Abstract
Bone morphogenetic protein (BMP) inhibits neural specification and induces epidermal differentiation during ectodermal patterning. However, the mechanism of this process is not well understood. Here we show that AP2γ, a transcription factor activator protein (AP)-2 family member, is upregulated by BMP4 during neural differentiation of pluripotent stem cells. Knockdown of AP2γ facilitates mouse embryonic stem cell (ESC) neural fate determination and impairs epidermal differentiation, whereas AP2γ overexpression inhibits neural conversion and promotes epidermal commitment. In the early chick embryo, AP2γ is expressed in the entire epiblast before HH stage 3 and gradually shifts to the putative epidermal ectoderm during HH stage 4. In the future neural plate AP2γ inhibits excessive neural expansion and it also promotes epidermal development in the surface ectoderm. Moreover, AP2γ knockdown in ESCs and chick embryos partially rescued the neural inhibition and epidermal induction effects of BMP4. Mechanistic studies showed that BMP4 directly regulates AP2γ expression through Smad1 binding to the AP2γ promoter. Taken together, we propose that during the early stages of ectodermal patterning in the chick embryo, AP2γ acts downstream of the BMP pathway to restrict precocious neural expansion in the prospective neural plate and initiates epidermal differentiation in the future epidermal ectoderm.
Keywords: AP2γ, BMP, neural and epidermal development, ectodermal patterning, ESC, chick embryo
Introduction
During gastrulation, an early phase in vertebrate embryonic development, cell movements result in a massive reorganization of the embryo from the single-layered blastula to the gastrula with three germ layers: the outer ectoderm, the inner endoderm and the interstitial mesoderm. The ectodermal precursor cells are patterned under the action of inductive signals from the neighboring tissues to produce neural or epidermal cells1,2. It has been shown in the chick embryo that different ectodermal regions have differential competency to respond to inductive signals3,4,5,6. Studies in Xenopus, chick and zebrafish demonstrate that bone morphogenetic protein (BMP) signaling plays important roles in neural/epidermal fate determination7,8,9. The “default model” proposes that BMP inhibits the “default” neural tendency of the ectodermal cells and induces them to adopt an epidermal fate, while BMP antagonists (noggin, chordin, and follistatin) from the organizer inhibit BMP signaling to protect the default neural fate7,10,11. The study in mouse embryo indicates that BMP signaling is required for inhibiting premature neural differentiation12, and the involvement of Fgf and Wnt signaling pathways in neural induction has been established by the evidence from Xenopus and chick embryo1,2,13,14,15,16.
BMP signal is transduced from the extracellular environment to the nucleus via Smad1/5/8 phosphorylation to regulate the expression of many target genes. Among them, Id1 sustains mouse embryonic stem cell (ESC) self-renewal17, and Dlx5 and Tlx2 act downstream of BMP to regulate mesoderm development18. Only Xenopus Msx119 and zebrafish ΔNp6320 are negative regulators of neural differentiation. To the best of our knowledge, there are no reports on BMP downstream targets that are involved in neural and epidermal differentiation of mammalian cells, and the functional effectors downstream of BMP signaling in ectodermal patterning remain unclear.
AP2γ (also known as Tcfap2c) belongs to the AP2 transcription factor family21, which plays important roles in proliferation, differentiation and embryonic development with diverse expression patterns22,23,24. Among the AP2 factors, AP2α, AP2β and AP2γ are reported to be the key regulators in neural crest development25,26,27,28, which is generally thought to occur following the neural plate formation. Conditional disruption of Smad4 in neural crest cells results in AP2α downregulation from embryonic day 9.5 (E9.5) in mouse embryo29. AP2α also regulates neural border specification during the neuralization stage in Xenopus and lamprey30,31. Mouse AP2γ is expressed in both extraembryonic and embryonic tissues32,33 and displays multiple functions in extraembryonic development, neural crest induction and terminal epidermal differentiation26,34,35. Moreover, disruption of AP2γ leads to mouse embryonic lethality at approximately E7.5, showing extraembryonic cell defects and abnormal embryonic gastrulation36. However, it is unclear whether AP2γ is involved in ectodermal patterning at earlier stages of embryonic development and what is the relationship between AP2γ and BMP signaling.
