Abstract
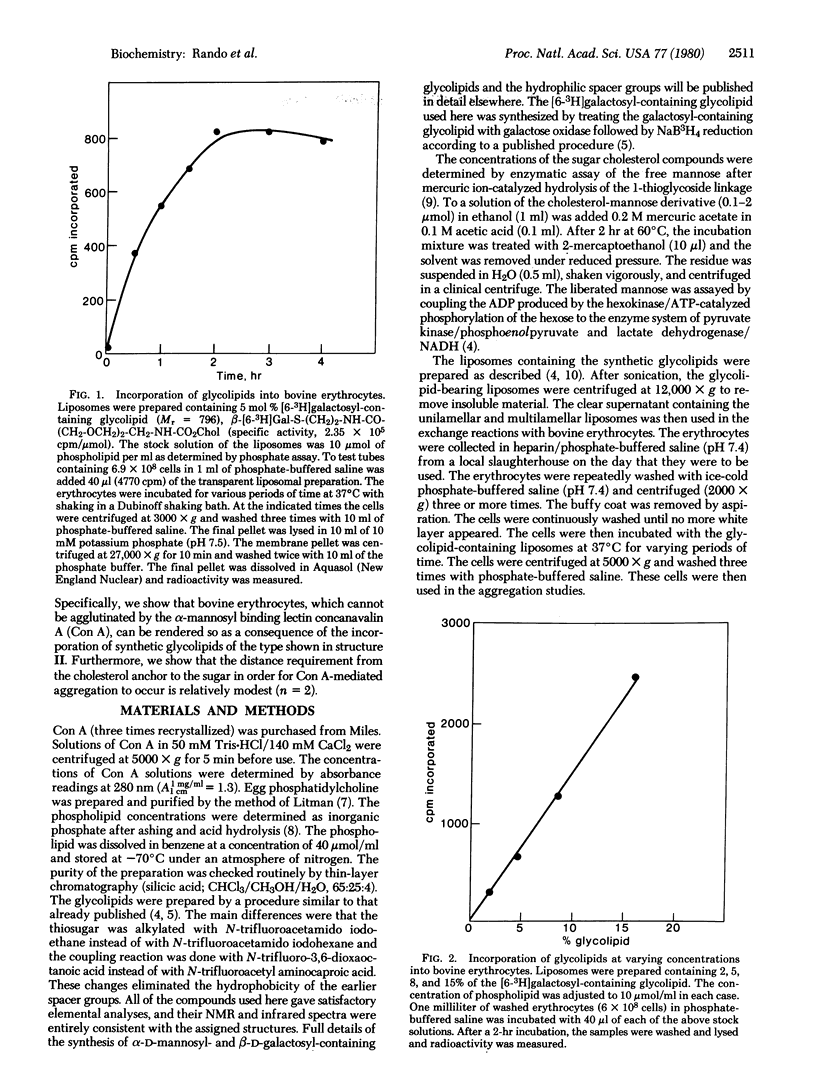
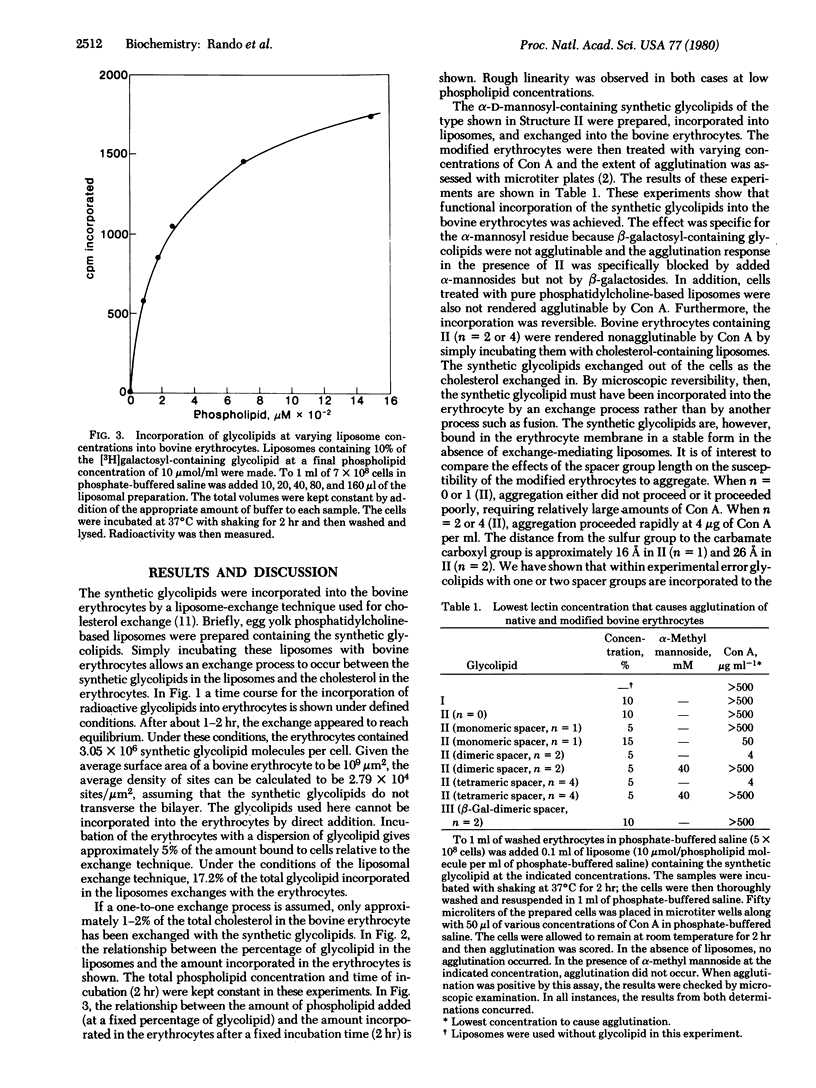
Synthetic glycolipids containing an alpha-mannoside group linked by a hydrophilic spacer arm to cholesterol were incorporated into bovine erythrocytes by exchange from glycolipid-containing liposomes. When the distance between the sugar and the cholesterol moieties was approximately 26 A, functional incorporation of these glycolipids could be easily detected, as revealed by the concanavalin A-mediated agglutination of these cells. Bovine erythrocytes are not themselves susceptible to concanavalin A-mediated agglutination. The minimal concentration of concanavalin A required for agglutination of modified erythrocytes, containing 9.15 x 10(6) glycolipid molecules per cell, was 4 microgram/ml. Under these conditions, only approximately 4% of the membrane-bound cholesterol had been exchanged for the synthetic glycolipid. The observed aggregation was reversible in the presence of alpha-methyl mannoside and did not occur when beta-galactosyl-containing glycolipids were used in place of their alpha-mannoside isomers. These studies demonstrate a technique of sugar incorporation into cell membranes which should be of great advantage in studies on the roles of cell surface sugars in biological recognition. Furthermore, they demonstrate that the sugars need only be a short distance (26 A) from the membrane in order to functionally bind concanavalin A.
