HMGR) (9, 10). These activities attributable to AMPK together with regulation of insulin signaling make it an attractive target for the management of hepatic metabolic disorders and insulin resistance (11, 12). The hypothalamic functions involved in the regulation of satiety may impact obesity and diabetes (13). AMPK activation is associated with the stimulation of hepatic fatty acid oxidation; inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis (14); inhibition of adipocyte lipolysis and lipogenesis (15); stimulation of skeletal muscle fatty acid oxidation and muscle glucose uptake (16); and modulation of insulin secretion by pancreatic β-cells (17). All these attributes of AMPK show promise and offer opportunity to develop a selective AMPK activator to treat lipid and glucose abnormalities. This review highlights the multifaceted actions of AMPK and its role in the liver and other tissues, with special emphasis on lipid metabolism, inflammation, and mode of action.
AMPK STRUCTURE AND REGULATION
Mammalian AMPK exists as a heterotrimeric complex consisting of three subunits that occur as multiple isoforms. The catalytic α-subunits (α1/α2) and the regulatory β- (β1/β2) and γ-subunits (γ1/γ2/γ3) are encoded by seven different genes. The catalytic α subunit contains a highly conserved Ser/Thr kinase domain near the N-terminus in the activation loop (18). Phosphorylation of Thr-172 within this loop is critical for enzyme activity (18). In addition to regulation through phosphorylation, AMPKα-subunit activity has been shown to be self-regulated by a region identified C-terminal to the kinase domain (19, 20). This autoinhibitory sequence (AIS) is similar to ubiquitin-associated domains, which are highly conserved sequences within the AMPK subfamily of kinases. Studies performed using AIS deletion constructs show a greater than 10-fold increase in kinase activity as compared with constructs containing the AIS (19, 20).
The AMPKβ-subunits do not display catalytic activity, but they appear to be critical for AMPK αβγ complex assembly and glycogen sensing in the cell (21–24). Domains located near the C terminus of the β-subunit have been shown to interact with regions on the α- and γ-subunits, which suggests that the β-subunit, in part, may function as a scaffold to support AMPK heterotrimeric complex assembly. The β-subunits also contain glycogen-binding domains (GBD) that mediate AMPK's association with glycogen, which could facilitate colocalization of AMPK with its substrate glycogen synthase (GS). This might allow the cell to coordinate the control of glycogen synthesis with glycogen levels and energy availability (21–24). Additionally, a potential role for β-subunit myristoylation has been suggested to be important for appropriate AMPK cell membrane localization and activation (25).
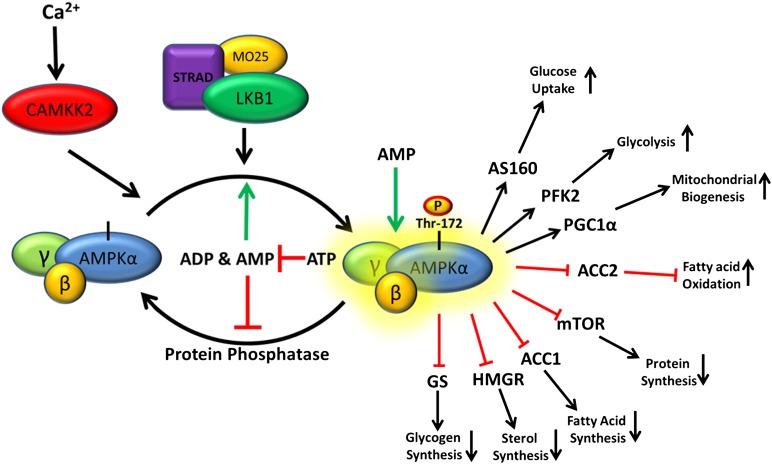
The AMPKγ-subunits regulate enzyme activity through sensing relative intracellular ATP, ADP, and AMP concentrations. This is accomplished through complex interactions of adenine nucleotide with four repeated cystathionone-β-synthase (CBS) motifs occurring in pairs called Bateman domains (6, 26, 27). It is now thought that the CBS motifs arrange in a manner that results in the formation of four adenine binding sites. Site 4 has a very high affinity for AMP and does not readily exchange for ATP or ADP, whereas sites 1 and 3 bind AMP, ADP, and ATP competitively (28). Site 2 seems to be largely unoccupied, and its role in regulating AMPK activity has not been fully elucidated (29, 30). Initially, AMPK activation was thought to be mediated solely by AMP binding; however, recent work has shown that both AMP and ADP binding result in conformational changes which activate AMPK in two ways: i) promoting Thr-172 phosphorylation by upstream kinases, and ii) antagonizing its dephosphorylation by protein phosphatase(s) (31, 32). Conversely, only AMP has been shown to directly increase phosphorylated AMPK (Thr-172) activity through an allosteric mechanism. Kinetic enzyme assays have shown that allosteric activation by AMP results in a greater than 10-fold increase in activity, while the activation resulting from Thr-172 phosphorylation of the α-subunit is greater than 100-fold. In combination, these two activation mechanisms yield a greater than 1000-fold increase in activity (33). In cells, intracellular adenine nucleotide ratios are very tightly regulated, and AMPK activation appears to be sensitive to small changes in these ratios. Although many ATP consuming processes produce ADP, adenylate kinase maintains AMP and ADP concentrations close to equilibrium in most cell types. Therefore, an increase in the ADP:ATP ratio is associated with a concomitant increase in AMP concentration, and the intracellular AMP:ATP ratio also increases. Given that both AMP and ADP activate AMPK through increasing phosphorylation and that ADP concentrations usually significantly exceed AMP concentrations (34–37), it is possible ADP may be the physiologically relevant adenine nucleotide activator of AMPK through its ability to maintain AMPK in its phosphorylated state (38–40).
AMPK phosphorylation/activation
Multiple AMPK kinases have been shown to mediate AMPKα Thr-172 phosphorylation in vitro; however, only two have proven to be physiologically relevant in vivo. The major AMPK kinase is the ubiquitously expressed tumor suppressor liver kinase B1 (LKB1) (41, 42). Although LKB1 activity is not dependent on phosphorylation of its activation loop, significant activity requires binding of the scaffold protein MO25 (43) and stabilization by Ste20-related adaptor (STRAD) protein binding (44). Together, these three proteins form a constitutively active trimeric LKB1-STRAD-MO25 complex that has been shown to mediate AMPK Thr-172 phosphorylation in multiple mammalian systems. Quick to follow the discovery of LKB1 as an AMPK kinase was the identification of Ca2+/calmodulin-dependent kinase β (CaMKKβ), which phosphorylates Thr-172 in response to increased cytosolic Ca2+ concentrations independent of changes in adenine nucleotides (45–48). It is thought that this mechanism may couple ATP-requiring processes, which often signal through increases in cytosolic Ca2+, with AMPK-mediated restoration of energy charge before a significant decrease in ATP levels occur (49). The physiological significance of another AMPK kinase, TAK1, remains to be established (50).
DOWNSTREAM METABOLIC TARGETS OF AMPK
AMPK maintains energy homeostasis by activating catabolic pathways (ATP producing) and inhibiting anabolic pathways (ATP consuming). Downstream effects of AMPK activation can be tissue-dependent and impact, directly or indirectly, a broad range of cellular processes, including lipid and glucose metabolism, energy expenditure, immune response, and cell growth and polarity.
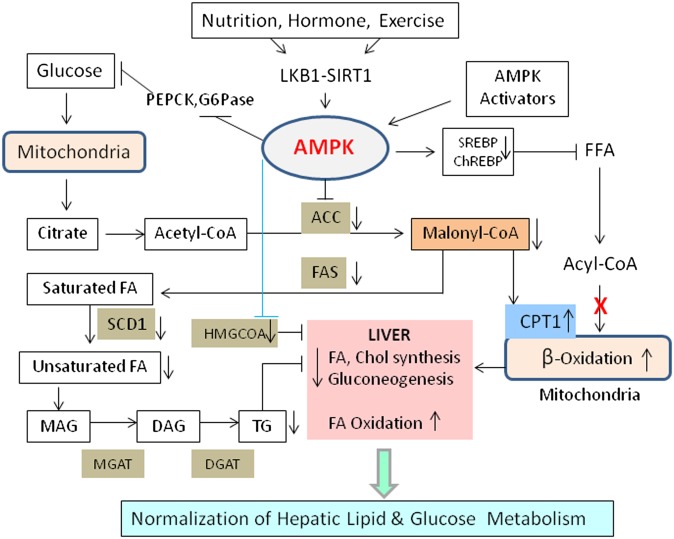
Key lipid metabolic enzymes in the liver that are substrates for AMPK include ACC1 (51), and HMGR (52), which are the rate-limiting enzymes of fatty acid and sterol synthesis, respectively. Glycerol phosphate acyl transferase (GPAT), a key enzyme in triglyceride and phospholipid synthesis, has also been shown to be susceptible to AMPK-mediated inhibitory phosphorylation (53). Additionally, AMPK activation in the liver was shown to directly increase fatty acid uptake through promoting FAT/CD36 translocation to the plasma membrane (30) and to enhance fatty acid oxidation through the inhibitory phosphorylation of ACC2, the enzyme responsible for producing the CPT1 inhibitor malonyl-CoA (54). In adipose tissue, AMPK phosphorylates lipases including hormone-sensitive lipase (HSL) (55) and adipocyte-triglyceride lipase (ATGL) (56). AMPK also coordinates long-term adaptation of lipid metabolism by downregulating mRNA levels of the transcriptional factor sterol regulatory element binding protein 1 (SREBP1) (29, 57) and protein levels of hepatic nuclear factor-4α (HNF-4α) (58), which reduces lipogenic genes including PK, ACC1, and FAS. AMPK also regulates at the intersection of lipid and carbohydrate metabolism by phosphorylating and reducing DNA binding of the glucose-sensitive ChREBP (59), resulting in effects on hepatic lipogenic gene targets, which significantly overlap with effects observed on SREBP1 (Fig. 1).
Fig. 1.
Current model for AMPK activation. Constitutively active LKB1-STRAD-MO25 complex continuously phosphorylates AMPK, which in the absence of low energy signals is rapidly dephosphorylated by protein phosphatases. In times of energy stress, when the rate of ATP consumption exceeds the rate of production, the ADP:ATP ratio increases. Through the action of adenylate kinase, increases in ADP concentrations are associated with concomitant increases in AMP concentrations, and the intracellular AMP:ATP ratio also increases. This energy stress favors binding of ADP/AMP over ATP to the γ-subunit, leading to a conformational change that increases LKB1-STRAD-MO25-dependent phosphorylation and decreases susceptibility to protein phosphatase-dependent dephosphorylation. The combined effect of increased AMPKα phosphorylation and AMP allosteric activation can yield > 1,000-fold increase in activity. Activated AMPK then mediates its control on metabolism by phosphorylation of many downstream targets, resulting in restored energy homeostasis.
AMPK regulates carbohydrate metabolism by increasing GLUT 4-dependent glucose uptake through i) phosphorylation of the Rab-GTPase-activating proteins AS160/TBC1D1 (60) and ii) increasing the glycolysis-stimulator fructose-2,6-bisphosphate concentrations through phosphorylation of specific 6-phosphofructose-2-kinase isoforms (61). Additionally, AMPK inhibits glycogen synthase via phosphorylation (62). AMPK activation in the liver also results in the downregulation of the gluconeogenic genes glucose-6-phosphatase and phosphoenolpyruvate carboxykinase (PEPCK) through multiple mechanisms, including phosphorylation of CREB-regulated transcription coactivator-2 (CRTC2) (63) and class IIA histone deacetylases (HDAC-4, 5, and 7), which results in decreases in HDAC3 nuclear recruitment and FOXO activation (64).
Independent of effects on lipid and carbohydrate metabolism, AMPK also coordinates energy homeostasis by increasing cellular catabolic capacity through enhancing mitochondrial function and serving as a metabolic checkpoint for cell growth. Mitochondrial function is enhanced through upregulation of the transcriptional coactivator PPAR-γ coactivator-1α (PGC-1α) (65), which promotes the expression of nuclear-encoded genes and mitochondrial biogenesis. It has recently been shown that AMPK also promotes mitophagy, a process that recycles damaged or surplus mitochondria (see Ref. 66 for review). AMPK serves as a metabolic checkpoint inhibiting energy-demanding cellular functions, such as cell growth, migration, and immune response, when energy availability is limited. This has been shown to be mediated either by inhibition of the protein synthesis activator target-of-rapamycin complex-1 (TORC1) through the direct phosphorylation of its Raptor subunit (67) and its regulator tumor suppressor tuberous sclerosis complex 2 (TSC2) (68) or by indirect inhibition of NF-κB signaling.
ROLE OF AMPK IN HEPATIC LIPID AND GLUCOSE METABOLISM
Hepatic lipid and glucose dysregulation in metabolic syndrome
Liver is a key organ involved in the lipid and glucose metabolism. It is the major site for storage and release of carbohydrates as well as for the synthesis of fatty acid; thus playing a key role in the control of whole-body energy metabolism (11). During over- and under-nutritional states, AMPK coordinates activity of enzymes in lipid and glucose metabolism (69), and it regulates the partitioning of fatty acids between oxidative and biosynthetic pathways. Derangement in lipid and glucose metabolism in the liver, characterized by enhanced production and impaired catabolism, contributes to insulin resistance and dyslipidemia/hyperlipidemia, leading to diabetes and liver disease (70) and setting the stage of MetS (71) and associated cardiovascular complications (2). The pathophysiological conditions leading to the abnormal metabolic pathways and eventually diabetes and obesity are often caused by the hepatic elevation of the enzymes synthesizing lipid and glucose (72). Hyperlipidemia in MetS appears to occur as a result of overproduction of VLDL and glucose by the liver through lipogenic and gluconeogenic pathways (2, 73).
The role of liver in insulin resistance and lipoprotein overproduction has been demonstrated in animal disease models of excess energy intake (74, 75) and in genetic animal models of diabetes, obesity, and hyperlipidemia (76), in which the production of lipids and glucose by liver is elevated and clearance is impaired (2, 4). Therefore, liver not only plays an important role in dyslipidemia but also in the development of insulin resistance (11) through imbalances in energy status. AMPK plays an important role in balancing this liver-centric dysregulation by functioning as an energy sensor and augmenting fatty acid oxidation and inhibiting the biosynthesis of cholesterol, triglycerides, and glucose.
Hepatic AMPK in glucose metabolism
While AMPK-mediated effects are observed in multiple tissues, liver appears to be the major organ responsible for lipids and glucose production. AMPK-mediated balancing of lipid and glucose production in the liver is a key step in maintaining hepatic metabolism. This function of AMPK in the liver is brought about by modulating a number of genes, both at the enzyme phosphorylation level as well as transcriptional level (51, 52, 57, 58). For instance, phosphorylation of ChREBP by AMPK mediates inhibition of glucose-induced gene transcription (59). Over-function of SREBP1, a lipogenic transcription factor, is associated with the increased prevalence of dyslipidemia in type-2 diabetes (77). A key contributing factor in type-2 diabetes is the failure of insulin to suppress gluconeogenesis and hepatic glucose production. AMPK prevents overproduction through downregulation of the SREBP1 and inhibition of lipogenic and gluconeogenic enzymes as evidenced by the liver-specific AMPKα2 knockout mice that develop hyperglycemia and glucose intolerance as a result of increased hepatic glucose production (78). Conversely, the stimulation of AMPK in wild-type mice dramatically reduces hepatic glucose output (79).
Results from animal models confirm the physiological importance of hepatic AMPK for whole-body glucose homeostasis. For instance, systemic infusion of an AMPK activator, 5-aminoimidazole-4-carboxamide riboside (AICAR), in normal and insulin-resistant obese rats decreased hepatic glucose production (80), further corroborated by blood glucose lowering in mouse models of diabetes following liver-specific short-term activation of AMPK using adenovirus-mediated expression of a constitutively active form of AMPKα2 (79). Additionally, liver-specific deletion of AMPKα2 caused mild hyperglycemia and glucose intolerance as a result of enhanced gluconeogensis (78). These results demonstrate that hepatic AMPKα2 is essential to inhibit gluconeogenesis and maintain blood glucose levels in the physiological range. AMPK appears to be acting through LKB1, because lack of hepatic LKB1 abolished AMPK activity and resulted in increased levels of blood glucose (81, 82). Furthermore, treatment with metformin, an AMPK activator, failed to reduce blood glucose in mice lacking LKB1, again suggesting the key role of LKB1 in the hepatic AMPK activation (81). Additionally, liver-specific deletion of AMPK α subunits (α1 and α2) in mice showed lack of efficacy following AICAR treatment, indicating crucial role of hepatic AMPK in the AICAR-mediated control of blood glucose levels (83). The suppression of gluconeogenesis by AMPK results from the inhibition of the transcription of PEPCK, the key regulatory gluconeogenic enzyme (84). In addition, AMPK attenuates the synthesis of glycogen in hepatocytes by deactivation of glycogen synthase (85). Thus, AMPK downregulates expression of enzymes that are centrally involved in fatty acid synthesis and gluconeogenesis by inhibiting the transcription factors SREBP-1c (57), ChREBP (59), and HNF-4α (58), and by attenuating the activity of transcriptional coactivators (65, 67), which inhibits the transcription of gluconeogenic enzymes. Thus, in the liver, AMPK normalizes glucose metabolism in disease states.
Hepatic AMPK in lipid metabolism
Lipid overproduction in the MetS is caused by elevated activity of enzymes of fatty acid and cholesterol synthesis, and these enzymes are controlled by AMPK (9, 10, 86). Leptin-deficient ob/ob mice provide an animal model of type-2 diabetes, exhibiting hyperglycemia, hyperinsulinemia, and obesity as a result of a high level of hepatic lipogenesis linked with increased expression of lipogenic and glycolytic genes (76). Overexpression of constitutively active (CA)-AMPKα2 in the liver of ob/ob mice normalizes the expression pattern of these genes. Importantly, AMPK activation reduces expression of SREBP1c (57) and ChREBP (59) transcription factors, which play a key role in the transcriptional regulation of lipogenic and glycolytic genes, respectively. The role of ChREBP in hepatic lipogenesis was confirmed by improved hepatic steatosis and insulin resistance in ob/ob mice with liver-specific inhibition of ChREBP (87) and activation of AMPK. This was further corroborated by the liver-specific AMPKα2 deletion in mice showing enhanced hepatic lipogenesis and increased plasma triglyceride levels and hepatic glucose production (78). Conversely, overexpression of AMPKα2 in hepatocytes decreases plasma triglyceride levels (79).
