Abstract
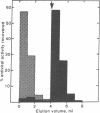
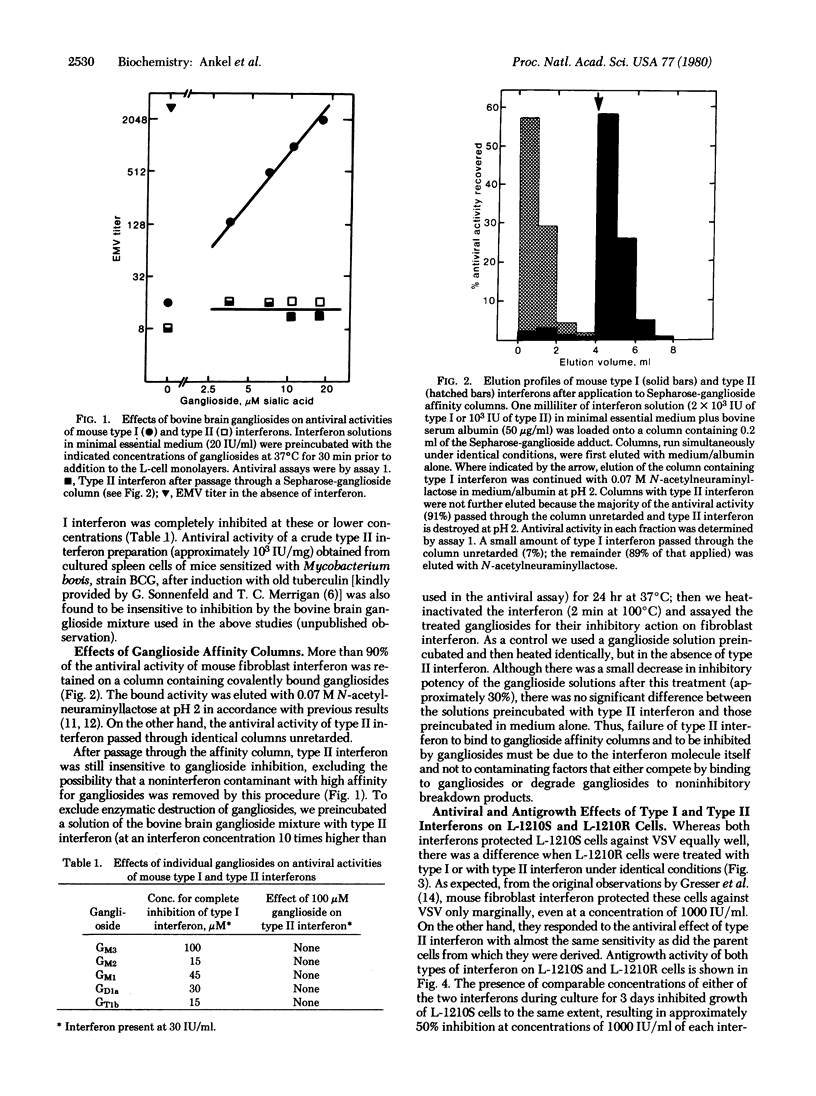
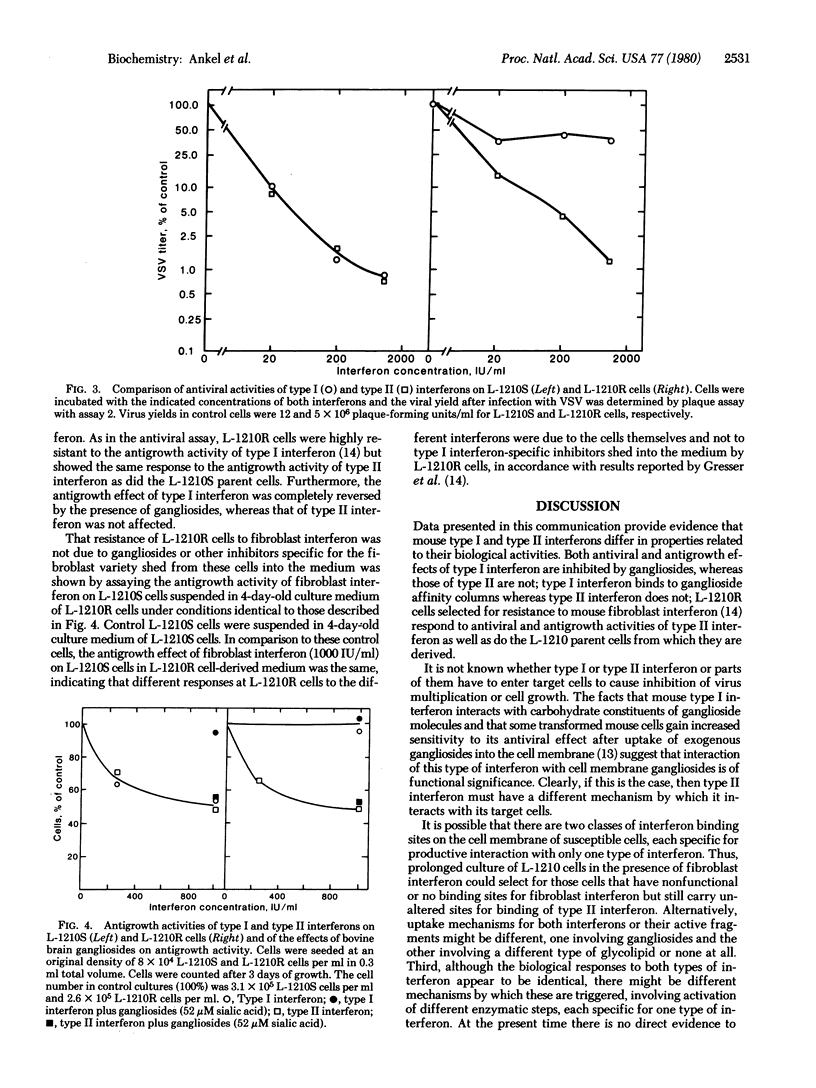
Different interferons can be obtained from the same animal species depending on the cells and (or) the inducers used. Interferons of type I and type II differ not only antigenically but also in molecular weight and stability at low pH. We have investigated whether mouse type I and type II interferons also differ in properties relating to their biological action. We present evidence which suggests that the molecular mechanism leading to antiviral and antigrowth effects induced by both types of interferon in susceptible cells must differ in at least one important step. Antiviral and antigrowth activities of type I but not of type II interferon are both inhibited when gangliosides are added to cell cultures together with the interferon. Whereas type I interferon strongly binds to ganglioside affinity columns and can be eluted with solutions of N-acetylneuraminyllactose, type II interferon passes through such columns unretarded. L-1210 mouse leukemia cells (L-1210S) respond equally well to antiviral and antigrowth activities of type I and type II interferons. Type I interferon-resistant L-1210 cells (L-1210R), derived from L-1210S cells after continuous culture in the presence of mouse fibroblast interferon, lack antiviral and antigrowth response to mouse type I interferon [Gresser, I., Bandu, H.T. & Brouty-Boyé, D. (1974) J. Natl. Cancer Inst. 52, 553-559]. However, these cells display the same sensitivity toward type II interferon as do the parent L-1210S cells from which they were derived and respond equally well to its antiviral and antigrowth activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besancon F., Ankel H., Basu S. Specificity and reversibility of interferon ganglioside interaction. Nature. 1976 Feb 19;259(5544):576–578. doi: 10.1038/259576a0. [DOI] [PubMed] [Google Scholar]
- Besancon F., Ankel H. Binding of interferon to gangliosides. Nature. 1974 Dec 6;252(5483):478–480. doi: 10.1038/252478a0. [DOI] [PubMed] [Google Scholar]