Full text
PDF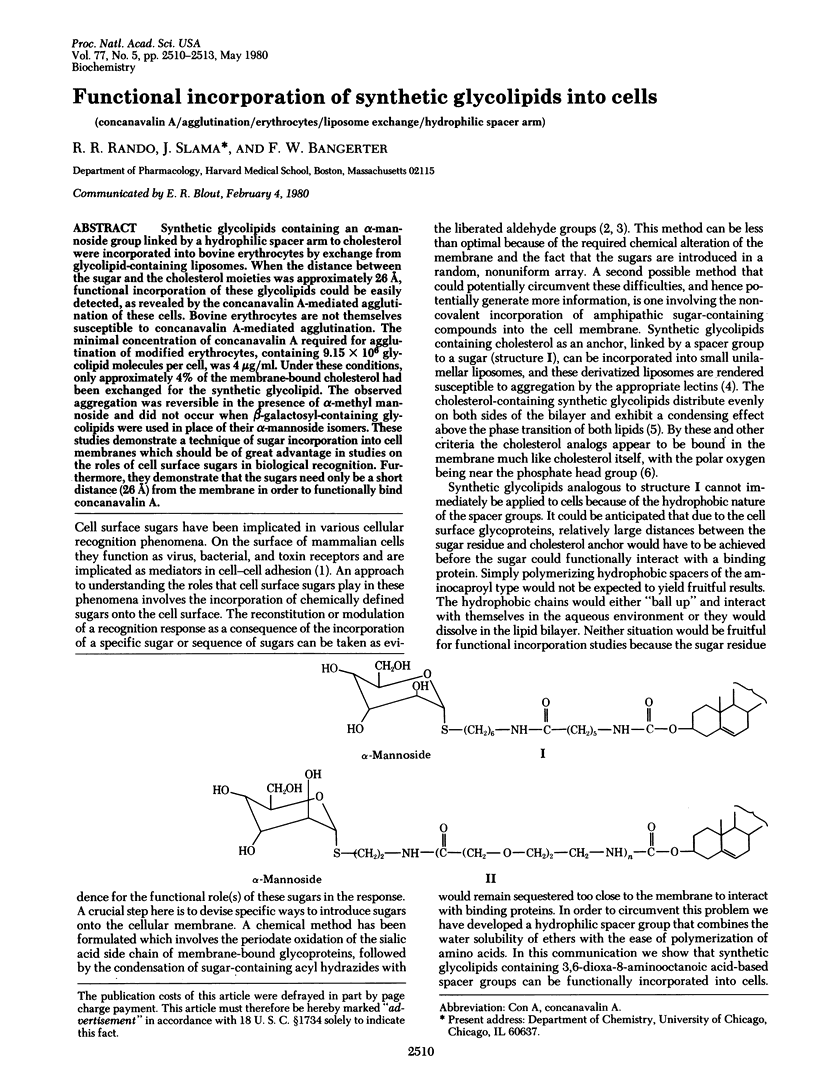

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Barenholz Y., Gibbes D., Litman B. J., Goll J., Thompson T. E., Carlson R. D. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977 Jun 14;16(12):2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- Huang C., Charlton J. P., Shyr C. I., Thompson T. E. Studies on phosphatidylcholine vesicles with thiocholesterol and a thiocholesterol-linked spin label incorporated in the vesicle wall. Biochemistry. 1970 Aug 18;9(17):3422–3426. doi: 10.1021/bi00819a021. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Lee Y. C. Quantitative hydrolysis of thioglycosides. Anal Biochem. 1976 Mar;71(1):318–321. doi: 10.1016/0003-2697(76)90044-0. [DOI] [PubMed] [Google Scholar]
- Litman B. J. Lipid model membranes. Characterization of mixed phospholipid vesicles. Biochemistry. 1973 Jun 19;12(13):2545–2554. doi: 10.1021/bi00737a028. [DOI] [PubMed] [Google Scholar]
- Orr G. A., Rando R. R., Bangerter F. W. Synthetic glycolipids and the lectin-mediated aggregation of liposomes. J Biol Chem. 1979 Jun 10;254(11):4721–4725. [PubMed] [Google Scholar]
- Orr G. A., Rando R. R. Synthetic concanavalin A receptors and erythrocyte agglutination. Nature. 1978 Apr 20;272(5655):722–725. doi: 10.1038/272722a0. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Bangerter F. W. Threshold effects on the lectin-mediated aggregation of synthetic glycolipid-containing liposomes. J Supramol Struct. 1979;11(3):295–309. doi: 10.1002/jss.400110304. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Orr G. A., Bangerter F. W. Threshold effects on the concanavalin A-mediated agglutination of modified erythrocytes. J Biol Chem. 1979 Sep 10;254(17):8318–8323. [PubMed] [Google Scholar]