Phosphorylation of ACC1 at Ser-79 and ACC2 at Ser-218 by AMPK leads to inhibition of ACC activity and decreased malonyl-CoA content, leading to reduced fatty acid biosynthesis and increased CPT1, the rate-limiting step in the import and oxidation of fatty acids in mitochondria (54). Thus, a reduction in malonyl-CoA concentration and a subsequent increase in β-oxidation results in decreased triglyceride synthesis (Fig. 2). ACC2 knockout mice show increased fatty acid oxidation in muscle, heart, and liver, and they are lean and have reduced fat content despite eating 20–30% more food than the WT mice (88). The liver triglycerides were also reduced substantially in these mice. Further evidence of AMPK-mediated triglyceride lowering was obtained by infusion of AICAR in lean and obese rodents (80). These results are consistent with ex vivo findings demonstrating the AICAR-induced inhibition of mitochondrial GPAT activity and subsequent inhibition of triacylglycerol synthesis (89). Further evidences supporting the role of AMPK in triglycerides lowering came from two separate studies: i) overexpression of AMPKα2 in the liver decreased plasma triglyceride and increased plasma ketone bodies, a surrogate marker for hepatic β-oxidation (79); and ii) liver-specific AMPKα2 deletion increased plasma triglyceride levels and reduced plasma ketone bodies (78). These observations further emphasize the critical role for AMPKα2 subunit in balancing between hepatic lipogenesis and β-oxidation. Thus, diminished AMPK activity may be an important contributing factor in the reduced mitochondrial function and deregulated intracellular lipid metabolism associated with hepatic insulin resistance.
Fig. 2.
Hepatic regulation of lipid and carbohydrate metabolism. Nutritional, hormonal, and pharmacological stimuli modulate AMPK activation, resulting in inhibition of FA and cholesterol synthesis and stimulation of FA oxidation. ACC inhibition lowers malonyl CoA, which in turn inhibits lipid synthesis and activates CPT1. Hepatic activation of AMPK also reduces glucose output. Both ACC and SCD1 are key enzymes in controlling liver lipids.
Another player in the AMPK activation pathway is SIRT1. In human hepatoma cells, AMPK activation through SIRT1 decreased glucose induced FAS and triglyceride accumulation (90). Overexpression of SIRT1 increased, while shRNA silencing of SIRT1 dramatically decreased LKB1, AMPK, and ACC phosphorylation, and glucose stimulated triglyceride accumulation (91), suggesting an important role of AMPK-SIRT1 axis in hepatic lipid metabolism. Decreased phosphorylation of SIRT1 and LKB1 in mice fed high-fat diet, and subsequent phosphorylation of AMPK and ACC further support this concept. This study suggests a regulatory role for SIRT1 in high-fat diet-induced regulation of AMPK-dependent effects (92). Furthermore, liver-specific SIRT1 knockout mice developed hepatic steatosis, possibly through AMPK-mediated pathway (93). Similarly, liver-specific deletion of LKB1 in mice dramatically reduced the phosphorylation of AMPK, leading to decreased glucose clearance, increased TORC2, and increased expression of gluconeogenic enzymes PEPCK and G6Pase, suggesting the importance of AMPK-LKB1-SIRT1 axis in regulating hepatic lipid metabolism (94). Additionally, expression of lipogenic genes, such as Fas, Acc, Srebp1c, and ChREBP, were elevated in liver-specific LKB1-deficient mice. Taken together, these data support the role of SIRT1, LKB1, and AMPK in suppressing gluconeogenic and lipogenic pathways in the acute regulation of energy balance in the liver.
AMPK and hepatic steatosis
Obesity is a risk factor for developing chronic nonalcoholic fatty liver disease (NAFLD), which is caused by the overexpression of the lipogenic genes srebp1c, acc1, and diacylglycerol acyltransferase 2 (dgat2) (75). Transgenic mice overexpressing srebp1 show massive fatty liver and increased accumulation of cholesteryl ester and triglycerides (95). AMPK activation reduces the lipogenic enzymes and transcription factors, including SREBP1, and increases mitochondrial oxidation of fatty acids in the liver. AMPK activator S17834, a synthetic polyphenol, regulates SREBP1 at the posttranscriptional level by phosphorylating SREBP1c at Ser-372, leading to inhibition of SREBP activity and attenuating hepatic steatosis and atherosclerosis in diet-induced insulin-resistance mice (96). AMPK is also involved in the mitochondrial biogenesis in the liver. For instance, AMPK activator resveratrol increases mitochondrial number in liver in association with AMPK activation, and mouse liver lacking AMPK shows reduced mitochondrial biogenesis (97). Thus, AMPK-mediated improvement in mitochondrial function together with inhibition of hepatic fatty acid synthesis and increase in fatty acid oxidation contributes to improvements in hepatic steatosis.
High sucrose-fed rats develop NAFLD concomitant with decreases in AMPK activity in the liver (98). Activation of AMPK in the liver leads to reduced lipid synthesis and increased fatty acid oxidation (Fig. 2). Transgenic mice expressing constitutively active (CA)-AMPK-α1 in the liver exhibited resistance to weight gain and accumulation of liver lipids on high-fat diet (99). Similar to ACC2−/− mice, steroyl CoA desaturase 1-deficient (SCD1−/−) mice on high-fat diet shows reduced body weight, body fat mass, hepatic lipids, and increased oxygen consumption. These mice also showed increased expression of genes involved in fatty acid oxidation and improved insulin sensitivity (100). This effect is thought to occur through increased AMPK phosphorylation (52%) and activity (40%), leading to increased ACC phosphorylation (62%) and CPT1 activity (63%) (101). Absence of SCD1 in ob/ob mice ameliorated the severe obesity observed in the ob/ob mice (101), suggesting the inhibition of ACC as one mechanism accounting for increased fatty acid oxidation in the livers of SCD1-deficient mice, as phosphorylation and activity of AMPK and ACC increased in SCD1−/− mice (100), resulting in decreased levels of malonyl-CoA and activation of CPT1, leading to increased palmitate oxidation. Leptin-deficient ob/ob mice with SCD1 mutations were significantly less obese than ob/ob controls and had reduced triglyceride storage in liver (101). SCD1 is therefore a component of the novel metabolic response to hepatic lipid accumulation. These results suggest that the inhibition of SCD1 leads to the activation of AMPK and downstream effects. Thus, SCD1 deficiency appears to be involved in AMPK activation and hepatic lipid metabolism.
More importantly, the antidiabetic drug metformin activates AMPK and markedly reduces hepatic lipid content in ob/ob mice (102). The metabolic improvements of adiponectin are linked to the activation of AMPK, resulting in decreased liver lipids (103). AMPK activators AICAR and thienopyridine reduce hepatic fat content in rodents through AMPK activation (104). Another protein, fetuin A, produced exclusively from the liver has been suggested to regulate fat-derived hormone adiponectin. Fetuin A inhibits insulin receptor kinase and induces insulin resistance (105), and fetuin A-deficient mice show improved insulin resistance (106). Conversely, wild-type mice treated with fetuin A develop insulin resistance, consistent with association of fetuin A and insulin resistance in humans (107). This effect of fetuin A is thought to be linked to adiponectin, as individuals with NAFLD have lower adiponectin (108). These studies suggest a cross-talk between adipose tissue and liver involving AMPK, fetuin A, and adiponectin. In humans with nonalcoholic steatotic hepatitis, metformin administration improved liver function tests and decreased liver size (109), suggesting a key role of AMPK in improving hepatic steatosis, consistent with its mechanism of action. Thus, hepatic steatosis presents a pathological condition of deregulated lipogenesis in the liver in the insulin resistance and diabetic individuals, leading to increased hepatic triglycerides. AMPK activation improves hepatic steatosis directly by inhibition of lipogenesis and indirectly by lowering malonyl-CoA, leading to increased CPT1 and fatty acid oxidation.
AMPK ROLE IN WHOLE-BODY ENERGY BALANCE
AMPK in white adipose
White adipose tissue (WAT) is a primary depot of lipid storage. In lean healthy subjects, this compartment maintains a reserve of energy substrate for utilization during energy depleting states. However, excess nutrients by diets high in fat and carbohydrate can lead to excessive adipose storage of triglycerides, resulting in obesity. During ATP-consuming states with increased lipolysis, such as fasting, exercise, and hypoxia, AMPK is activated in WAT to generate substrate for ATP production (15, 110–112). AMPK activation suppresses the lipogenic pathway in WAT. Additionally, the downstream reduction of malonyl-CoA levels (a repressor of CPT1) leads to increases in CPT1 and uncoupling protein 1 (UCP1)-dependent fatty acid oxidation (15, 113). Mitochondrial β-oxidation restores the AMP:ATP ratio. Adipokines, leptin, and adiponectin, which are secreted by the adipose, promote positive feedback by activating AMPK, thereby enhancing oxidation of fatty acids (114, 115).
In terms of β-adrenergic effects on AMPK and triglyceride lipolysis, some studies in primary rat adipocytes and F442a adipocytes demonstrate inhibition of lipolysis with AMPK activators, such as AICAR (116–119), via inactivation of HSL (120). Yet other researchers have described phosphorylation of HSL by AMPK, leading to lipolysis and resulting in reduced plasma triglycerides and fatty acids (115, 121). Furthermore, AMPKa2−/− mice fed a high-fat diet showed increased white adipose tissue mass associated with increases in lipid content, supporting a role of AMPK facilitated lipolysis (113, 122). The role of AMPK on lipolysis is complex and requires further research to elucidate clinical relevance.
AMPK is also involved in the phenotypic changes associated with mitochondrial uncoupling. Mice with hyperactivated brown adipose thermogenesis (123) as well as mice having ectopic expression of UCP1 in white adipose (124, 125) have increased AMPK. These animals exhibit associated decreases in lipogenesis and lipolysis and increases in fatty acid oxidation and glycolysis, resulting in decreased body weight. Similarly, 3T3-L1 adipocytes overexpressing UCP1 have decreased triglyceride content, accompanied by decreased lipogenesis and increased glycolysis (126).
In addition to the direct effects of AMPK in adipocytes, adipose tissue mediates a critical role in whole-body energy balance through secretion of adipokines leptin and adiponectin (127). Leptin, when administered to normal rats via adenovirus transfer, results in whole-body fat loss associated with lipid depletion of adipocytes, as well as increased circulating nonesterified fatty acids and ketones derived from increased fatty acid oxidation (114).
Mouse AMPK activity is localized to epididymal fat rather than subcutaneous fat (111). AMPK has lower expression and is less active in visceral fat than subcutaneous fat in morbidly obese humans (128, 129). WAT biopsies from obese insulin-resistant subjects and body-weight-matched obese insulin-sensitive subjects revealed an association of lower AMPK activity in WAT from insulin-resistant subjects (128). Adiponectin expression and lipolysis rates are highly correlated to AMPK expression in subcutaneous fat (129). Subjects with low acylation-stimulating protein (ASP) and triglycerides have associated increases in adipose AMPK, UCP1, and CPT1 as well as decreased ACC (130). Finally, a recent study involving type-2 diabetic subjects receiving oral doses of either metformin or sulfonylurea monotherapy demonstrated enhanced AMPK and ACC phosphorylation with metformin treatment (131). However, there was no associated improvement in BMI, blood pressure, circulating lipids, or glucose outcomes compared with the sulfonylurea group. Additional studies in larger populations of obese type-2 diabetics are needed to fully understand the therapeutic efficacy of AMPK activation in obesity.
AMPK in the hypothalamus
AMPK phosphorylation in metabolically active tissues such as adipose and muscle yields a lean phenotype with decreased adipose stores. However, tissue-specific AMPK activation in the hypothalamus may increase appetite and food consumption. AMPK is activated during fasting, yet suppressed during refeeding (132). During fasting, AMPK-mediated phosphorylation of ACC (inhibition) reduces malonyl-CoA levels in the hypothalamus and increases food consumption (133). Subsequently, CPT1 activity is stimulated resulting in increased mitochondrial fatty acid oxidation (134). Fasting induces phosphorylation of ACC and AMPK in rats, and it suppresses FAS mRNA expression in the ventromedial nuclei subsequently, yielding decreased malonyl-CoA in the hypothalamus (133). Expression of active AMPK in the hypothalamus of mice increases food consumption, while hypothalamus of AMPK dominant negative mice results in a hypophagic phenotype (132). Treatment of mice with AMPK inhibitor, compound C, decreases food consumption and body weight, and treatment with AICAR, an activator of AMPK increases feeding behavior (135, 136), suggesting a direct role of AMPK in feeding behavior.
The role of hypothalamic AMPK on control of feeding behavior is promoted by the hormones leptin and ghrelin. Leptin, secreted by the WAT in response to feeding, has been shown to inhibit AMPK activity in the hypothalamus, resulting in satiety of appetite (137). Other groups have demonstrated that centrally administered ghrelin can increase feeding behavior via AMPK activation, independent of leptin (133). Ghrelin, secreted by the stomach, stimulates signaling in neuropeptide Y (NPY) neurons and the cannabinoid pathway via mechanisms involving AMPK activation (138). Intracerebroventricular (ICV) administration of ghrelin in rats increases phosphorylation of both AMPK and ACC causing increased food consumption, an effect prevented by coadministration with the AMPK inhibitor compound C (133). These findings suggest that the orexigenic activity of ghrelin may be mediated directly via AMPK activation. The net result of AMPK activation in the hypothalamus is increased appetite (49, 133, 139, 140). Based on these findings, it is possible that AMPK activators capable of crossing the blood-brain barrier may increase feeding behavior. Conversely, therapeutic AMPK activators with exposure limited to tissues such as liver, muscle, or adipose may yield beneficial reductions in peripheral lipid stores without affecting food consumption. Thus, tissue-selective AMPK activators provide an attractive therapeutic approach to reduce adipocity, body weight, circulating lipids, hepatic steatosis, or insulin resistance without affecting appetite.
AMPK in skeletal muscle
AMPK is activated by the increase in AMP:ATP ratio associated with ATP consumption during exercise, muscle contraction, and hypoxia (123, 141, 142). During stress conditions in which ATP levels are reduced, AMPK increases catabolic processes, such as glycolysis and fatty acid oxidation, and represses anabolic processes, including glycogen, protein, and lipid synthesis (141, 142). By the ACC/malonyl-CoA/CPT1 pathway, AMPK enhances fatty acid oxidation in muscle, depleting stored triacylglycerides and diacylglycerides contributing to insulin resistance (143). AMPK restores the AMP:ATP ratio through ATP production mediated by increased fatty acid uptake and lipid oxidation (113, 141, 142, 144, 145). Leptin secreted from adipose contributes to increased lipid catabolism and energy expenditure in resting muscle (146, 147).
Similar to liver, AMPK also mediates changes in cellular glucose homeostasis in muscle cells. In response to muscle contraction, AMPK activation phosphorylates and stimulates 14-3-3 binding to TBC1D1, as well as TBC1D4 (AS160), thereby inhibiting this Rab-GTPase-activating protein repressor of GLUT4 translocation, and resulting in GLUT4 translocation to the plasma membrane of myocytes to facilitate glucose uptake (148, 149).
Acute effects of ATP depletion stimulate AMPK phosphorylation; however, chronic responses include AMPK-derived expression including increased mitochondrial hexokinase II (HKII) involved in glucose transport (150), PGC-1α involved in mitochondrial biogenesis, and CPT1 involved in fatty acid oxidation via α1- and α2-AMPK subunits (151). Increased HKII expression is associated with the translocation of GLUT4 in fast-twitch muscle fibers but not slow-twitch (152). Furthermore, AICAR stimulates glucose uptake in mouse muscle via AMPK γ3 subunit expressed strictly in glycolytic skeletal muscle (153). HKII also mediates glucose phosphorylation to glucose-6-phosphate, a process that is diminished in insulin resistant subjects as a result of impaired glucose tolerance (154). These findings suggest AMPK may play a role in the regulation of fuel balance from triacylglycerol and diacylglycerol stores (increased fat oxidation) to glycogen stores, thereby increasing the glycolytic potential of skeletal muscle and potentially reducing insulin resistance.
During endurance exercise, AMPK mediates a transition from fast-twitch to the oxidative slow-twitch muscle fiber phenotype (145) by directly phosphorylating PGC-1α (113, 155). AICAR treatment of rats inactivates GS via AMPK phosphorylation (156). Because glycogen surplus results in reduced glucose uptake, the inhibition of GS should favorably increase glucose tolerance in muscle.
The role of AMPK activity has been assessed in muscle of obese and type-2 diabetic human subjects in several contexts. While one study reported that obese diabetics had an exercise time- and intensity- associated reduction in AMPK activity and AS160 phosphorylation, type-2 diabetics had lower basal PGC-1α gene expression (157). Other research groups have reported no differences in AMPK activities or fatty acid oxidation in a similar setting (158). Reduced basal glycerol release, muscle biopsy HSL protein levels, HSL phosphorylation, and increased skeletal muscle triglyceride have been observed in obese subjects (159). Finally, a recent study explored the effect of short- and long-term fasting on AMPK activity in healthy human subjects and reported elevated lipid and glycogen content in muscle and suppression of AS160 phosphorylation, with no change in AMPK phosphorylation (160). Thus, the mixed data in human subjects warrants a more careful, appropriately designed and adequately powered study.
ROLE OF AMPK IN CARDIAC FUNCTION
Although the expression of AMPK in the normal functioning heart is very low compared with skeletal muscle, the role of AMPK signaling in heart has been suggested in physiological (161) and pathological conditions like hypertrophy (162, 163) and ischemia (164). One of the primary functions of AMPK activation is to induce glucose uptake, which has been observed in hypertrophied hearts of animal models (165) and humans (166), suggesting a functional role of AMPK in cardiac hypertrophy (162, 167) and ischemia (168, 169). Further evidence of AMPK in cardiac function comes from AMPK mutation that leads to myocardial metabolic storage disease with coexistence of hypertrophy and defects in conduction system (167). Using a muscle-specific α2 kinase dead (KD) transgenic mouse model, Russell 3rd et al. (169) demonstrated that AMPK in heart is involved in glucose uptake during low flow ischemia and reperfusion, thus playing a cardioprotective role by reducing ischemia and reperfusion associated damage. Indeed, infusion of an AMPK activator, AICAR, in ischemia-induced rats caused myocardial AMPK activation and GLUT-4 translocation (170). Thus, AMPK-mediated glucose metabolism is suggested to have protective effects in cardiac hypertrophy (171).
The protective role of AMPK in failing heart has been extensively studied (reviewed in Ref. 172). While a vast amount of literature exists on the role of AMPK on heart function, here we focus on two known and widely studied AMPK activators, AICAR and metformin, in relation to cardiac function. Acute therapy of metformin imparts cardioprotection against myocardial infarction both in diabetic and nondiabetic mice, suggesting direct action of AMPK activator on the ventricular muscle (173). Long-term activation of AMPK by AICAR in pressure overload-induced cardiac hypertrophy rats showed attenuation of cardiac hypertrophy and improved cardiac function (163). To directly address the cardioprotective effect of AMPK, Nagata et al. (174) produced a constitutively active mutant of AMPK by replacing Thr-172 with Asp, and they demonstrated that overexpression of this construct in HUVECs inhibited anoxia-induced apoptosis, suggesting AMPK's role in protecting against ischemic cardiac injury. As the major effect of metformin is in glucose metabolism, it is possible that the cardioprotective effect of metformin occurs primarily via enhanced glucose metabolism in the heart. Glucose-independent long-term effect of metformin was investigated in nondiabetic rat model of post-MI heart failure (175). In this animal model, metformin showed attenuation of the development of heart failure after myocardial infarction, suggesting glucose-independent effect of AMPK activator on cardiac function. Altered substrate metabolism is associated with advanced heart failure (HF), and metformin is known to affect substrate metabolism leading to improved outcome in diabetic HF. Kazdova et al. (176) examined the effect of metformin on the improvement of cardiac function in nondiabetic volume-overload HF rat model and observed normalization of NEFA and increased palmitate oxidation but no effect on mitochondrial respiration, cardiac morphology, and mortality, suggesting that the use of metformin is safe in diabetic HF.