- Besancon F., Ankel H. Inhibition of interferon action by plant lectins. Nature. 1974 Aug 30;250(5469):784–786. doi: 10.1038/250784a0. [DOI] [PubMed] [Google Scholar]
- Besançon F., Ankel H. Decreased sensitivity of an interferon-resistant subline of murine leukemia L-1210 cells to toxic effects of ricin and abrin. Biochem Biophys Res Commun. 1979 Jun 13;88(3):818–825. doi: 10.1016/0006-291x(79)91481-5. [DOI] [PubMed] [Google Scholar]
- Besançon F., Ankel H. Membrane receptors for interferon. Tex Rep Biol Med. 1977;35:282–292. [PubMed] [Google Scholar]
- Boldt D. H., Speckart S. F., Richards R. L., Alving C. R. Interactions of lectins with glycolipids in liposomes. Biochem Biophys Res Commun. 1977 Jan 10;74(1):208–214. doi: 10.1016/0006-291x(77)91395-x. [DOI] [PubMed] [Google Scholar]
- Bourgeade M. F., Chany C., Merigan T. C. Type I and type II interferons: differential antiviral actions in transformed cells. J Gen Virol. 1980 Feb;46(2):449–454. doi: 10.1099/0022-1317-46-2-449. [DOI] [PubMed] [Google Scholar]
- CRAIGHEAD J. E., SHELOKOV A. Encephalomyocarditis virus hemagglutination-inhibition test using antigens prepared in HeLa cell cultures. Proc Soc Exp Biol Med. 1961 Dec;108:823–826. doi: 10.3181/00379727-108-27080. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I., Hollenberg M. D. Affinity chromatography and structural analysis of Vibrio cholerae enterotoxin-ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. Biochemistry. 1973 Oct 9;12(21):4253–4264. doi: 10.1021/bi00745a033. [DOI] [PubMed] [Google Scholar]
- De Maeyer-Guignard J., Tovey M. G., Gresser I., De Maeyer E. Purification of mouse interferon by sequential affinity chromatography on poly(U)--and antibody--agarose columns. Nature. 1978 Feb 16;271(5646):622–625. doi: 10.1038/271622a0. [DOI] [PubMed] [Google Scholar]