Obesity is associated with insulin resistance and glucose intolerance, which is linked to increased cardiac injury. A fat-derived plasma protein, C1q/TNF-related protein 9 (CTRP9), has shown beneficial effect on glucose metabolism (177) and vascular function (178). Decreased levels of CTRP9 have been observed in mouse model of ischemia-reperfusion injury and in DIO mice (179). Delivery of adenovirus expressing CTRP9 attenuated infarct size after ischemia reperfusion, and this effect was found to be mediated via AMPK signaling. In another study, activation of AMPK by metformin in mouse model of HF showed improvement in left ventricular function, attenuation of cardiac hypertrophy, and survival (180). These cardioprotective effects were associated with phosphorylation of AMPK and eNOS and increases in PGC1-α. Furthermore, metformin-mediated improvements in cardiac function were found to be dampened in AMPK-deficient mice, suggesting the important role of AMPK in reducing cardiac damage. In a canine pacing-induced HF model (181), AMPK activators AICAR and metformin attenuated oxidative stress-induced cardiomyocyte death and improved cardiac function via AMPK-mediated mechanism, as the beneficial effects of cardioprotection was blunted by Compound C, an inhibitor of AMPK (180, 181). Together, these findings suggest a cardioprotective role of AMPK activators in established animal models of cardiac injury and dysfunction.
ROLE OF AMPK IN IMMUNE RESPONSE ASSOCIATED WITH METABOLIC DISORDERS
Anti-inflammatory effects of AMPK signaling have been demonstrated in multiple in vivo models of inflammatory diseases with AMPK activators AICAR and metformin. AICAR-mediated activation of AMPK was shown to largely reduce inflammation and minimize organ damage in models of acute lung injury, acute and chronic colitis, and autoimmune encephalomyelitis (182–184).
In patients with type-2 diabetes or mild metabolic syndrome, treatment with metformin reduces plasma levels of high-sensitivity C-reactive protein (hsCRP) as well as levels of IL-6, ICAM-1, MIF, and TNF-α (185, 186). Interestingly, while metformin affects hsCRP levels in much greater extent than insulin secretagoge glibenclamide (185, 187), both metformin and PPAR-γ agonist rosiglitazone, which also promotes AMPK activity (29), were similarly effective in controlling inflammatory markers in addition to metabolic parameters (186).
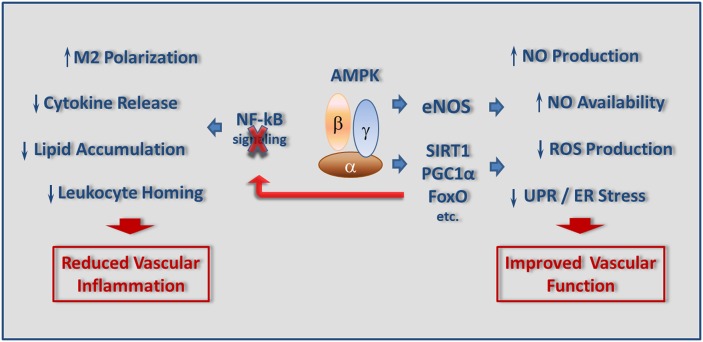
The most common attributes of chronic inflammation associated with obesity, type-2 diabetes, and atherosclerosis include i) phenotypic changes in circulating and resident monocyte-macrophages; ii) leukocyte migration, lipid accumulation, and foam cell formation in subendothelial space of the diseased arteries; iii) activation of NF-kB signaling; iv) unfolded protein response (UPR); and v) local and systemic oxidative stress (188, 189) (Fig. 3).
Fig. 3.
Downstream anti-inflammatory effects of AMPK signaling. Activation of AMPK reduces vascular inflammation via inhibiting NF-κB signaling pathway, leading to decreases in cytokine production, lipid peroxidation, and leukocyte homing. On the other hand, AMP activation improves vascular function by influencing eNOS and acting on the SIRT1-PGC1α-FoxO axis.
Polarization of resident macrophages toward pro-inflammatory M1 or mixed M1/M2 phenotypes is a hallmark of low-grade inflammation associated with metabolic disorders (188). Similar to other tissues, AMPK is also believed to act as a “master switch” of macrophage functional polarization, as it can be rapidly activated or inactivated upon exposure to anti-inflammatory (IL-10, TGF-β) or pro-inflammatory (LPS) stimuli, respectively (190). In murine macrophages, constitutively active AMPKα1 significantly lowered production of LPS-induced TNF-α and IL-6, whereas IL-10 secretion remained substantially upregulated coincidently with modulation of IkB-B, Akt/GSKβ, mTOR, and CREB (190), known downstream mediators of AMPK signaling (191). Interestingly, AMPK/angiotensin-converting enzyme (ACE) axis also has been recently shown to support macrophage M2 polarization. Although controversial, this study demonstrates that unidentified lipid mediators secreted by adipocytes from nonobese individuals stimulate AMPK phosphorylation and ACE expression and promote macrophage phenotypic transition toward anti-inflammatory state (192).
In the subendothelial space of the large and small arteries, differentiation of monocyte-derived macrophages into lipid-laden foam cells is considered to be a crucial event for the development and progression of atherosclerotic lesion (193). While uncontrolled uptake of modified LDL by macrophage scavenger receptors is largely responsible for this process, cholesterol efflux mediated by the ATP-binding cassette transporters ABCA1 and ABCG1 is recognized as a critical mechanism to balance tissue cholesterol homeostasis (194). AMPK activation attenuates oxLDL-induced lipid accumulation in murine macrophages by upregulation of ABCG1 expression and promoting cholesterol efflux from lipid-laden cells (195). Furthermore, infusion of AICAR in vivo augments ABCG1 expression levels and markedly reduces atherosclerosis in ApoE−/− mice (195).
Migration of circulating monocytes into vascular intima, followed by proliferation of monocyte/macrophage lineage within atherosclerotic lesion, represents a key feature of human atheroma as well as plaque instability and rupture (193, 196, 197). Consistent with the ability of AMPK to inhibit growth and proliferation of numerous mesenchymal cell types (198), AMPK activation also suppresses oxLDL-induced macrophage proliferation by directly causing cell cycle arrest independent of p38 MAPK/Akt signaling (199). Migration and homing of leukocytes into inflammatory sites is an energy consuming function that is highly dependent on availability of energy substrates (200) and, therefore, can potentially lead to a shortfall in energy supply (201). AMPK activation in monocyte-like cell line rapidly reduced random cell migration as well as chemotaxis toward SDF1α gradient independent of CXCR4 receptor binding (201) or subcellular localization of the Rho GTPases, RhoA, and cdc42, emphasizing the role of AMPK in control of cell movement via change in energy dynamics at the site of inflammation (201).
Energy deficiency, hypoxia, and a highly aggressive oxidative environment at the area of inflammation trigger significant endoplasmic reticulum (ER) stress and unfolded protein response (UPR) in multiple resident cell types tangled with variety of human pathologies, including diabetes and obesity (202, 203). Circulating oxidized and glycated LDLs (HOG-LDL) damage and induce apoptosis in vascular endothelial cells (EC), further promoting progression of atherosclerosis as well as other vascular complications of metabolic disorders (204, 205). HOG-LDL-mediated impairment of vascular endothelium has been linked to increased ER stress via oxidation and inhibition of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) (206). AMPKα2-deficient mice revealed higher expression of ER stress markers, increased levels of intracellular Ca2+, and significantly reduced SERCA activity in aortic EC, strongly supporting protective role for AMPKα2 subunit in maintaining SERCA function (207). Consistently, deletion of AMPKα2 subunit in ApoE−/− or LDLR−/− mice results in escalation of vascular inflammation, oxidative stress, and largely increased atherosclerotic burden due to impaired endothelium-dependent vasorelaxation and SERCA activity (206, 207).
Activation of the NADPH oxidase system is aligned with metabolic disorders and believed to function as a primary mechanism of endothelial dysfunction and chronic vascular spasm (208). Reduced availability of nitric oxide (NO) coupled with generation of multiple highly reactive oxidant species (ROS) modulates the activity of diverse intracellular signaling molecules via “redox signaling” (209). Endothelial NO synthase (eNOS) is a direct AMPK target (12) linked to immune response and vascular inflammation. Several studies implicate AMPK activation as an effective pathway in improving a disturbed redox balance (12, 209) through restoration of vascular function via NO-dependent vasorelaxation and inhibition of leukocyte adhesion (210, 211). Metformin and AICAR treatments result in increased superoxide dismutase mRNA expression and inhibition of hyperglycemia-induced intracellular and mitochondrial ROS production (212). AMPK upregulates UCP-2 expression in cultured EC and in aorta from diabetic mice, resulting in decreased superoxide and 3-nitrotyrosine production and increased NO bioactivity (213). Interestingly, recent data suggest that antioxidant effect of rosiglitazone is strictly dependent on its ability to activate AMPK and is not mediated by PPAR-γ (214).
There is now evidence that in human neutrophils, AMPK inhibits NADPH oxidase and suppresses superoxide production, suggesting that activation of AMPK could be an important mechanism for regulating an immune response when energy supply is limited (215).
Mechanistically, AMPK directly phosphorylates and controls activity of numerous key metabolic regulators and transcription factors predominantly involved in control of glucose and lipid metabolism (12). Multiple studies demonstrated that AMPK activation downregulates NF-kB system, a prime mechanism for low-grade chronic inflammation associated with obesity, type-2 diabetes, and atherosclerosis (188, 189, 193). However, inhibitory effects of AMPK on NF-kB signaling are likely to be indirect and governed by downstream mediators, such as SIRT1 and PGC1α (216). Exposure of macrophages to pro-inflammatory stimuli results in decreased sirtuin levels associated with increased activation of RelA/p65 subunit of NF-kB and increased pro-inflammatory cytokine release (217). Constitutively active AMPKα1 mimics the effect of SIRT1 on deacetylating NF-kB and, as a result, significantly inhibits LPS- and free fatty acid (FFA)-induced TNF-α expression and restores macrophage anti-inflammatory phenotype (218). Consistently, myeloid-specific deletion of SIRT1 gene results in increased accumulation of activated macrophages in liver and adipose tissue of mice challenged with a high-fat diet and promotes development of systemic insulin resistance and metabolic imbalance (219).
The p65 subunit of NF-kB constitutively bound to PGC-1α and NF-kB activation increases physical interaction between p65 and PGC1α, resulting in reduction in PGC1α expression and subsequent dysregulation of glucose oxidation (220). Adenovirus-mediated overexpression of the PGC1α gene in human aortic smooth muscle cells (SMC) and EC alleviates intracellular and mitochondrial ROS production and NF-kB activity, as well as MCP-1 and VCAM-1 expression, supporting the possibility that signaling molecules stimulating PGC-1α in the vasculature, such as AMPK, will exert beneficial effects on vascular inflammation and development of atherosclerosis (221). Thus, anti-inflammatory consequences of AMPK activation strongly support a rational to develop novel pharmacological interventions designed to stimulate AMPK activity and potentially provide additional clinical benefits for patients with metabolic disorders by reducing local and systemic inflammation linked to vascular complications of the disease.
PHARMACOLOGICAL ACTIVATORS OF AMPK
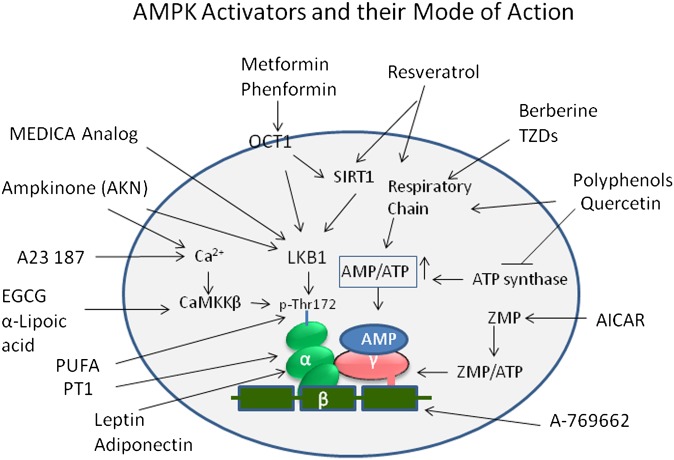
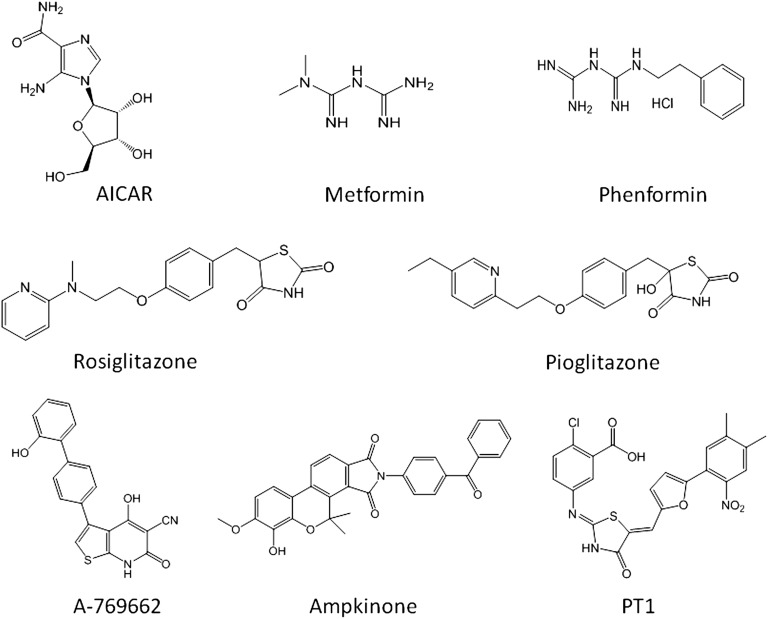
The effects on glucose and lipid metabolism described in the previous sections suggest that AMPK activation may be a potential mechanism in the effective treatment of type-2 diabetes and the MetS (222) and perhaps even obesity. Numerous new compounds have been reported as a result of searching novel AMPK activators by rational drug design, screening of chemical libraries, and testing of plant extracts. While a wide variety of agents activate AMPK, in many cases, the mechanisms remain poorly understood. Some agents directly interact with one or more of the AMPK protein subunits, while others influence AMPK activation indirectly through a number of players in the metabolic and signaling pathways (Fig. 4). The indirect activators of AMPK include pharmacological agents, nutritional staging, hormones, natural bioactives, and long chain fatty acids. The direct activators are selective small molecules.
Fig. 4.
As shown, many activators, such as resveratrol, berberine, and TZDs, inhibit mitochondrial ATP production and thus activate AMPK indirectly by increasing the cellular AMP:ATP ratio. Resveratrol also acts via SIRT1-mediated mechanism to influence LKB1 and, eventually, phosphorylation of AMPK. The biguanide drugs metformin and phenformin require the transporter OCT1 to enter cells but also work through modulation of AMP:ATP ratio. EGCG and α-lipoic acid activate AMPK via CaMKKβ. AICAR is taken up by cells and converted to ZMP, an AMP mimetic that binds to the AMPKγ subunit. A-769662, a small molecule, mimics the effect of AMP but binds to a different site, probably involving the β subunit. Activation of AMPK by PT1 occurs by relieving inhibition by the auto-inhibitory domain on the α subunit. Adipokines, leptin, and adiponectin activate AMPK by influencing α subunit indirectly.
In the context of how indirect and direct AMPK activators induce AMPK activation, it is important to mention here the two upstream kinases in mammals, the LKB1 (223) and the CaMKK-β (45–47), which phosphorylate the catalytic α subunit, leading to the activation of AMPK. The AMP:ATP ratio change by pharmacological activators induce either AMP-mediated allosteric activation and phosphorylation of AMPK or inhibition of dephosphorylation of Thr-172 by protein phosphatases (33, 224, 225). Both effects are caused by binding of AMP at two exchangeable sites on the γ subunits of AMPK complex and are inhibited by ATP by virtue of its binding at the same sites (226, 227). Activation of CaMKK-β by Ca2+/calmodulin provides an alternate pathway by which AMPK can be activated via increased intracellular Ca2+ levels, independent of AMP. A wide variety of stimuli and activators modulate AMPK activation through multiple mechanisms described above. As discussed below, some pharmacological activators of AMPK function through SIRT1- and PGC-1α-mediated pathways (Fig. 4). While AMPK activation in muscle leads to transcriptional activation of fatty acid β-oxidation via PGC-1α (228), liver AMPK activation controls glucose and lipid homeostasis primarily through downregulation of gluconeogenic genes, like PEPCK and G6Pase (57, 229), and lipogenic genes, like ACC1, FAS, SCD1, and SREBP1 (10, 51, 52).
A variety of known pharmacological agents, either synthetic or plant-derived and with different structural features, all lead to AMPK activation, albeit by different mechanisms (Fig. 4). The indirect AMPK activators are metformin and phenformin (57); phytohemicals derived from traditional medicines, like berberine (230); flavonoids and polyphenols, like quercetin (231, 232) derived from plants; and synthetic compounds, like thiazolidinediones (233, 234). The indirect activators (Figs. 5–7) activate AMPK by inhibiting complex 1 of the respiratory chain, whereas osmotic stress (116), and resveratrol (232, 235) activate AMPK without any change in cellular nucleotides. Metformin effects have also been seen where no changes in cellular nucleotides are detectable (234).
Fig. 5.
Synthetic direct and indirect activators of AMPK. These activators induce AMPK activation via a number of pathways, including LKB1, SIRT1, AMP:ATP ratio, CaMKKβ, and ATP synthase.
Fig. 7.
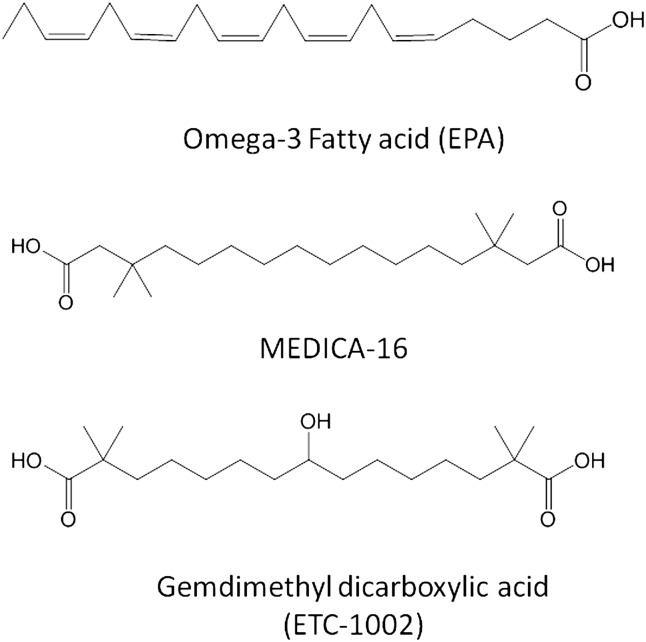
Long-chain fatty acids and their analogs as AMPK activators.
Hawley et al. (236) carried out elegant research to identify mechanism of AMPK activators using cells expressing γ-subunit variants. These researchers employed two different types of cells, one that showed AMP sensitivity and expressed AMPK complexes (termed as WT) and the other cell line that harbored a γ2 variant that was insensitive to AMP. The CBS domains bind ATP and AMP; the binding of AMP and ATP to AMPK occurs in a mutually exclusive manner. Binding of ATP keeps the activity of AMPK low, and when AMP levels increase, the exchange of AMP for ATP results in a several-fold increase in kinase activity. Using these two cell lines, these authors tested indirect AMPK activators like metformin, phenformin, troglitazone, quercetin, resveratrol, and berberine, and direct activators like AICAR, A-769662, and Ca2+ ionophore A23187, which increases cytoplasmic Ca2+ and activates upstream kinase CaMKKβ. In this study, while the majority of compounds tested showed inhibition of mitochondrial ATP production and thereby activation of AMPK indirectly, metformin appeared to activate AMPK by an additional mechanism involving LKB1 (237). Similarly, resveratrol and quercetin, in addition to modulating the AMP:ATP ratio partly via inhibition of ATP synthase, also showed activation of AMPK through SIRT1 (232), although some researchers consider this effect as secondary to the activation of AMPK (144).
AMPK ACTIVATORS
Indirect AMPK activators
Metformin.
Metformin, a derivative of guanidine (Fig. 5), is taken up by the cells via organic cation transporter 1 (OCT1), which is highly expressed in the liver and facilitates the selective uptake of metformin into hepatocytes (238). In mice lacking OCT1, the effect of metformin on AMPK activation and gluconeogenesis is dampened, and the hypoglycemic effect of the biguanide is abolished (239), suggesting that metformin's primary action is the inhibition of hepatic gluconeogenesis and to some extent glucose uptake in skeletal muscles (81). Among the mechanisms of action of metformin suggested, the direct inhibition of the respiratory chain complex 1 constitutes the more convincing molecular mechanism, leading to reduced cellular ATP and increased AMP (82, 240, 241). The main antidiabetic effect of metformin is attributed to hepatic activation of AMPK followed by the inhibition of gluconeogenesis. In contrast to skeletal muscle, the effect of metformin in the liver is mediated by LKB1, as the liver-specific knockout of LKB1 in diabetic mice did not respond to metformin (242). Thus, the AMPK-mediated hypoglycemic effects of metformin in mice appear to require the upstream kinase LKB1. Available experimental evidence suggests that metformin activates AMPK via modulating the AMP:ATP ratio as well as LKB1 mediated pathway (242), although an AMPK-independent effect has also been suggested (243). In human subjects, metformin reduces blood glucose levels mainly via inhibition of hepatic gluconeogenesis (239).
Thiazolidinediones.
Thiazolidinediones (TZD) are another class of antidiabetic drugs that have been shown to stimulate AMPK activity. Members of this group, such as rosiglitazone and pioglitazone, cause rapid increase in AMPK activity. Similar to the biguanides, the acute effects of TZDs on AMPK activity is mediated indirectly via inhibition of complex I of the respiratory chain (82) and subsequent alterations in cellular adenine nucleotide levels. However, TZDs have also been shown to be high affinity ligands for peroxisome proliferator-activated receptor-γ (PPAR-γ) (244). Indeed, many pharmacological effects of rosiglitazone have been attributed to its PPAR-γ activating properties (245, 246). TZDs acutely activate AMPK by a mechanism independent of PPAR-γ-regulated gene transcription, which appears to be associated with change in cellular energy state (82, 247). TZDs downregulate lipolysis through the reduction of TNF-α and reduce fatty acids in circulation by accelerated uptake by adipocytes (248). In addition to TNF-α, these drugs also inhibit the release of several adipokines from adipose cells, such as interleukin-6 and resistin, which induce muscle insulin resistance. Concomitantly, TZDs augment the secretion of the insulin-sensitizing factor adiponectin from adipose tissue in man and rodents (249). Adiponectin stimulates glucose uptake and fatty acid oxidation in skeletal muscles and inhibits gluconeogenesis in the liver by activating AMPK (103, 250). Thus, TZDs might exert their therapeutic benefit by activating AMPK via two independent mechanisms. In the skeletal muscle of diabetic patients treated with TZDs, an increase in PGC-1α was noticed over six months (251). Importantly, TZD effects were observed in adipocytes in which PPAR-γ expression was blocked (233), which was further corroborated using a TZD derivative, BLX-1002, that does not bind to PPAR-γ, but stimulates AMPK activity and induces insulin secretion in β-cells (252). PPAR-γ sparing activity of another TZD, MSDC-0602, was recently shown to have attributes consistent with AMPK activation (253).
Plant-derived AMPK activators
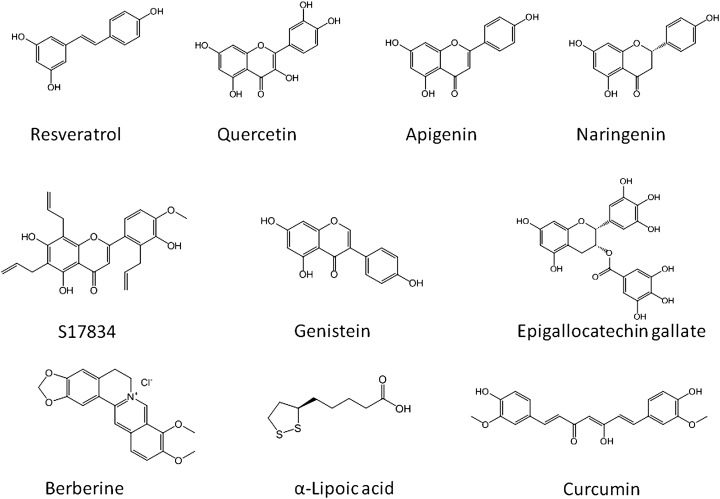
Polyphenols.
A number of plant-derived agents, like genistein, quercetin, isoginkgetin, and epigallocatechin-3-gallate (EGCG), all contain isoflavone and isoflavone-like moieties in their structures, and have been shown to activate AMPK (Fig. 6). These are polyphenolic substances found in a variety of plants. The lipid-lowering effects of the phytoestrogen genistein and its ability to decrease adiposity were found to be related to the activation of AMPK (231). Plant-derived phytoestrogens and polyphenols activate AMPK in 3T3-L1 preadipocytes and adipocytes and in primary mouse hepatocytes (254).
Fig. 6.
Plant-derived AMPK activators. One of the main features of plant-derived AMPK activators is the presence of multiple phenolic groups that make them hydrophilic and easy to absorb in the gut. However, a plant-derived AMPK activator having decent pharmacokinetic properties is yet to be identified.
The activation of AMPK by EGCG has been suggested to be mediated via CaMKK-β (255). Green and black teas are enriched with a variety of polyphenolic compounds, including EGCG (256). The consumption of both green and black teas lowers cholesterol in preclinical animal models and also in man (224, 257, 258). Singh et al. (259) demonstrated that cholesterol-lowering effect of green and black tea extracts enriched in catechins and epigallocatechins occurs through phosphorylation of AMPK, concomitant with phosphorylation of HMG-CoA reductase, similar to that seen in AICAR-treated cells. Polyphenols, such as resveratrol and EGCG, are shown to be potent activators for AMPK in vitro and in vivo (235, 255). Some of the beneficial metabolic actions of polyphenols are mediated by their ability to activate SIRT1 (235), as resveratrol, a polyphenolic substance, activated SIRT1, leading to the modulation of LKB1/AMPK-signaling axis (91). SIRT1 activation by polyphenols leads to LKB1-dependent AMPK activation by direct deacetylation of LKB1 by SIRT1 (260). EGCG-induced increase in AMPK phosphorylation is mediated by CaMKK-β activation (255). Furthermore, the SIRT1 regulation of LKB1/AMPK signaling appears to be tissue-specific as resveratrol-stimulated AMPK activation in neurons was independent of SIRT1 (261).
Another group of AMPK activators derived from plants has been identified. For example, capsaicinoids, extracted from hot peppers, are shown to induce fatty acid oxidation and decrease body fat accumulation in diabetic mice. The synthetic structural ester isomer isodihydrocapsiate activates LKB1 both in vitro and in vivo (262). In L6 myotubes, these substances increased glucose uptake and reduced blood glucose in the myocytes of diabetic mice. Inhibition of salidroside-induced AMPK activity by Compound C in L6 myotubes suggests that this compound exerts its effects by activating AMPK (263).
Curcumin, a polyphenolic natural product of the plant Curcuma longa, was originally described as an anticancer agent (264), but later it was also shown to have AMPK-activating properties in ovarian cancer cells (265). It prevented cytokine-induced death in vitro and in vivo in isolated mouse pancreatic islets in an AMPK-dependent manner (266), and it reduced hepatic glucose production (267).
D-xylose and lipophilic D-xylose derivatives
Winder et al. introduced AMPK as a target for developing novel drugs for the treatment of type-2 diabetes in 1999 (222). Since then, numerous molecules that activate AMPK, directly or indirectly, have been synthesized or extracted from plants. One such compound is D-xylose and its derivatives (268). These compounds activate AMPK phosphorylation and glucose uptake by L6 myotubes at very high concentrations (10–20 mM). More potent derivatives of D-xylose, such as 2,4;3,5-dibenzylidene-D-xylose-diethyl-dithioacetal, 2,4-benzylidene-D-xylose-diethyl-dithioacetal, and 2,4-benzylidene-D-xylose-3-O-methyl-diethyl-dithioacetal, were synthesized that showed significant stimulation of glucose uptake in myotubes at concentrations ranging from 5 to 100 μM. This effect occurs via AMPK activation through AMPK phosphorylation at Thr-172 and inhibited by the AMPK inhibitor Compound C, suggesting involvement of AMPK activation. Some of these compounds reduced blood glucose levels in the genetically diabetic KKAy mice (268) and show potential as antihyperglycemic compounds.
Berberine
Berberine, an isoquinoline alkaloid derived from Hydrastic canadensis, and its synthetic derivative dihydroberberine are claimed to be effective antilipogenic and hypoglycemic agents in rodents (230, 269). Berberine has been shown to have antidiabetic effects in vitro as well (270). To investigate the mode of action of berberine, Lee et al. (230) used two animal models of insulin resistance. In db/db mice, berberine improved glucose tolerance without affecting food consumption. In high fat-fed Wistar rats, berberine downregulated lipogenic genes and upregulated genes that are involved in energy expenditure. In adipocytes and L6 cells, treatment with berberine showed AMPK activation, leading to the phosphorylation of ACC and inhibition of fatty acid synthesis. These researchers also suggested AMPK activation in primary mouse adipocytes via activation of p38 (230). The antidiabetic effect of berberine may also result from the anti-inflammatory activities as suggested by Jeong et al. (271).
alpha-Lipoic acid
α-Lipoic acid (Fig. 6) is a plant-derived antioxidant, which has been shown to decrease lipid accumulation in rat skeletal muscle and steatosis in the obese rat liver via AMPK phosphorylation (272). The inhibition of lipogenesis by α-lipoic acid was suggested to occur by AMPK-dependent and AMPK-independent pathways. In C2C12 myotubes, α-lipoic acid increased the AMPK activity by Thr-172 phosphorylation of the AMPKα2 subunit, which resulted in the phosphorylation of ACC at Ser-218. Investigation of the effect of α-lipoic acid revealed that AMPK phosphorylation occurs through the CaMKK pathway as the inhibition of CaMKK-β with the selective inhibitor STO-609 abolished α-lipoic acid-stimulated AMPK activation, with a concomitant reduction of ACC phosphorylation. This was further corroborated by studies using a short interfering-RNA against CaMKK-β, which abolished α-lipoic acid-induced AMPK activation. These data suggest that CaMKK-β is possibly the target for α-lipoic acid-induced AMPK activation in myotubes (273).
Long chain-fatty acids, PUFA, and fatty acid analogs
Activation of AMPK by long chain fatty acid (LCFA) (274) and polyunsaturated fatty acids (PUFA) (69, 275) has been shown in animal models. In skeletal muscles (276) and L6 myotubes (277), fatty acids stimulate AMPK activation. These effects are short-lived and transient due to efficient β-oxidation. Substituted α,ω-dicarboxylic acids of 14-18C fatty acids (MEDICA analogs) (Fig. 7), which are not metabolized beyond their acyl-CoA thioesters, inhibit lipid synthesis (278) and show hypolipidemic and antidiabetogenic activities (279). In a more recent study (280), MEDICA analogs are shown to activate recombinant AMPK in a cell-free system, in cell lines such as HepG2 and 3T3-L1 and in diabetic db/db mice. Activation of AMPK in the latter animal model normalized blood glucose and suppressed hepatic glucose production. These effects were suggested to occur via decreased energy charge in cultured cells as a result of mitochondrial uncoupling by a long chain fatty acyl analog (281) and by direct activation of LKB1-dependent phosphorylation of AMPK- Thr-172 by the MEDICA-CoA or LCFA-CoA thioester but not by free acid.
A new class of fatty acid analogs, gemdimethyl dicarboxylic acids, have been shown as potent inhibitors of fatty acid and cholesterol synthesis in primary rat hepatocytes and in in vivo lipogenesis assay (282). A series of cell-based studies revealed that an optimized compound, 2,2,14,14-tetramethyl-8-hydroxypentadecanedioic acid (ETC-1002) (Fig. 7), stimulated AMPK phosphorylation, leading to the phosphorylation of ACC and HMG-CoA reductase and associated with inhibition of lipogenesis and cholesterol synthesis (283). Further investigation into the mechanistic insights suggested that ETC-1002 free acid was able to activate AMPK in a cell line independent of xeno-CoA formation (Stephen Pinkosky et al., unpublished observations). The unique properties of ETC-1002, including AMPK activation, are expected to have beneficial effects in normalizing lipid and carbohydrate abnormalities. Indeed, many of the expected benefits of AMPK activation were seen in a diet-induced hyperlipidemic hamster model treated with ETC-1002, including lowering of triglycerides, LDL-C, adiposity, and improvement in hepatic steatosis (284). The liver-selective effect of ETC-1002 was also seen in KKAy mice with the lowering of glucose and improvement in glucose intolerance. The KKAy mouse is an animal model of diabetes and obesity for evaluating antidiabetic and hypolipidemic agents (245). As expected from a selective direct activator of AMPK (104), ETC-1002 showed antidiabetic effect and improved hepatic steatosis in this model (285). Not surprising that these favorable attributes of ETC-1002 in a number of animal disease models also translated into humans and showed significant improvements in circulating lipids in normal (286) and dyslipidemic individuals without any apparent adverse effects. Furthermore, ETC-1002 dramatically reduced aortic lipids in LDL receptor knockout mice in a lipid-dependent and independent manner (287). ETC-1002 displays activities and attributes as good as a selective small-molecule AMPK activator A-769662 in terms of its IC50 of fatty acid synthesis in primary rat hepatocytes (3–5 µM) and efficacy in animal model of diabetes (104). Interestingly, the 30 mg/kg/day efficacy of the selective AMPK activator A-769662 in animal model of diabetes and obesity was very similar to the 30 mg/kg/day dose of ETC-1002 in multiple animal models of disease. While ETC-1002 was safe and efficacious in human subjects, another AMPK activator, A-769662, is yet to be evaluated in humans for safety and efficacy. Thus, ETC-1002 offers promises to emerge as a monotherapy for patients with lipid and carbohydrate abnormalities, and because of LDL-lowering activities in human subjects (286), it may also offer hope to those patients who are statin intolerant.
DIRECT ACTIVATORS
AICAR
The nucleoside AICAR has been widely used as a reference compound to investigate the downstream effects following activation of AMPK (116, 119). The effect of AICAR on mitochondrial respiration in intact hepatocytes at higher concentrations of AICAR (>100 μM) was not mediated by AMPK, as this effect was also observed in hepatocytes isolated from AMPKα1α2 liver-specific knockout mice (203). AMPK-independent effects of AICAR in the hepatic and muscle glucose uptake have been reported (54, 288). AICAR gets metabolized to 5 amino imidazole-4-carboxamide ribonucleoside monophosphate (ZMP) as a result of uptake of AICAR (analog of adenosine) by the cells via an adenosine transport system, followed by conversion of AICAR to ZMP via adenosine kinase. Like AMP, ZMP also binds to the γ subunit of AMPK and induces allosteric changes in AMPK conformation, allowing kinase activation (54) and inhibition of dephosphorylation, both leading to increase in the AMP:ATP ratio. Thus, AMPK activation by AICAR may be described as a direct activator, as the metabolite of AICAR, ZMP, directly interacts with the AMPK γ-subunit similar to AMP. Because of the structural similarities of ZMP with 5′-AMP, it mimics all of the allosteric effects of 5′AMP on the AMPK system (116). Some researchers, however, have claimed that ZMP can also regulate glucose metabolism by direct inhibition of fructose-1,6-biphosphatase in hepatocytes, independent of the activation of AMPK (289). AICAR exhibited antidiabetic effects in disease models by improving blood glucose level, lipid profile, hepatic glucose output, and glucose disposal (290). In both healthy and diabetic individuals, AICAR infusion increased skeletal muscle glucose uptake (291). The inhibition of IL-6 and IL-8 secretion by adipose and skeletal muscle following AICAR treatment suggests that AICAR may also improve insulin sensitivity in the periphery (292).
A-769662
A new class of AMPK activators, known as non-nucleoside thienopyridone, has been identified using partially purified AMPK from rat liver (104). The lead molecule A769662 showed specificity to AMPK activation when screened against a panel of 76 protein kinases at 10 μM concentration (293). Unlike other AMPK activators, A-769662 directly activates native AMPK complex purified from rat liver in cell-free assays by mimicking both the effects of AMP on allosteric activation, as revealed by the AMPKα subunit and LKB1 knockout cell lines, and inhibition of dephosphorylation of AMPK complex (225, 293). Further activation of AMPK by A-769662 at saturating AMP concentrations suggests that the binding site of A-769622 and AMP may not be identical (104, 293). Additionally, A-769662 allosterically activates AMPK complexes harboring a mutation in the γ1 subunit that abolishes allosteric activation by AMP (225). Interestingly, an AMPK complex lacking the glycogen-binding domain of the β subunit or containing a mutation of Ser-108 to alanine (an autophosphorylation site within the glycogen binding domain of the β1 subunit) completely abolished the allosteric effect of A-769662, while only partially reducing AMPK activation (225). Most importantly, A769662 activates AMPK complexes that exclusively contain the β1 subunit (294). This property limits the potential therapeutic use of A-769662 to tissues expressing the ubiquitous β1 subunit but not to tissues, like skeletal muscle, that assemble predominantly AMPKβ2 complexes.
The main target of A769662 appears to be the liver, in which an activation of β1-containing AMPK complex reduces the expression of gluconeogenic enzymes and hepatic glucose production. AMPK β1, but not β2-containing complexes, were dose-dependently activated by A-769662 in muscles. AMPK activation by A-769662 was independent of the upstream kinase utilized (225, 293). Importantly, unlike indirect AMPK activators, neither the changes in adenine nucleotide levels (79) nor alterations in mitochondrial oxidative phosphorylation following treatment with A-769662 have been detected in hepatocytes. Addition of A-769662 to primary mouse hepatocytes stimulates AMPK activity and phosphorylation of its known downstream targets, but it was completely abolished in hepatocytes lacking AMPKα1 and α2 catalytic subunits.