- Falcoff R. Some properties of virus and immune-induced human lymphocyte interferons. J Gen Virol. 1972 Aug;16(2):251–253. doi: 10.1099/0022-1317-16-2-251. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-Boyé D. Interferon and cell division. IX. Interferon-resistant L1210 cells: characteristics and origin. J Natl Cancer Inst. 1974 Feb;52(2):553–559. doi: 10.1093/jnci/52.2.553. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Kloppel T. M., Keenan T. W., Freeman M. J., Morré D. J. Glycolipid-bound sialic acid in serum: increased levels in mice and humans bearing mammary carcinomas. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3011–3013. doi: 10.1073/pnas.74.7.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L. C., Georgiades J. A., Johnson H. M. Large-scale production and partial purification of mouse immune interferon. Infect Immun. 1979 Jan;23(1):80–86. doi: 10.1128/iai.23.1.80-86.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoukalian J., Zwingelstein G., Abdul-Malak N., Doré J. F. Alteration of gangliosides in plasma and red cells of humans bearing melanoma tumors. Biochem Biophys Res Commun. 1978 Dec 14;85(3):916–920. doi: 10.1016/0006-291x(78)90630-7. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Sica V., Parikh I., Nola E., Puca G. A., Cuatrecasas P. Affinity chromatography and the purification of estrogen receptors. J Biol Chem. 1973 Sep 25;248(18):6543–6558. [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. The immunosuppressive effect of type II mouse interferon preparations on antibody production. Cell Immunol. 1977 Dec;34(2):193–206. doi: 10.1016/0008-8749(77)90243-x. [DOI] [PubMed] [Google Scholar]
- Surolia A., Bachhawat B. K., Podder S. K. Interaction between lectin from Ricinus communis and liposomes containing gangliosides. Nature. 1975 Oct 30;257(5529):802–804. doi: 10.1038/257802a0. [DOI] [PubMed] [Google Scholar]
- Vengris V. E., Reynolds F. H., Jr, Hollenberg M. D., Pitha P. M. Interferon action: role of membrane gangliosides. Virology. 1976 Jul 15;72(2):486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wietzerbin J., Stefanos S., Lucero M., Falcoff E., Thang D. C., Thang M. N. Presence of a polynucleotide binding site on murine immune interferon (T-type). Biochem Biophys Res Commun. 1978 Nov 14;85(1):480–489. doi: 10.1016/s0006-291x(78)80067-9. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]