A769662 showed an IC50 of 3.2 and 3.6 µM in primary rat and mouse hepatocytes, respectively, in an assay measuring 14C-acetate incorporation in fatty acids. As expected from an AMPK activator, chronic treatment with A-769662 lowered plasma triglyceride, glucose, and liver lipids in ob/ob mice, which was associated with the mild downregulation of PEPCK, G6Pase, and FAS. The effect of metformin on these genes was not consistent with AMPK activation in this study. Another patented thienopyridone derivative was recently reported (295), which also contains a thienopyridone moiety and activates AMPK, most likely by interacting with the β1 subunits. Since the identification and elucidation of the mechanism of efficacy of A-769662 in an animal model in 2006, no further progress on the development of this molecule has been reported.
Furanothiazolidine (PT1)
During the screening of chemical library against the α-subunit of AMPK, Pang et al. (296) discovered a furanothiazolidine derivative PT1 as a direct activator of AMPK (Fig. 5). When tested in HepG2 cells, it lowered lipid content and activated AMPKα1394, AMPKα1335, and AMPKα2398, as well as the α1β1γ1 heterotrimer in a dose-dependent manner with an EC50 of about 8 µM using inactive form of human AMPK α1394. As expected from the mechanism used for screening, it interacted with the autoinhibitory domain in the α1 subunit and converted AMPK to a constitutively active complex by relieving the autoinhibition. Experiments with L6 myotubes showed that PT1 effectively increased the phosphorylation of AMPK with no apparent changes in the ATP:ADP ratio in the cells. In HeLa cells, PT1 stimulated AMPK and ACC phosphorylation in the absence of LKB1 and without increasing the AMP:ATP ratio. It should be noted that a concentration of 40 µM was used for the activation of AMPK in L6 myotubes and in HeLa cells, which is rather high for a direct activator of AMPK. Because HeLa cells express CaMKK-β, but not LKB1, as the upstream kinases, this suggests that the AMPK phosphorylation in the HeLa cells by PT1 occurs via CaMKK-β-mediated mechanism. This was further corroborated by loss of PT1-induced phosphorylation of AMPK in cells cotreated with STO-609, a specific CaMKK-β inhibitor, suggesting a critical role for Ca2+-dependent CaMKK-β activation in this process. In vivo efficacy and safety in appropriate animal models are awaited to ascertain the potential of this novel chemotype for development as antidiabetic drug that targets the autoinhibitory domain of the AMPK α subunit.
Ampkinones
A small molecule activator of AMPK was identified by Oh et al. (297), which was termed as ampkinone (Fig. 5). These amkinones phosphorylate AMPK at Thr-172 in a number of cell lines, leading to ACC phosphorylation. While these data were generated with the 10 µM concentration, a dose-response in terms of AMPK phosphorylation was noticed up to 100 µM concentration with an EC50 of 4.3 µM. Further studies with appropriate cell lines and specific inhibitors demonstrated that AMPK activation by ampkinone occurs via LKB1-mediated mechanism. In a diet-induced obese mouse model, amkinone at 10 mg/kg/day dose showed improvement in glucose tolerance and insulin sensitivity concomitant with the reduction in adiposity and body weight without affecting food consumption. More follow-up in vivo studies are needed to show efficacy in established diabetes models, and safety associated with this class of molecules.
LIMITATIONS OF KNOWN AMPK ACTIVATORS TO TREAT METABOLIC DISORDERS
Plant-derived products have not shown adequate therapeutic use primarily as a result of poor pharmacokinetic properties and adverse effects of some of the natural products at higher doses. Synthetic molecules like AICAR suffer from unfavorable pharmacokinetic properties as well (i.e., high effective concentration, poor bioavailability, and short half-life) and severe metabolic complications like lactic acidosis and massive uric acid production (298). Another serious shortcoming of AICAR is the lack of a strict specificity for AMPK. In fact, AICAR interacts with other enzymes, such as S-adenosylhomocysteine hydrolase and glycogen phosphorylase (299). To solve these problems, efforts have been made to develop more potent derivatives of AICAR. One example is the imidazo pyridine derivative S27847, which increased the activity of AMPK 7-fold in hepatocytes at micromolar concentrations (300), but it remains to be seen if it is associated with any adverse effects.
Metformin has been used to treat diabetes for 50 years, and is currently the front-line drug for the treatment of type-2 diabetes. Of concern is a recent report that links metformin-induced activation of AMPK to an increased biogenesis of Alzheimer's amyloid in mouse brain (301). Although metformin is relatively safe, it is not effective in all patients, perhaps due to variability in the efficiency of hepatic uptake by the OCT1 transporter (238). In addition, a number of reduced function polymorphisms have been identified in the OCT1 gene that exhibit impaired metformin uptake (302), suggesting that genetic variation may alter responsiveness to the drug in human patients. Intestinal epithelial cells also express transporters of the OCT1 family (Oct1–3), and inhibition of the respiratory chain in the gut may be responsible for the unpleasant gastrointestinal side effects of biguanides, as well as the more dangerous side effect of lactic acidosis that led to the withdrawal of phenformin, a more potent biguanide, in 1994. There is evidence that much of the lactic acid produced in animals treated with biguanides is derived from the gut (136). It therefore seems quite likely that a drug that activated the AMPK system more directly would be more efficacious in the treatment of type-2 diabetes and the metabolic syndrome than the biguanides.
Small molecule selective activators of AMPK also suffer from poor bioavailability, and overall unfavorable pharmacokinetic properties. The first selective small molecule, A-769662, activator of AMPK discovered, although had reasonable EC50, the in vivo EC50 in acute lipogenesis was similar to fatty acid analogs, thus showing no advantage over reported AMPK activators. Additionally, the safety of this molecule is unknown. The finding that A769662 activates AMPK complexes that exclusively contain the β1 subunit (104) may limit the potential therapeutic use of this compound to tissues expressing the ubiquitous β1 subunit but not to tissue-like skeletal muscle with AMPKβ2 complexes. Indeed, unlike metformin, A769662 was unable to stimulate glucose uptake in skeletal muscle (303). Other selective small molecule activators of AMPK, like PT1 and ampkinone, are yet to be tested in adequate animal models for efficacy and safety before it can advance to the clinic. One small molecule ETC-1002, which has shown efficacy in multiple animal models consistent with effects expected from an AMPK activator and shown to be safe and efficacious in clinical studies involving dyslipidemic subjects, offers tremendous promise as a new novel treatment.
CHALLENGES OF DEVELOPING AMPK ACTIVATORS
Despite significant efforts toward finding efficacious AMPK activators for therapeutic use in patients with MetS, most of the identified compounds prepared either from synthetic route or extracted from plant sources suffer from one or more drawbacks. Initially, lack of a robust assay to screen AMPK activators impeded discovery efforts. Due to the lack of an assay system predictive of its usefulness in the in vivo models, even today researchers are relying on the partially purified AMPK from rat liver, which is not a high-throughput assay method but is expected to provide meaningful data to justify follow-up in vivo efficacy studies. Unfortunately, small molecules with specificity and selectivity have not shown good pharmacokinetic properties. The other challenge in the discovery of AMPK activators is the multiple mechanisms of AMPK activators in multiple tissues and their differential effects on biochemical pathways in those tissues (i.e., liver, muscle, and adipose). Thus, in the development of a screening paradigm for new compounds, it is challenging to decide which tissue to target and what endpoint to measure in an in vivo setting. As an example, the main target of A769662 is most likely the liver, in which an activation of β1-containing AMPK complex reduces the expression of gluconeogenic enzymes. The question then remains about other tissues, like muscle, which is a key metabolic target organ for glucose metabolism. To date, except ETC-1002, small molecules that selectively activate AMPK have yet to show efficacy in the clinic. Future studies are likely to explain whether the limited success in targeting AMPK is related to the identified molecule's safety or the complex biology of this novel target in specific tissues that results in the regulation of energy imbalances in lipid and carbohydrate metabolism.
FUTURE DIRECTIONS
New compounds that activate AMPK in multiple tissues or those directed to the liver are needed as novel antidiabetic, antihyperlipidemic, and antiobesity drugs to normalize metabolic imbalances. The ideal AMPK activator should have multiple beneficial properties on several pathways, which are deranged in MetS. While the new chemical entity should have adequate specificity to activate AMPK at a lower concentration than marketed agents, it should preferably activate AMPK in the liver and desirably in other tissues, like muscle. Thus, the activation of AMPK should be targeted to liver, adipocytes, and skeletal muscles, but not to the brain. Oral formulation of drugs having better bioavailability and overall favorable pharmacokinetic properties is preferred. With the success of ETC-1002 in the clinic in terms of safety and efficacy, it is reasonable to hope that the ongoing efforts in several laboratories may lead to discovering a novel class of drugs to treat diabetes and related metabolic abnormalities in the future.
Footnotes
Abbreviations:
- ACC
- acetyl CoA carboxylase
- ACE
- angiotensin-converting enzyme
- AICAR
- 5-aminoimidazole-4-carboxamide riboside
- AIS
- autoinhibitory sequence
- AMPK
- 5′ adenosine monophosphate-activated protein kinase
- ATGL
- adipocyte-triglyceride lipase
- CaMKKβ
- Ca2+ /calmodulin-dependent kinase β
- CBS
- cystathionone-β-synthase
- CDC42
- cell division control protein 42 homolog
- ChREBP
- carbohydrate response element binding protein 1
- CNS
- central nervous system
- CPT1
- carnitine palmitoyltransferase 1
- CRTC2
- CREB-regulated transcription coactivator-2
- CTRP9
- C1q/TNF-related protein 9
- CXCR4
- C-X-C chemokine receptor type 4
- DDI
- drug-drug interaction
- DGAT2
- diacylglycerol acyltransferase 2
- DIO
- diet-induced obese
- EGCG
- epigallocathechin-3-gallate
- eNOS
- endothelial nitric oxide synthase
- FOXO
- forkhead box
- GBD
- glycogen-binding domain
- GPAT
- glycerol phosphate acyl transferase
- GS
- glycogen synthase
- HDAC
- class IIA histone deacetylases
- HF
- heart failure
- HKII
- hexokinase II
- HMGR
- 3-hydroxy-3-methylglutaryl-CoA reductase
- HNF-4α
- hepatic nuclear factor-4α
- hsCRP
- high-sensitivity C-reactive protein
- HSL
- hormone-sensitive lipase
- HUVEC
- human umbilical vein endothelial cell
- ICAM-1
- intercellular adhesion molecule-1
- IL
- interleukin
- LCFA
- long chain fatty acid
- LKB1
- liver kinase B1
- LPS
- lipopolysaccharide
- MetS
- metabolic syndrome
- MGAT
- monoacylglycerol acyltransferase
- MIF
- macrophage inhibitory factor
- mTOR
- mammalian target of rapamycin
- NAFLD
- nonalcoholic fatty liver disease
- NF-kB
- nuclear factor kappa B
- OCT1
- Organic cation transporter 1
- PEPCK
- phosphoenolpyruvate carboxylase
- PGC1-α
- PPAR-γ coactivator-1α
- SCD1
- steroyl CoA desaturase 1
- SDF1α
- stromal cell-derived factor 1 α
- SERCA
- sarcoplasmic/endoplasmic reticulum Ca2+ ATPase
- SIRT1
- sirtuin (silent mating type information regulation 2 homolog) 1
- SREBP1
- sterol regulatory element binding protein 1
- STRAD
- ste20 related adapter
- TBC1D4
- TBC1 domain family member 4
- TGF-β
- transforming growth factor-β
- TNF-α
- tumor necrosis factor-α
- TORC1
- target-of-rapamycin complex-1
- TSC2
- tuberous sclerosis complex 2
- UCP1
- uncoupling protein 1
- UPR
- unfolded protein response
- VCAM-1
- vascular cell adhesion molecule-1
- WAT
- white adipose tissue
REFERENCES
- 1.Grundy S. M. 2000. Metabolic complications of obesity. Endocrine. 13: 155–165 [DOI] [PubMed] [Google Scholar]
- 2.Han S., Liang C. P., Westerterp M., Senokuchi T., Welch C. L., Wang Q., Matsumoto M., Accili D., Tall A. R. 2009. Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J. Clin. Invest. 119: 1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd S. T., Scott D. M., Pick A. M. 2009. Pill burden in low-income patients with metabolic syndrome and diabetes. J. Pharm. Technol. 25: 297–302 [Google Scholar]
- 4.Srivastava R. A., Srivastava N. 2004. Search for obesity drugs: targeting central and peripheral pathways. Curr. Med. Chem. Immunol. Endocr. Metab. Agents. 4: 75–90 [Google Scholar]
- 5.Hardie D. G. 2004. The AMP-activated protein kinase pathway—new players upstream and downstream. J. Cell Sci. 117: 5479–5487 [DOI] [PubMed] [Google Scholar]
- 6.Cheung P. C., Salt I. P., Davies S. P., Hardie D. G., Carling D. 2000. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem. J. 346: 659–669 [PMC free article] [PubMed] [Google Scholar]
- 7.Birnbaum M. J. 2005. Activating AMP-activated protein kinase without AMP. Mol. Cell. 19: 289–290 [DOI] [PubMed] [Google Scholar]
- 8.Hardie D. G. 2003. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 144: 5179–5183 [DOI] [PubMed] [Google Scholar]
- 9.Beg Z. H., Allmann D. W., Gibson D. M. 1973. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and wth protein fractions of rat liver cytosol. Biochem. Biophys. Res. Commun. 54: 1362–1369 [DOI] [PubMed] [Google Scholar]
- 10.Carlson C. A., Kim K. H. 1973. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J. Biol. Chem. 248: 378–380 [PubMed] [Google Scholar]
- 11.Viollet B., Foretz M., Guigas B., Horman S., Dentin R., Bertrand L., Hue L., Andreelli F. 2006. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J. Physiol. 574: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towler M. C., Hardie D. G. 2007. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 100: 328–341 [DOI] [PubMed] [Google Scholar]
- 13.Kola B. 2008. Role of AMP-activated protein kinase in the control of appetite. J. Neuroendocrinol. 20: 942–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie D. G. 2004. AMP-activated protein kinase: a master switch in glucose and lipid metabolism. Rev. Endocr. Metab. Disord. 5: 119–125 [DOI] [PubMed] [Google Scholar]
- 15.Daval M., Foufelle F., Ferre P. 2006. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 574: 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardie D. G., Pan D. A. 2002. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 30: 1064–1070 [DOI] [PubMed] [Google Scholar]
- 17.Düfer M., Noack K., Krippeit-Drews P., Drews G. 2010. Activation of the AMP-activated protein kinase enhances glucose-stimulated insulin secretion in mouse beta-cells. Islets. 2: 156–163 [DOI] [PubMed] [Google Scholar]
- 18.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. 1996. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 271: 27879–27887 [DOI] [PubMed] [Google Scholar]
- 19.Crute B. E., Seefeld K., Gamble J., Kemp B. E., Witters L. A. 1998. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J. Biol. Chem. 273: 35347–35354 [DOI] [PubMed] [Google Scholar]
- 20.Pang T., Xiong B., Li J. Y., Qiu B. Y., Jin G. Z., Shen J. K., Li J. 2007. Conserved alpha-helix acts as autoinhibitory sequence in AMP-activated protein kinase alpha subunits. J. Biol. Chem. 282: 495–506 [DOI] [PubMed] [Google Scholar]
- 21.Bendayan M., Londono I., Kemp B. E., Hardie G. D., Ruderman N., Prentki M. 2009. Association of AMP-activated protein kinase subunits with glycogen particles as revealed in situ by immunoelectron microscopy. J. Histochem. Cytochem. 57: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson E. R., Pan D. A., James J., Lucocq J. M., Hawley S. A., Green K. A., Baba O., Terashima T., Hardie D. G. 2003. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr. Biol. 13: 861–866 [DOI] [PubMed] [Google Scholar]
- 23.McBride A., Ghilagaber S., Nikolaev A., Hardie D. G. 2009. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 9: 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polekhina G., Gupta A., Michell B. J., van Denderen B., Murthy S., Feil S. C., Jennings I. G., Campbell D. J., Witters L. A., Parker M. W., et al. 2003. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr. Biol. 13: 867–871 [DOI] [PubMed] [Google Scholar]
- 25.Oakhill J. S., Chen Z. P., Scott J. W., Steel R., Castelli L. A., Ling N., Macaulay S. L., Kemp B. E. 2010. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc. Natl. Acad. Sci. USA. 107: 19237–19241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp B. E. 2004. Bateman domains and adenosine derivatives form a binding contract. J. Clin. Invest. 113: 182–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bateman A. 1997. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 22: 12–13 [DOI] [PubMed] [Google Scholar]
- 28.Kemp B. E., Oakhill J. S., Scott J. W. 2007. AMPK structure and regulation from three angles. Structure. 15: 1161–1163 [DOI] [PubMed] [Google Scholar]
- 29.Morrison A., Yan X., Tong C., Li J. 2011. Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, and JNK signaling in nondiabetic mice. Am. J. Physiol. Heart Circ. Physiol. 301: H895–H902 [DOI] [PubMed] [Google Scholar]
- 30.Bonen A., Han X. X., Habets D. D., Febbraio M., Glatz J. F., Luiken J. J. 2007. A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am. J. Physiol. Endocrinol. Metab. 292: E1740–E1749 [DOI] [PubMed] [Google Scholar]
- 31.Davies S. P., Helps N. R., Cohen P. T., Hardie D. G. 1995. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 377: 421–425 [DOI] [PubMed] [Google Scholar]
- 32.Sanders M. J., Grondin P. O., Hegarty B. D., Snowden M. A., Carling D. 2007. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 403: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. 2006. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 281: 32207–32216 [DOI] [PubMed] [Google Scholar]
- 34.Chen Z. P., Stephens T. J., Murthy S., Canny B. J., Hargreaves M., Witters L. A., Kemp B. E., McConell G. K. 2003. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 52: 2205–2212 [DOI] [PubMed] [Google Scholar]
- 35.Frederich M., Balschi J. A. 2002. The relationship between AMP-activated protein kinase activity and AMP concentration in the isolated perfused rat heart. J. Biol. Chem. 277: 1928–1932 [DOI] [PubMed] [Google Scholar]
- 36.Hellsten Y., Richter E. A., Kiens B., Bangsbo J. 1999. AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J. Physiol. 520: 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. 1979. Cytosolic phosphorylation potential. J. Biol. Chem. 254: 6538–6547 [PubMed] [Google Scholar]
- 38.Jin X., Townley R., Shapiro L. 2007. Structural insight into AMPK regulation: ADP comes into play. Structure. 15: 1285–1295 [DOI] [PubMed] [Google Scholar]
- 39.Hardie D. G., Carling D., Gamblin S. J. 2011. AMP-activated protein kinase: also regulated by ADP? Trends Biochem. Sci. 36: 470–477 [DOI] [PubMed] [Google Scholar]
- 40.Carling D., Mayer F. V., Sanders M. J., Gamblin S. J. 2011. AMP-activated protein kinase: nature's energy sensor. Nat. Chem. Biol. 7: 512–518 [DOI] [PubMed] [Google Scholar]
- 41.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13: 2004–2008 [DOI] [PubMed] [Google Scholar]
- 42.Brajenovic M., Joberty G., Kuster B., Bouwmeester T., Drewes G. 2004. Comprehensive proteomic analysis of human Par protein complexes reveals an interconnected protein network. J. Biol. Chem. 279: 12804–12811 [DOI] [PubMed] [Google Scholar]
- 43.Boudeau J., Baas A. F., Deak M., Morrice N. A., Kieloch A., Schutkowski M., Prescott A. R., Clevers H. C., Alessi D. R. 2003. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 22: 5102–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baas A. F., Boudeau J., Sapkota G. P., Smit L., Medema R., Morrice N. A., Alessi D. R., Clevers H. C. 2003. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 22: 3062–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. 2005. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2: 9–19 [DOI] [PubMed] [Google Scholar]
- 46.Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. 2005. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2: 21–33 [DOI] [PubMed] [Google Scholar]
- 47.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. 2005. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280: 29060–29066 [DOI] [PubMed] [Google Scholar]
- 48.Hawley S. A., Selbert M. A., Goldstein E. G., Edelman A. M., Carling D., Hardie D. G. 1995. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 270: 27186–27191 [DOI] [PubMed] [Google Scholar]
- 49.Hardie D. G. 2011. Sensing of energy and nutrients by AMP-activated protein kinase. Am. J. Clin. Nutr. 93: 891S–896S [DOI] [PubMed] [Google Scholar]
- 50.Momcilovic M., Hong S. P., Carlson M. 2006. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 281: 25336–25343 [DOI] [PubMed] [Google Scholar]
- 51.Munday M. R., Campbell D. G., Carling D., Hardie D. G. 1988. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur. J. Biochem. 175: 331–338 [DOI] [PubMed] [Google Scholar]
- 52.Clarke P. R., Hardie D. G. 1990. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 9: 2439–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muoio D. M., Seefeld K., Witters L. A., Coleman R. A. 1999. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem. J. 338: 783–791 [PMC free article] [PubMed] [Google Scholar]
- 54.Merrill G. F., Kurth E. J., Hardie D. G., Winder W. W. 1997. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 273: E1107–E1112 [DOI] [PubMed] [Google Scholar]
- 55.Watt M. J., Holmes A. G., Pinnamaneni S. K., Garnham A. P., Steinberg G. R., Kemp B. E., Febbraio M. A. 2006. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 290: E500–E508 [DOI] [PubMed] [Google Scholar]
- 56.Ahmadian M., Abbott M. J., Tang T., Hudak C. S., Kim Y., Bruss M., Hellerstein M. K., Lee H. Y., Samuel V. T., Shulman G. I., et al. 2011. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13: 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108: 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leclerc I., Lenzner C., Gourdon L., Vaulont S., Kahn A., Viollet B. 2001. Hepatocyte nuclear factor-4alpha involved in type 1 maturity-onset diabetes of the young is a novel target of AMP-activated protein kinase. Diabetes. 50: 1515–1521 [DOI] [PubMed] [Google Scholar]
- 59.Kawaguchi T., Osatomi K., Yamashita H., Kabashima T., Uyeda K. 2002. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J. Biol. Chem. 277: 3829–3835 [DOI] [PubMed] [Google Scholar]
- 60.Sakamoto K., Holman G. D. 2008. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 295: E29–E37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marsin A. S., Bouzin C., Bertrand L., Hue L. 2002. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 277: 30778–30783 [DOI] [PubMed] [Google Scholar]
- 62.Jørgensen S. B., Nielsen J. N., Birk J. B., Olsen G. S., Viollet B., Andreelli F., Schjerling P., Vaulont S., Hardie D. G., Hansen B. F., et al. 2004. The alpha2–5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 53: 3074–3081 [DOI] [PubMed] [Google Scholar]
- 63.Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., et al. 2005. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 437: 1109–1111 [DOI] [PubMed] [Google Scholar]
- 64.Mihaylova M. M., Vasquez D. S., Ravnskjaer K., Denechaud P. D., Yu R. T., Alvarez J. G., Downes M., Evans R. M., Montminy M., Shaw R. J. 2011. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 145: 607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zong H., Ren J. M., Young L. H., Pypaert M., Mu J., Birnbaum M. J., Shulman G. I. 2002. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA. 99: 15983–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardie D. G. 2011. AMPK and autophagy get connected. EMBO J. 30: 634–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 30: 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inoki K., Zhu T., Guan K. L. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 115: 577–590 [DOI] [PubMed] [Google Scholar]
- 69.Suchankova G., Tekle M., Saha A. K., Ruderman N. B., Clarke S. D., Gettys T. W. 2005. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem. Biophys. Res. Commun. 326: 851–858 [DOI] [PubMed] [Google Scholar]
- 70.Reaven G. M. 1995. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 75: 473–486 [DOI] [PubMed] [Google Scholar]
- 71.Watanabe S., Yaginuma R., Ikejima K., Miyazaki A. 2008. Liver diseases and metabolic syndrome. J. Gastroenterol. 43: 509–518 [DOI] [PubMed] [Google Scholar]
- 72.Muoio D. M., Newgard C. B. 2006. Obesity-related derangements in metabolic regulation. Annu. Rev. Biochem. 75: 367–401 [DOI] [PubMed] [Google Scholar]
- 73.Boizard M., Le Liepvre X., Lemarchand P., Foufelle F., Ferre P., Dugail I. 1998. Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J. Biol. Chem. 273: 29164–29171 [DOI] [PubMed] [Google Scholar]
- 74.Srivastava R. A., Jahagirdar R., Azhar S., Sharma S., Bisgaier C. L. 2006. Peroxisome proliferator-activated receptor-alpha selective ligand reduces adiposity, improves insulin sensitivity and inhibits atherosclerosis in LDL receptor-deficient mice. Mol. Cell. Biochem. 285: 35–50 [DOI] [PubMed] [Google Scholar]
- 75.Srivastava R. A., He S. 2010. Anti-hyperlipidemic and insulin sensitizing activities of fenofibrate reduces aortic lipid deposition in hyperlipidemic Golden Syrian hamster. Mol. Cell. Biochem. 345: 197–206 [DOI] [PubMed] [Google Scholar]
- 76.Godbole V. Y., Grundleger M. L., Thenen S. W. 1980. Early development of lipogenesis in genetically obese (ob/ob) mice. Am. J. Physiol. 239: E265–E268 [DOI] [PubMed] [Google Scholar]
- 77.Kaplan M. L., Leveille G. A. 1981. Development of lipogenesis and insulin sensitivity in tissues of the ob/ob mouse. Am. J. Physiol. 240: E101–E107 [DOI] [PubMed] [Google Scholar]
- 78.Andreelli F., Foretz M., Knauf C., Cani P. D., Perrin C., Iglesias M. A., Pillot B., Bado A., Tronche F., Mithieux G., et al. 2006. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 147: 2432–2441 [DOI] [PubMed] [Google Scholar]
- 79.Foretz M., Ancellin N., Andreelli F., Saintillan Y., Grondin P., Kahn A., Thorens B., Vaulont S., Viollet B. 2005. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 54: 1331–1339 [DOI] [PubMed] [Google Scholar]
- 80.Bergeron R., Previs S. F., Cline G. W., Perret P., Russell R. R., 3rd, Young L. H., Shulman G. I. 2001. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 50: 1076–1082 [DOI] [PubMed] [Google Scholar]
- 81.Sakamoto K., Goransson O., Hardie D. G., Alessi D. R. 2004. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am. J. Physiol. Endocrinol. Metab. 287: E310–E317 [DOI] [PubMed] [Google Scholar]
- 82.Brunmair B., Staniek K., Gras F., Scharf N., Althaym A., Clara R., Roden M., Gnaiger E., Nohl H., Waldhausl W., et al. 2004. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 53: 1052–1059 [DOI] [PubMed] [Google Scholar]
- 83.Jørgensen S. B., Viollet B., Andreelli F., Frosig C., Birk J. B., Schjerling P., Vaulont S., Richter E. A., Wojtaszewski J. F. 2004. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J. Biol. Chem. 279: 1070–1079 [DOI] [PubMed] [Google Scholar]
- 84.Shirai T., Inoue E., Ishimi Y., Yamauchi J. 2011. AICAR response element binding protein (AREBP), a key modulator of hepatic glucose production regulated by AMPK in vivo. Biochem. Biophys. Res. Commun. 414: 287–291 [DOI] [PubMed] [Google Scholar]
- 85.Bultot L., Guigas B., Von Wilamowitz-Moellendorff A., Maisin L., Vertommen D., Hussain N., Beullens M., Guinovart J. J., Foretz M., Viollet B., et al. 2012. AMP-activated protein kinase phosphorylates and inactivates liver glycogen synthase. Biochem. J. 44: 193–203 [DOI] [PubMed] [Google Scholar]
- 86.Carlson C. L., Winder W. W. 1999. Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise. J. Appl. Physiol. 86: 669–674 [DOI] [PubMed] [Google Scholar]
- 87.Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J. R., Girard J., Postic C. 2006. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 55: 2159–2170 [DOI] [PubMed] [Google Scholar]
- 88.Choi C. S., Savage D. B., Abu-Elheiga L., Liu Z. X., Kim S., Kulkarni A., Distefano A., Hwang Y. J., Reznick R. M., Codella R., et al. 2007. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc. Natl. Acad. Sci. USA. 104: 16480–16485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruderman N., Prentki M. 2004. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 3: 340–351 [DOI] [PubMed] [Google Scholar]
- 90.Imai K., Inukai K., Ikegami Y., Awata T., Katayama S. 2006. LKB1, an upstream AMPK kinase, regulates glucose and lipid metabolism in cultured liver and muscle cells. Biochem. Biophys. Res. Commun. 351: 595–601 [DOI] [PubMed] [Google Scholar]
- 91.Hou X., Xu S., Maitland-Toolan K. A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T. J., et al. 2008. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 283: 20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoneda M., Guo Y., Ono H., Nakatsu Y., Zhang J., Cui X., Iwashita M., Kumamoto S., Tsuchiya Y., Sakoda H., et al. 2010. Decreased SIRT1 expression and LKB1 phosphorylation occur with long-term high-fat diet feeding, in addition to AMPK phosphorylation impairment in the early phase. Obes. Res. Clin. Pract. 4: e201–e207 [DOI] [PubMed] [Google Scholar]
- 93.Purushotham A., Schug T. T., Xu Q., Surapureddi S., Guo X., Li X. 2009. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 9: 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fulco M., Sartorelli V. 2008. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 7: 3669–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimano H., Horton J. D., Hammer R. E., Shimomura I., Brown M. S., Goldstein J. L. 1996. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. 98: 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y., Xu S., Mihaylova M. M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J. Y., et al. 2011. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13: 376–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Um J. H., Park S. J., Kang H., Yang S., Foretz M., McBurney M. W., Kim M. K., Viollet B., Chung J. H. 2010. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 59: 554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song Z., Deaciuc I., Zhou Z., Song M., Chen T., Hill D., McClain C. J. 2007. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G894–G902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J., Maika S., Craddock L., King J. A., Liu Z. M. 2008. Chronic activation of AMP-activated protein kinase-alpha1 in liver leads to decreased adiposity in mice. Biochem. Biophys. Res. Commun. 370: 248–253 [DOI] [PubMed] [Google Scholar]
- 100.Ntambi J. M., Miyazaki M., Stoehr J. P., Lan H., Kendziorski C. M., Yandell B. S., Song Y., Cohen P., Friedman J. M., Attie A. D. 2002. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA. 99: 11482–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dobrzyn P., Dobrzyn A., Miyazaki M., Cohen P., Asilmaz E., Hardie D. G., Friedman J. M., Ntambi J. M. 2004. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc. Natl. Acad. Sci. USA. 101: 6409–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim Y. D., Park K. G., Lee Y. S., Park Y. Y., Kim D. K., Nedumaran B., Jang W. G., Cho W. J., Ha J., Lee I. K., et al. 2008. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes. 57: 306–314 [DOI] [PubMed] [Google Scholar]
- 103.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., et al. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8: 1288–1295 [DOI] [PubMed] [Google Scholar]
- 104.Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., et al. 2006. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3: 403–416 [DOI] [PubMed] [Google Scholar]
- 105.Rauth G., Poschke O., Fink E., Eulitz M., Tippmer S., Kellerer M., Haring H. U., Nawratil P., Haasemann M., Jahnen-Dechent W., et al. 1992. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur. J. Biochem. 204: 523–529 [DOI] [PubMed] [Google Scholar]
- 106.Mathews S. T., Rakhade S., Zhou X., Parker G. C., Coscina D. V., Grunberger G. 2006. Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem. Biophys. Res. Commun. 350: 437–443 [DOI] [PubMed] [Google Scholar]
- 107.Stefan N., Hennige A. M., Staiger H., Machann J., Schick F., Krober S. M., Machicao F., Fritsche A., Haring H. U. 2006. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 29: 853–857 [DOI] [PubMed] [Google Scholar]
- 108.Musso G., Gambino R., Durazzo M., Biroli G., Carello M., Faga E., Pacini G., De Michieli F., Rabbione L., Premoli A., et al. 2005. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology. 42: 1175–1183 [DOI] [PubMed] [Google Scholar]
- 109.Sofer E., Boaz M., Matas Z., Mashavi M., Shargorodsky M. 2011. Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Metabolism. 60: 1278–1284 [DOI] [PubMed] [Google Scholar]
- 110.Park H., Kaushik V. K., Constant S., Prentki M., Przybytkowski E., Ruderman N. B., Saha A. K. 2002. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J. Biol. Chem. 277: 32571–32577 [DOI] [PubMed] [Google Scholar]
- 111.Sponarova J., Mustard K. J., Horakova O., Flachs P., Rossmeisl M., Brauner P., Bardova K., Thomason-Hughes M., Braunerova R., Janovska P., et al. 2005. Involvement of AMP-activated protein kinase in fat depot-specific metabolic changes during starvation. FEBS Lett. 579: 6105–6110 [DOI] [PubMed] [Google Scholar]
- 112.Gauthier M. S., Miyoshi H., Souza S. C., Cacicedo J. M., Saha A. K., Greenberg A. S., Ruderman N. B. 2008. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 283: 16514–16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Viollet B., Athea Y., Mounier R., Guigas B., Zarrinpashneh E., Horman S., Lantier L., Hebrard S., Devin-Leclerc J., Beauloye C., et al. 2009. AMPK: lessons from transgenic and knockout animals. Front. Biosci. 14: 19–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Orci L., Cook W. S., Ravazzola M., Wang M. Y., Park B. H., Montesano R., Unger R. H. 2004. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc. Natl. Acad. Sci. USA. 101: 2058–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yin W., Mu J., Birnbaum M. J. 2003. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3–L1 adipocytes. J. Biol. Chem. 278: 43074–43080 [DOI] [PubMed] [Google Scholar]
- 116.Corton J. M., Gillespie J. G., Hawley S. A., Hardie D. G. 1995. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229: 558–565 [DOI] [PubMed] [Google Scholar]
- 117.Dagon Y., Avraham Y., Berry E. M. 2006. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem. Biophys. Res. Commun. 340: 43–47 [DOI] [PubMed] [Google Scholar]
- 118.Daval M., Diot-Dupuy F., Bazin R., Hainault I., Viollet B., Vaulont S., Hajduch E., Ferre P., Foufelle F. 2005. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 280: 25250–25257 [DOI] [PubMed] [Google Scholar]
- 119.Sullivan J. E., Brocklehurst K. J., Marley A. E., Carey F., Carling D., Beri R. K. 1994. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 353: 33–36 [DOI] [PubMed] [Google Scholar]
- 120.Djouder N., Tuerk R. D., Suter M., Salvioni P., Thali R. F., Scholz R., Vaahtomeri K., Auchli Y., Rechsteiner H., Brunisholz R. A., et al. 2010. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 29: 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Su C. L., Sztalryd C., Contreras J. A., Holm C., Kimmel A. R., Londos C. 2003. Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J. Biol. Chem. 278: 43615–43619 [DOI] [PubMed] [Google Scholar]
- 122.Villena J. A., Viollet B., Andreelli F., Kahn A., Vaulont S., Sul H. S. 2004. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 53: 2242–2249 [DOI] [PubMed] [Google Scholar]
- 123.Klaus S., Keipert S., Rossmeisl M., Kopecky J. 2012. Augmenting energy expenditure by mitochondrial uncoupling: a role of AMP-activated protein kinase. Genes Nutr. 7: 369–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matejkova O., Mustard K. J., Sponarova J., Flachs P., Rossmeisl M., Miksik I., Thomason-Hughes M., Grahame Hardie D., Kopecky J. 2004. Possible involvement of AMP-activated protein kinase in obesity resistance induced by respiratory uncoupling in white fat. FEBS Lett. 569: 245–248 [DOI] [PubMed] [Google Scholar]
- 125.Rossmeisl M., Flachs P., Brauner P., Sponarova J., Matejkova O., Prazak T., Ruzickova J., Bardova K., Kuda O., Kopecky J. 2004. Role of energy charge and AMP-activated protein kinase in adipocytes in the control of body fat stores. Int. J. Obes. Relat. Metab. Disord. 28(Suppl. 4): S38–S44 [DOI] [PubMed] [Google Scholar]
- 126.Si Y., Shi H., Lee K. 2009. Metabolic flux analysis of mitochondrial uncoupling in 3T3–L1 adipocytes. PLoS ONE. 4: e7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friedman J. 2002. Fat in all the wrong places. Nature. 415: 268–269 [DOI] [PubMed] [Google Scholar]
- 128.Gauthier M. S., O'Brien E. L., Bigornia S., Mott M., Cacicedo J. M., Xu X. J., Gokce N., Apovian C., Ruderman N. 2011. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem. Biophys. Res. Commun. 404: 382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martínez-Agustin O., Hernandez-Morante J. J., Martinez-Plata E., Sanchez de Medina F., Garaulet M. 2010. Differences in AMPK expression between subcutaneous and visceral adipose tissue in morbid obesity. Regul. Pept. 163: 31–36 [DOI] [PubMed] [Google Scholar]
- 130.MacLaren R. E., Cui W., Lu H., Simard S., Cianflone K. 2010. Association of adipocyte genes with ASP expression: a microarray analysis of subcutaneous and omental adipose tissue in morbidly obese subjects. BMC Med. Genomics. 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boyle J. G., Logan P. J., Jones G. C., Small M., Sattar N., Connell J. M., Cleland S. J., Salt I. P. 2011. AMP-activated protein kinase is activated in adipose tissue of individuals with type 2 diabetes treated with metformin: a randomised glycaemia-controlled crossover study. Diabetologia. 54: 1799–1809 [DOI] [PubMed] [Google Scholar]
- 132.Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferre P., Birnbaum M. J., et al. 2004. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 428: 569–574 [DOI] [PubMed] [Google Scholar]
- 133.López M., Lage R., Saha A. K., Perez-Tilve D., Vazquez M. J., Varela L., Sangiao-Alvarellos S., Tovar S., Raghay K., Rodriguez-Cuenca S., et al. 2008. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 7: 389–399 [DOI] [PubMed] [Google Scholar]
- 134.Kahn B. B., Alquier T., Carling D., Hardie D. G. 2005. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1: 15–25 [DOI] [PubMed] [Google Scholar]
- 135.Ronnett G. V., Aja S. 2008. AMP-activated protein kinase in the brain. Int J Obes (Lond). 32(Suppl. 4): S42–S48 [DOI] [PubMed] [Google Scholar]
- 136.Xue B., Kahn B. B. 2006. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 574: 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim C. T., Kola B., Korbonits M. 2010. AMPK as a mediator of hormonal signalling. J. Mol. Endocrinol. 44: 87–97 [DOI] [PubMed] [Google Scholar]
- 138.Kola B., Farkas I., Christ-Crain M., Wittmann G., Lolli F., Amin F., Harvey-White J., Liposits Z., Kunos G., Grossman A. B., et al. 2008. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One 3: e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ahima R. S., Qi Y., Singhal N. S. 2006. Adipokines that link obesity and diabetes to the hypothalamus. Prog. Brain Res. 153: 155–174 [DOI] [PubMed] [Google Scholar]
- 140.Lage R., Dieguez C., Vidal-Puig A., Lopez M. 2008. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 14: 539–549 [DOI] [PubMed] [Google Scholar]
- 141.Hardie D. G. 2008. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. (Lond.) 32(Suppl 4): S7–S12 [DOI] [PubMed] [Google Scholar]
- 142.Zhang B. B., Zhou G., Li C. 2009. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 9: 407–416 [DOI] [PubMed] [Google Scholar]
- 143.Lowell B. B., Shulman G. I. 2005. Mitochondrial dysfunction and type 2 diabetes. Science. 307: 384–387 [DOI] [PubMed] [Google Scholar]
- 144.Cantó C., Jiang L. Q., Deshmukh A. S., Mataki C., Coste A., Lagouge M., Zierath J. R., Auwerx J. 2010. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11: 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McGee S. L., Hargreaves M. 2010. AMPK-mediated regulation of transcription in skeletal muscle. Clin. Sci. (Lond.). 118: 507–518 [DOI] [PubMed] [Google Scholar]
- 146.Kus V., Prazak T., Brauner P., Hensler M., Kuda O., Flachs P., Janovska P., Medrikova D., Rossmeisl M., Jilkova Z., et al. 2008. Induction of muscle thermogenesis by high-fat diet in mice: association with obesity-resistance. Am. J. Physiol. Endocrinol. Metab. 295: E356–E367 [DOI] [PubMed] [Google Scholar]
- 147.Minokoshi Y., Kim Y. B., Peroni O. D., Fryer L. G., Muller C., Carling D., Kahn B. B. 2002. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 415: 339–343 [DOI] [PubMed] [Google Scholar]
- 148.Karagounis L. G., Hawley J. A. 2009. The 5′ adenosine monophosphate-activated protein kinase: regulating the ebb and flow of cellular energetics. Int. J. Biochem. Cell Biol. 41: 2360–2363 [DOI] [PubMed] [Google Scholar]
- 149.Pehmøller C., Treebak J. T., Birk J. B., Chen S., Mackintosh C., Hardie D. G., Richter E. A., Wojtaszewski J. F. 2009. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14–3-3 binding in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 297: E665–E675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Holmes B. F., Kurth-Kraczek E. J., Winder W. W. 1999. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J. Appl. Physiol. 87: 1990–1995 [DOI] [PubMed] [Google Scholar]
- 151.Jørgensen S. B., Wojtaszewski J. F., Viollet B., Andreelli F., Birk J. B., Hellsten Y., Schjerling P., Vaulont S., Neufer P. D., Richter E. A., et al. 2005. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 19: 1146–1148 [DOI] [PubMed] [Google Scholar]
- 152.Wright D. C., Geiger P. C., Holloszy J. O., Han D. H. 2005. Contraction- and hypoxia-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in slow-twitch rat soleus muscle. Am. J. Physiol. Endocrinol. Metab. 288: E1062–E1066 [DOI] [PubMed] [Google Scholar]
- 153.Barnes B. R., Marklund S., Steiler T. L., Walter M., Hjalm G., Amarger V., Mahlapuu M., Leng Y., Johansson C., Galuska D., et al. 2004. The 5′-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J. Biol. Chem. 279: 38441–38447 [DOI] [PubMed] [Google Scholar]
- 154.Lehto M., Huang X., Davis E. M., Le Beau M. M., Laurila E., Eriksson K. F., Bell G. I., Groop L. 1995. Human hexokinase II gene: exon-intron organization, mutation screening in NIDDM, and its relationship to muscle hexokinase activity. Diabetologia. 38: 1466–1474 [DOI] [PubMed] [Google Scholar]
- 155.Jäger S., Handschin C., St-Pierre J., Spiegelman B. M. 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA. 104: 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wojtaszewski J. F., Jorgensen S. B., Hellsten Y., Hardie D. G., Richter E. A. 2002. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 51: 284–292 [DOI] [PubMed] [Google Scholar]
- 157.Sriwijitkamol A., Coletta D. K., Wajcberg E., Balbontin G. B., Reyna S. M., Barrientes J., Eagan P. A., Jenkinson C. P., Cersosimo E., DeFronzo R. A., et al. 2007. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 56: 836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Steinberg G. R., Smith A. C., Van Denderen B. J., Chen Z., Murthy S., Campbell D. J., Heigenhauser G. J., Dyck D. J., Kemp B. E. 2004. AMP-activated protein kinase is not down-regulated in human skeletal muscle of obese females. J. Clin. Endocrinol. Metab. 89: 4575–4580 [DOI] [PubMed] [Google Scholar]
- 159.Jocken J. W., Roepstorff C., Goossens G. H., van der Baan P., van Baak M., Saris W. H., Kiens B., Blaak E. E. 2008. Hormone-sensitive lipase serine phosphorylation and glycerol exchange across skeletal muscle in lean and obese subjects: effect of beta-adrenergic stimulation. Diabetes. 57: 1834–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vendelbo M. H., Clasen B. F., Treebak J. T., Moller L., Krusenstjerna-Hafstrom T., Madsen M., Nielsen T. S., Stodkilde-Jorgensen H., Pedersen S. B., Jorgensen J. O., et al. 2012. Insulin resistance after a 72 hour fast is associated with impaired AS160 phosphorylation and accumulation of lipid and glycogen in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 302: E190–E200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Musi N., Hirshman M. F., Arad M., Xing Y., Fujii N., Pomerleau J., Ahmad F., Berul C. I., Seidman J. G., Tian R., et al. 2005. Functional role of AMP-activated protein kinase in the heart during exercise. FEBS Lett. 579: 2045–2050 [DOI] [PubMed] [Google Scholar]
- 162.Tian R., Musi N., D'Agostino J., Hirshman M. F., Goodyear L. J. 2001. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 104: 1664–1669 [DOI] [PubMed] [Google Scholar]
- 163.Li H. L., Yin R., Chen D., Liu D., Wang D., Yang Q., Dong Y. G. 2007. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J. Cell. Biochem. 100: 1086–1099 [DOI] [PubMed] [Google Scholar]
- 164.Li J., Coven D. L., Miller E. J., Hu X., Young M. E., Carling D., Sinusas A. J., Young L. H. 2006. Activation of AMPK alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am. J. Physiol. Heart Circ. Physiol. 291: H1927–H1934 [DOI] [PubMed] [Google Scholar]
- 165.Zhang J., Duncker D. J., Ya X., Zhang Y., Pavek T., Wei H., Merkle H., Ugurbil K., From A. H., Bache R. J. 1995. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation. 92: 1274–1283 [DOI] [PubMed] [Google Scholar]
- 166.Nuutila P., Maki M., Laine H., Knuuti M. J., Ruotsalainen U., Luotolahti M., Haaparanta M., Solin O., Jula A., Koivisto V. A., et al. 1995. Insulin action on heart and skeletal muscle glucose uptake in essential hypertension. J. Clin. Invest. 96: 1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arad M., Benson D. W., Perez-Atayde A. R., McKenna W. J., Sparks E. A., Kanter R. J., McGarry K., Seidman J. G., Seidman C. E. 2002. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J. Clin. Invest. 109: 357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim A. S., Miller E. J., Wright T. M., Li J., Qi D., Atsina K., Zaha V., Sakamoto K., Young L. H. 2011. A small molecule AMPK activator protects the heart against ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 51: 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Russell R. R., 3rd, Li J., Coven D. L., Pypaert M., Zechner C., Palmeri M., Giordano F. J., Mu J., Birnbaum M. J., Young L. H. 2004. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Invest. 114: 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Russell R. R., 3rd, Bergeron R., Shulman G. I., Young L. H. 1999. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. 277: H643–H649 [DOI] [PubMed] [Google Scholar]
- 171.Kolwicz S. C., Jr, Tian R. 2011. Glucose metabolism and cardiac hypertrophy. Cardiovasc. Res. 90: 194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beauloye C., Bertrand L., Horman S., Hue L. 2011. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc. Res. 90: 224–233 [DOI] [PubMed] [Google Scholar]
- 173.Calvert J. W., Gundewar S., Jha S., Greer J. J., Bestermann W. H., Tian R., Lefer D. J. 2008. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 57: 696–705 [DOI] [PubMed] [Google Scholar]
- 174.Nagata D., Kiyosue A., Takahashi M., Satonaka H., Tanaka K., Sata M., Nagano T., Nagai R., Hirata Y. 2009. A new constitutively active mutant of AMP-activated protein kinase inhibits anoxia-induced apoptosis of vascular endothelial cell. Hypertens. Res. 32: 133–139 [DOI] [PubMed] [Google Scholar]
- 175.Yin M., van der Horst I. C., van Melle J. P., Qian C., van Gilst W. H., Sillje H. H., de Boer R. A. 2011. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am. J. Physiol. Heart Circ. Physiol. 301: H459–H468 [DOI] [PubMed] [Google Scholar]
- 176.Benes J., Kazdova L., Drahota Z., Houstek J., Medrikova D., Kopecky J., Kovarova N., Vrbacky M., Sedmera D., Strnad H., et al. 2011. Effect of metformin therapy on cardiac function and survival in a volume-overload model of heart failure in rats. Clin. Sci. (Lond.). 121: 29–41 [DOI] [PubMed] [Google Scholar]
- 177.Wong G. W., Krawczyk S. A., Kitidis-Mitrokostas C., Ge G., Spooner E., Hug C., Gimeno R., Lodish H. F. 2009. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 23: 241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zheng Q., Yuan Y., Yi W., Lau W. B., Wang Y., Wang X., Sun Y., Lopez B. L., Christopher T. A., Peterson J. M., et al. 2011. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler. Thromb. Vasc. Biol. 31: 2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kambara T., Ohashi K., Shibata R., Ogura Y., Maruyama S., Enomoto T., Uemura Y., Shimizu Y., Yuasa D., Matsuo K., et al. 2012. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J. Biol. Chem. 287: 18965–18973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gundewar S., Calvert J. W., Jha S., Toedt-Pingel I., Ji S. Y., Nunez D., Ramachandran A., Anaya-Cisneros M., Tian R., Lefer D. J. 2009. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ. Res. 104: 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sasaki H., Asanuma H., Fujita M., Takahama H., Wakeno M., Ito S., Ogai A., Asakura M., Kim J., Minamino T., et al. 2009. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 119: 2568–2577 [DOI] [PubMed] [Google Scholar]
- 182.Bai A., Ma A. G., Yong M., Weiss C. R., Ma Y., Guan Q., Bernstein C. N., Peng Z. 2010. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem. Pharmacol. 80: 1708–1717 [DOI] [PubMed] [Google Scholar]
- 183.Nath N., Giri S., Prasad R., Salem M. L., Singh A. K., Singh I. 2005. 5-aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J. Immunol. 175: 566–574 [DOI] [PubMed] [Google Scholar]
- 184.Zhao X., Zmijewski J. W., Lorne E., Liu G., Park Y. J., Tsuruta Y., Abraham E. 2008. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 295: L497–L504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Akbar D. H. 2003. Effect of metformin and sulfonylurea on C-reactive protein level in well-controlled type 2 diabetics with metabolic syndrome. Endocrine. 20: 215–218 [DOI] [PubMed] [Google Scholar]
- 186.Fidan E., Onder Ersoz H., Yilmaz M., Yilmaz H., Kocak M., Karahan C., Erem C. 2011. The effects of rosiglitazone and metformin on inflammation and endothelial dysfunction in patients with type 2 diabetes mellitus. Acta Diabetol. 48: 297–302 [DOI] [PubMed] [Google Scholar]
- 187.Ashcroft F. M. 2005. ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 115: 2047–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Olefsky J. M., Glass C. K. 2010. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72: 219–246 [DOI] [PubMed] [Google Scholar]
- 189.Schenk S., Saberi M., Olefsky J. M. 2008. Insulin sensitivity: modulation by nutrients and inflammation. J. Clin. Invest. 118: 2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sag D., Carling D., Stout R. D., Suttles J. 2008. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 181: 8633–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mihaylova M. M., Shaw R. J. 2011. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13: 1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kohlstedt K., Trouvain C., Namgaladze D., Fleming I. 2011. Adipocyte-derived lipids increase angiotensin-converting enzyme (ACE) expression and modulate macrophage phenotype. Basic Res. Cardiol. 106: 205–215 [DOI] [PubMed] [Google Scholar]
- 193.Rocha V. Z., Libby P. 2009. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 6: 399–409 [DOI] [PubMed] [Google Scholar]
- 194.Yvan-Charvet L., Wang N., Tall A. R. 2010. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30: 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li D., Wang D., Wang Y., Ling W., Feng X., Xia M. 2010. Adenosine monophosphate-activated protein kinase induces cholesterol efflux from macrophage-derived foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J. Biol. Chem. 285: 33499–33509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rekhter M. D., Gordon D. 1995. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am. J. Pathol. 147: 668–677 [PMC free article] [PubMed] [Google Scholar]
- 197.Koenig W., Khuseyinova N. 2007. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler. Thromb. Vasc. Biol. 27: 15–26 [DOI] [PubMed] [Google Scholar]
- 198.Hardie D. G. 2011. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 25: 1895–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ishii N., Matsumura T., Kinoshita H., Motoshima H., Kojima K., Tsutsumi A., Kawasaki S., Yano M., Senokuchi T., Asano T., et al. 2009. Activation of AMP-activated protein kinase suppresses oxidized low-density lipoprotein-induced macrophage proliferation. J. Biol. Chem. 284: 34561–34569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Carlier M. F., Pantaloni D. 2007. Control of actin assembly dynamics in cell motility. J. Biol. Chem. 282: 23005–23009 [DOI] [PubMed] [Google Scholar]
- 201.Kanellis J., Kandane R. K., Etemadmoghadam D., Fraser S. A., Mount P. F., Levidiotis V., Kemp B. E., Power D. A. 2006. Activators of the energy sensing kinase AMPK inhibit random cell movement and chemotaxis in U937 cells. Immunol. Cell Biol. 84: 6–12 [DOI] [PubMed] [Google Scholar]
- 202.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., Hotamisligil G. S. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 306: 457–461 [DOI] [PubMed] [Google Scholar]
- 203.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Napoli C. 2003. Oxidation of LDL, atherogenesis, and apoptosis. Ann. N. Y. Acad. Sci. 1010: 698–709 [DOI] [PubMed] [Google Scholar]
- 205.Artwohl M., Graier W. F., Roden M., Bischof M., Freudenthaler A., Waldhausl W., Baumgartner-Parzer S. M. 2003. Diabetic LDL triggers apoptosis in vascular endothelial cells. Diabetes. 52: 1240–1247 [DOI] [PubMed] [Google Scholar]
- 206.Dong Y., Zhang M., Liang B., Xie Z., Zhao Z., Asfa S., Choi H. C., Zou M. H. 2010. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 121: 792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dong Y., Zhang M., Wang S., Liang B., Zhao Z., Liu C., Wu M., Choi H. C., Lyons T. J., Zou M. H. 2010. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 59: 1386–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fernández-Sánchez A., Madrigal-Santillan E., Bautista M., Esquivel-Soto J., Morales-Gonzalez A., Esquivel-Chirino C., Durante-Montiel I., Sanchez-Rivera G., Valadez-Vega C., Morales-Gonzalez J. A. 2011. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 12: 3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cave A. C., Brewer A. C., Narayanapanicker A., Ray R., Grieve D. J., Walker S., Shah A. M. 2006. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 8: 691–728 [DOI] [PubMed] [Google Scholar]
- 210.Ewart M. A., Kohlhaas C. F., Salt I. P. 2008. Inhibition of tumor necrosis factor alpha-stimulated monocyte adhesion to human aortic endothelial cells by AMP-activated protein kinase. Arterioscler. Thromb. Vasc. Biol. 28: 2255–2257 [DOI] [PubMed] [Google Scholar]
- 211.Wang S., Xu J., Song P., Viollet B., Zou M. H. 2009. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 58: 1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kukidome D., Nishikawa T., Sonoda K., Imoto K., Fujisawa K., Yano M., Motoshima H., Taguchi T., Matsumura T., Araki E. 2006. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 55: 120–127 [PubMed] [Google Scholar]
- 213.Xie Z., Zhang J., Wu J., Viollet B., Zou M. H. 2008. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 57: 3222–3230 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 214.Ceolotto G., Gallo A., Papparella I., Franco L., Murphy E., Iori E., Pagnin E., Fadini G. P., Albiero M., Semplicini A., et al. 2007. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 27: 2627–2633 [DOI] [PubMed] [Google Scholar]
- 215.Alba G., El Bekay R., Alvarez-Maqueda M., Chacon P., Vega A., Monteseirin J., Santa Maria C., Pintado E., Bedoya F. J., Bartrons R., et al. 2004. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 573: 219–225 [DOI] [PubMed] [Google Scholar]
- 216.Salminen A., Hyttinen J. M., Kaarniranta K. 2011. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. (Berl.) 89: 667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang S. R., Wright J., Bauter M., Seweryniak K., Kode A., Rahman I. 2007. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am. J. Physiol. Lung Cell. Mol. Physiol. 292: L567–L576 [DOI] [PubMed] [Google Scholar]
- 218.Yang Z., Kahn B. B., Shi H., Xue B. Z. 2010. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 285: 19051–19059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schug T. T., Xu Q., Gao H., Peres-da-Silva A., Draper D. W., Fessler M. B., Purushotham A., Li X. 2010. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol. 30: 4712–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Alvarez-Guardia D., Palomer X., Coll T., Davidson M. M., Chan T. O., Feldman A. M., Laguna J. C., Vazquez-Carrera M. 2010. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc. Res. 87: 449–458 [DOI] [PubMed] [Google Scholar]
- 221.Kim H. J., Park K. G., Yoo E. K., Kim Y. H., Kim Y. N., Kim H. S., Kim H. T., Park J. Y., Lee K. U., Jang W. G., et al. 2007. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid. Redox Signal. 9: 301–307 [DOI] [PubMed] [Google Scholar]
- 222.Winder W. W., Hardie D. G. 1999. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 277: E1–E10 [DOI] [PubMed] [Google Scholar]
- 223.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. 2003. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Davies M. J., Judd J. T., Baer D. J., Clevidence B. A., Paul D. R., Edwards A. J., Wiseman S. A., Muesing R. A., Chen S. C. 2003. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J. Nutr. 133: 3298S–3302S [DOI] [PubMed] [Google Scholar]
- 225.Sanders M. J., Ali Z. S., Hegarty B. D., Heath R., Snowden M. A., Carling D. 2007. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J. Biol. Chem. 282: 32539–32548 [DOI] [PubMed] [Google Scholar]
- 226.Boudeau J., Scott J. W., Resta N., Deak M., Kieloch A., Komander D., Hardie D. G., Prescott A. R., van Aalten D. M., Alessi D. R. 2004. Analysis of the LKB1-STRAD-MO25 complex. J. Cell Sci. 117: 6365–6375 [DOI] [PubMed] [Google Scholar]
- 227.Xiao B., Heath R., Saiu P., Leiper F. C., Leone P., Jing C., Walker P. A., Haire L., Eccleston J. F., Davis C. T., et al. 2007. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 449: 496–500 [DOI] [PubMed] [Google Scholar]
- 228.Suwa M., Nakano H., Kumagai S. 2003. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 95: 960–968 [DOI] [PubMed] [Google Scholar]
- 229.Lochhead P. A., Salt I. P., Walker K. S., Hardie D. G., Sutherland C. 2000. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 49: 896–903 [DOI] [PubMed] [Google Scholar]
- 230.Lee Y. S., Kim W. S., Kim K. H., Yoon M. J., Cho H. J., Shen Y., Ye J. M., Lee C. H., Oh W. K., Kim C. T., et al. 2006. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 55: 2256–2264 [DOI] [PubMed] [Google Scholar]
- 231.Ahn J., Lee H., Kim S., Park J., Ha T. 2008. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 373: 545–549 [DOI] [PubMed] [Google Scholar]
- 232.Suchankova G., Nelson L. E., Gerhart-Hines Z., Kelly M., Gauthier M. S., Saha A. K., Ido Y., Puigserver P., Ruderman N. B. 2009. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem. Biophys. Res. Commun. 378: 836–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fryer L. G., Parbu-Patel A., Carling D. 2002. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277: 25226–25232 [DOI] [PubMed] [Google Scholar]
- 234.Hawley S. A., Gadalla A. E., Olsen G. S., Hardie D. G. 2002. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 51: 2420–2425 [DOI] [PubMed] [Google Scholar]
- 235.Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., et al. 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 444: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hawley S. A., Ross F. A., Chevtzoff C., Green K. A., Evans A., Fogarty S., Towler M. C., Brown L. J., Ogunbayo O. A., Evans A. M., et al. 2010. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 11: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xie Z., Dong Y., Scholz R., Neumann D., Zou M. H. 2008. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 117: 952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jin H. E., Hong S. S., Choi M. K., Maeng H. J., Kim D. D., Chung S. J., Shim C. K. 2009. Reduced antidiabetic effect of metformin and down-regulation of hepatic Oct1 in rats with ethynylestradiol-induced cholestasis. Pharm. Res. 26: 549–559 [DOI] [PubMed] [Google Scholar]
- 239.Shu Y., Sheardown S. A., Brown C., Owen R. P., Zhang S., Castro R. A., Ianculescu A. G., Yue L., Lo J. C., Burchard E. G., et al. 2007. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Invest. 117: 1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.El-Mir M. Y., Nogueira V., Fontaine E., Averet N., Rigoulet M., Leverve X. 2000. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275: 223–228 [DOI] [PubMed] [Google Scholar]
- 241.Owen M. R., Doran E., Halestrap A. P. 2000. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348: 607–614 [PMC free article] [PubMed] [Google Scholar]
- 242.Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. 2005. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 310: 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guigas B., Bertrand L., Taleux N., Foretz M., Wiernsperger N., Vertommen D., Andreelli F., Viollet B., Hue L. 2006. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes. 55: 865–874 [DOI] [PubMed] [Google Scholar]
- 244.Spiegelman B. M. 1998. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 47: 507–514 [DOI] [PubMed] [Google Scholar]
- 245.Srivastava R. A. 2009. Fenofibrate ameliorates diabetic and dyslipidemic profiles in KKAy mice partly via down-regulation of 11beta-HSD1, PEPCK and DGAT2. Comparison of PPARalpha, PPARgamma, and liver X receptor agonists. Eur. J. Pharmacol. 607: 258–263 [DOI] [PubMed] [Google Scholar]
- 246.Srivastava R. A. 2011. Evaluation of anti-atherosclerotic activities of PPAR-alpha, PPAR-gamma, and LXR agonists in hyperlipidemic atherosclerosis-susceptible F(1)B hamsters. Atherosclerosis. 214: 86–93 [DOI] [PubMed] [Google Scholar]
- 247.Saha A. K., Avilucea P. R., Ye J. M., Assifi M. M., Kraegen E. W., Ruderman N. B. 2004. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem. Biophys. Res. Commun. 314: 580–585 [DOI] [PubMed] [Google Scholar]
- 248.Schimmack G., Defronzo R. A., Musi N. 2006. AMP-activated protein kinase: Role in metabolism and therapeutic implications. Diabetes Obes. Metab. 8: 591–602 [DOI] [PubMed] [Google Scholar]
- 249.Bays H., Mandarino L., DeFronzo R. A. 2004. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 89: 463–478 [DOI] [PubMed] [Google Scholar]
- 250.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K. 2007. Adiponectin and adiponectin receptors in obesity-linked insulin resistance. Novartis Found. Symp. 286: 164–176; discussion 176–182, 200–203 [DOI] [PubMed] [Google Scholar]
- 251.Mootha V. K., Lindgren C. M., Eriksson K. F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurila E., et al. 2003. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34: 267–273 [DOI] [PubMed] [Google Scholar]
- 252.Zhang F., Dey D., Branstrom R., Forsberg L., Lu M., Zhang Q., Sjoholm A. 2009. BLX-1002, a novel thiazolidinedione with no PPAR affinity, stimulates AMP-activated protein kinase activity, raises cytosolic Ca2+, and enhances glucose-stimulated insulin secretion in a PI3K-dependent manner. Am. J. Physiol. Cell Physiol. 296: C346–C354 [DOI] [PubMed] [Google Scholar]
- 253.Chen Z., Vigueira P. A., Chambers K. T., Hall A. M., Mitra M. S., Qi N., McDonald W. G., Colca J. R., Kletzien R. F., Finck B. N. 2012. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor gamma-sparing thiazolidinedione. J. Biol. Chem. 287: 23537–23548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cederroth C. R., Vinciguerra M., Gjinovci A., Kuhne F., Klein M., Cederroth M., Caille D., Suter M., Neumann D., James R. W., et al. 2008. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes. 57: 1176–1185 [DOI] [PubMed] [Google Scholar]
- 255.Collins Q. F., Liu H. Y., Pi J., Liu Z., Quon M. J., Cao W. 2007. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J. Biol. Chem. 282: 30143–30149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bhatia I. S., Ullah R. 1962. Metabolism of polyphenols in the tea leaf. Nature. 193: 658–659 [DOI] [PubMed] [Google Scholar]
- 257.Maron D. J., Lu G. P., Cai N. S., Wu Z. G., Li Y. H., Chen H., Zhu J. Q., Jin X. J., Wouters B. C., Zhao J. 2003. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch. Intern. Med. 163: 1448–1453 [DOI] [PubMed] [Google Scholar]
- 258.Koo S. I., Noh S. K. 2007. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 18: 179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Singh D. K., Banerjee S., Porter T. D. 2009. Green and black tea extracts inhibit HMG-CoA reductase and activate AMP kinase to decrease cholesterol synthesis in hepatoma cells. J. Nutr. Biochem. 20: 816–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lan F., Cacicedo J. M., Ruderman N., Ido Y. 2008. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 283: 27628–27635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dasgupta B., Milbrandt J. 2007. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA. 104: 7217–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hwang S. L., Yang B. K., Lee J. Y., Kim J. H., Kim B. D., Suh K. H., Kim D. Y., Kim M. S., Song H., Park B. S., et al. 2008. Isodihydrocapsiate stimulates plasma glucose uptake by activation of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 371: 289–293 [DOI] [PubMed] [Google Scholar]
- 263.Li H. B., Ge Y. K., Zheng X. X., Zhang L. 2008. Salidroside stimulated glucose uptake in skeletal muscle cells by activating AMP-activated protein kinase. Eur. J. Pharmacol. 588: 165–169 [DOI] [PubMed] [Google Scholar]
- 264.López-Lázaro M. 2008. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 52(Suppl. 1): S103–S127 [DOI] [PubMed] [Google Scholar]
- 265.Pan W., Yang H., Cao C., Song X., Wallin B., Kivlin R., Lu S., Hu G., Di W., Wan Y. 2008. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol. Rep. 20: 1553–1559 [PubMed] [Google Scholar]
- 266.Kanitkar M., Gokhale K., Galande S., Bhonde R. R. 2008. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br. J. Pharmacol. 155: 702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fujiwara H., Hosokawa M., Zhou X., Fujimoto S., Fukuda K., Toyoda K., Nishi Y., Fujita Y., Yamada K., Yamada Y., et al. 2008. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res. Clin. Pract. 80: 185–191 [DOI] [PubMed] [Google Scholar]
- 268.Gruzman A., Shamni O., Ben Yakir M., Sandovski D., Elgart A., Alpert E., Cohen G., Hoffman A., Katzhendler Y., Cerasi E., et al. 2008. Novel D-xylose derivatives stimulate muscle glucose uptake by activating AMP-activated protein kinase alpha. J. Med. Chem. 51: 8096–8108 [DOI] [PubMed] [Google Scholar]
- 269.Turner N., Li J. Y., Gosby A., To S. W., Cheng Z., Miyoshi H., Taketo M. M., Cooney G. J., Kraegen E. W., James D. E., et al. 2008. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 57: 1414–1418 [DOI] [PubMed] [Google Scholar]
- 270.Yin J., Hu R., Chen M., Tang J., Li F., Yang Y., Chen J. 2002. Effects of berberine on glucose metabolism in vitro. Metabolism. 51: 1439–1443 [DOI] [PubMed] [Google Scholar]
- 271.Jeong H. W., Hsu K. C., Lee J. W., Ham M., Huh J. Y., Shin H. J., Kim W. S., Kim J. B. 2009. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 296: E955–E964 [DOI] [PubMed] [Google Scholar]
- 272.Park K. G., Min A. K., Koh E. H., Kim H. S., Kim M. O., Park H. S., Kim Y. D., Yoon T. S., Jang B. K., Hwang J. S., et al. 2008. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology. 48: 1477–1486 [DOI] [PubMed] [Google Scholar]
- 273.Shen Q. W., Zhu M. J., Tong J., Ren J., Du M. 2007. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am. J. Physiol. Cell Physiol. 293: C1395–C1403 [DOI] [PubMed] [Google Scholar]
- 274.Clark H., Carling D., Saggerson D. 2004. Covalent activation of heart AMP-activated protein kinase in response to physiological concentrations of long-chain fatty acids. Eur. J. Biochem. 271: 2215–2224 [DOI] [PubMed] [Google Scholar]
- 275.de Lange P., Farina P., Moreno M., Ragni M., Lombardi A., Silvestri E., Burrone L., Lanni A., Goglia F. 2006. Sequential changes in the signal transduction responses of skeletal muscle following food deprivation. FASEB J. 20: 2579–2581 [DOI] [PubMed] [Google Scholar]
- 276.Fediuc S., Gaidhu M. P., Ceddia R. B. 2006. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. J. Lipid Res. 47: 412–420 [DOI] [PubMed] [Google Scholar]
- 277.Watt M. J., Steinberg G. R., Chen Z. P., Kemp B. E., Febbraio M. A. 2006. Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J. Physiol. 574: 139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bar-Tana J., Rose-Kahn G., Srebnik M. 1985. Inhibition of lipid synthesis by beta beta’-tetramethyl-substituted, C14–C22, alpha, omega-dicarboxylic acids in the rat in vivo. J. Biol. Chem. 260: 8404–8410 [PubMed] [Google Scholar]
- 279.Bar-Tana J., Ben-Shoshan S., Blum J., Migron Y., Hertz R., Pill J., Rose-Khan G., Witte E. C. 1989. Synthesis and hypolipidemic and antidiabetogenic activities of beta,beta,beta’,beta’-tetrasubstituted, long-chain dioic acids. J. Med. Chem. 32: 2072–2084 [DOI] [PubMed] [Google Scholar]
- 280.Za'tara G., Bar-Tana J., Kalderon B., Suter M., Morad E., Samovski D., Neumann D., Hertz R. 2008. AMPK activation by long chain fatty acyl analogs. Biochem. Pharmacol. 76: 1263–1275 [DOI] [PubMed] [Google Scholar]
- 281.Hermesh O., Kalderon B., Bar-Tana J. 1998. Mitochondria uncoupling by a long chain fatty acyl analogue. J. Biol. Chem. 273: 3937–3942 [DOI] [PubMed] [Google Scholar]
- 282.Cramer C. T., Goetz B., Hopson K. L., Fici G. J., Ackermann R. M., Brown S. C., Bisgaier C. L., Rajeswaran W. G., Oniciu D. C., Pape M. E. 2004. Effects of a novel dual lipid synthesis inhibitor and its potential utility in treating dyslipidemia and metabolic syndrome. J. Lipid Res. 45: 1289–1301 [DOI] [PubMed] [Google Scholar]
- 283.Pinkosky S. L., Cramer C. T., Hurley T. R., Bradshaw C. D., Spahr M. A., Srivastava R. A. K., Newton R. S.2011. ETC-1002 regulates key pathways of lipid and carbohydrate metabolism in primary rat hepatocytes through a novel acyl-CoA synthetase-dependent AMP-activated protein kinase mechanism (Abstract 331). ATVB Scientific Session. 117.
- 284.Srivastava R. A., Hurley T. R., Brant A. F., Bradshaw C. D., Hanselman J. C., Baker C., Naples M., Adeli K., Newton R. S.2011. A novel small molecule, ETC-1002, lowers proatherogenic lipoproteins, reduces adiposity, and improves hepatic steatosis in a hyperlipidemic hamster model (Abstract 97). ATVB Scientific Session: 55.
- 285.Hanselman J. C., Bradshaw C. D., Brant A. F., Cramer C. T., Hurley T. R., Pinkosky S. L., Spahr M. A., Washburn J. G., Newton R. S., Srivastava R. A. K. 2011 ETC-1002 reduces circulating and hepatic triglyceride content and improves glycemic control in KKAy mice (Abstract 554). ATVB Scientific Session: 177. [Google Scholar]
- 286.MacDougall D. E., DiCarlo L. A., Milad M. A., Vanderlugt J. T., Newton R. S.2011. ETC-1002 was safe, well tolerated and reduced LDL-C in a phase 1 multiple-dose study of subjects with mild dyslipidemia (Poster 344). ATVB Scientific Session: 121.
- 287.Cramer C. T., Srivastava R. A., Lutostanski J., Bisgaier C. L., Newton R. S.2011. ETC-1002: a novel small molecule inhibitor of lipogenesis improves plasma lipid profile and inhibits atherosclerosis progression in LDLr−/− Mouse by a lipid-dependent and independent mechanism (Abstract 405). ATVB Scientific Session: 137.
- 288.Mukhtar M. H., Payne V. A., Arden C., Harbottle A., Khan S., Lange A. J., Agius L. 2008. Inhibition of glucokinase translocation by AMP-activated protein kinase is associated with phosphorylation of both GKRP and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294: R766–R774 [DOI] [PubMed] [Google Scholar]
- 289.Vincent M. F., Erion M. D., Gruber H. E., Van den Berghe G. 1996. Hypoglycaemic effect of AICAriboside in mice. Diabetologia. 39: 1148–1155 [DOI] [PubMed] [Google Scholar]
- 290.Bergeron R., Russell R. R., 3rd, Young L. H., Ren J. M., Marcucci M., Lee A., Shulman G. I. 1999. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am. J. Physiol. 276: E938–E944 [DOI] [PubMed] [Google Scholar]
- 291.Babraj J. A., Mustard K., Sutherland C., Towler M. C., Chen S., Smith K., Green K., Leese G., Hardie D. G., Rennie M. J., et al. 2009. Blunting of AICAR-induced human skeletal muscle glucose uptake in type 2 diabetes is dependent on age rather than diabetic status. Am. J. Physiol. Endocrinol. Metab. 296: E1042–E1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lihn A. S., Pedersen S. B., Lund S., Richelsen B. 2008. The anti-diabetic AMPK activator AICAR reduces IL-6 and IL-8 in human adipose tissue and skeletal muscle cells. Mol. Cell. Endocrinol. 292: 36–41 [DOI] [PubMed] [Google Scholar]
- 293.Göransson O., McBride A., Hawley S. A., Ross F. A., Shpiro N., Foretz M., Viollet B., Hardie D. G., Sakamoto K. 2007. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J. Biol. Chem. 282: 32549–32560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Treebak J. T., Birk J. B., Hansen B. F., Olsen G. S., Wojtaszewski J. F. 2009. A-769662 activates AMPK beta1-containing complexes but induces glucose uptake through a PI3-kinase-dependent pathway in mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 297: C1041–C1052 [DOI] [PubMed] [Google Scholar]
- 295.Zhou G., Sebhat I. K., Zhang B. B. 2009. AMPK activators–potential therapeutics for metabolic and other diseases. Acta Physiol. (Oxf.). 196: 175–190 [DOI] [PubMed] [Google Scholar]
- 296.Pang T., Zhang Z. S., Gu M., Qiu B. Y., Yu L. F., Cao P. R., Shao W., Su M. B., Li J. Y., Nan F. J., et al. 2008. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J. Biol. Chem. 283: 16051–16060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Oh S., Kim S. J., Hwang J. H., Lee H. Y., Ryu M. J., Park J., Jo Y. S., Kim Y. K., Lee C. H., Kweon K. R., et al. 2010. Antidiabetic and antiobesity effects of Ampkinone (6f), a novel small molecule activator of AMP-activated protein kinase. J. Med. Chem. 53: 7405–7413 [DOI] [PubMed] [Google Scholar]
- 298.Goodyear L. J. 2008. The exercise pill—too good to be true? N. Engl. J. Med. 359: 1842–1844 [DOI] [PubMed] [Google Scholar]
- 299.Fabianowska-Majewska K., Duley J. A., Simmonds H. A. 1994. Effects of novel anti-viral adenosine analogues on the activity of S-adenosylhomocysteine hydrolase from human liver. Biochem. Pharmacol. 48: 897–903 [DOI] [PubMed] [Google Scholar]
- 300.Charton J., Girault-Mizzi S., Debreu-Fontaine M. A., Foufelle F., Hainault I., Bizot-Espiard J. G., Caignard D. H., Sergheraert C. 2006. Synthesis and biological evaluation of benzimidazole derivatives as potent AMP-activated protein kinase activators. Bioorg. Med. Chem. 14: 4490–4518 [DOI] [PubMed] [Google Scholar]
- 301.Chen Y., Zhou K., Wang R., Liu Y., Kwak Y. D., Ma T., Thompson R. C., Zhao Y., Smith L., Gasparini L., et al. 2009. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc. Natl. Acad. Sci. USA. 106: 3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Becker M. L., Visser L. E., van Schaik R. H., Hofman A., Uitterlinden A. G., Stricker B. H. 2009. Genetic variation in the multidrug and toxin extrusion 1 transporter protein influences the glucose-lowering effect of metformin in patients with diabetes: a preliminary study. Diabetes. 58: 745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Scott J. W., van Denderen B. J., Jorgensen S. B., Honeyman J. E., Steinberg G. R., Oakhill J. S., Iseli T. J., Koay A., Gooley P. R., Stapleton D., et al. 2008. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem. Biol. 15: 1220–1230 [DOI] [PubMed] [Google Scholar]