Here we show that AP2γ is upregulated by BMP4 during pluripotent stem cell differentiation and that AP2γ partially mediates the BMP4 functions of neural inhibition and epidermal promotion. In vivo, chick AP2γ (cAP2γ) is expressed in the epiblast before HH stage 3 and gradually shifts to the peripheral ectoderm during HH stage 4, and AP2γ acts downstream of BMP signaling to regulate neural and epidermal development in different regions of the epiblast in the early chick embryo.
Results
AP2γ knockdown facilitates neural differentiation and impairs epidermal commitment of ESCs
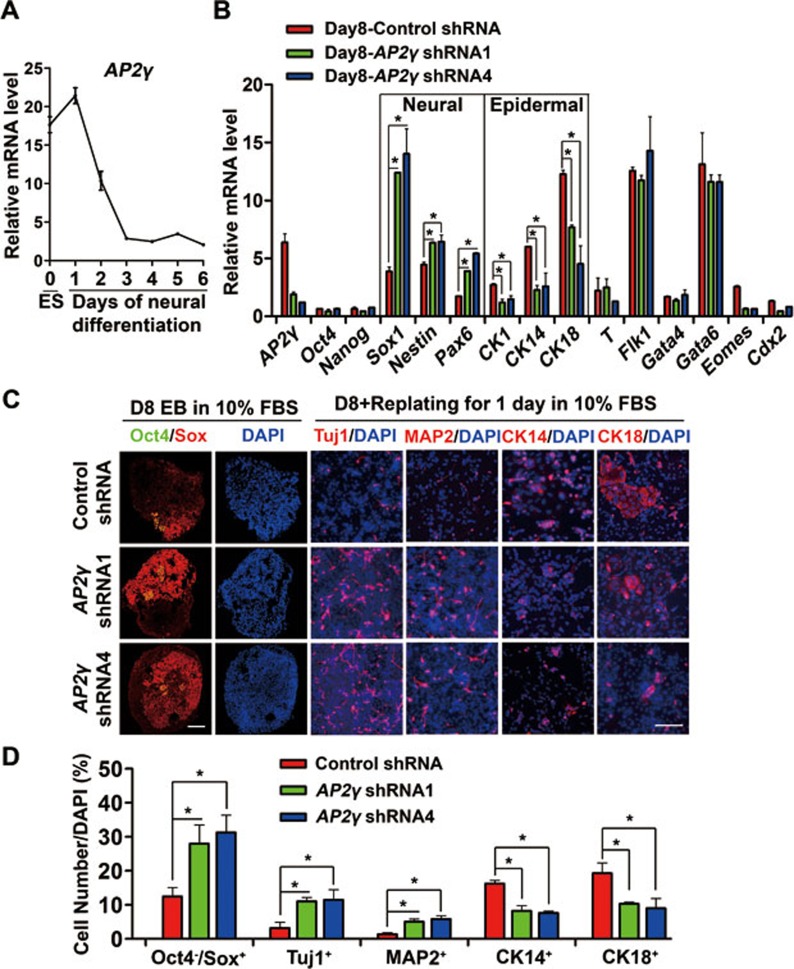
Previously, we established methods of inducing a high percentage of neural progenitor cells (NPCs) from P19 embryonic carcinoma cells and ESCs, and showed that BMP4 inhibited the neural differentiation of pluripotent stem cells37,38. To search for functional factors involved in neural inhibition by BMP4, differential gene expression microarray was performed with or without BMP4 treatment during P19 cell neural differentiation. Within the differentially expressed genes, AP2γ was found to be markedly upregulated by BMP4 at both mRNA and protein levels (Supplementary information, Figure S1). ESC neural differentiation in knockout serum replacement (KSR) medium was used as an in vitro model38,39 to study AP2γ function in early embryonic development. We found that AP2γ mRNA was expressed in undifferentiated mouse ESCs and that its level gradually decreased with progressing neural conversion (Figure 1A), suggesting that AP2γ might be involved in the neural differentiation of ESCs.
Figure 1.
AP2γ knockdown facilitates neural commitment and impairs epidermal fate determination during ESC differentiation. (A) qRT-PCR analysis of AP2γ mRNA level during neural differentiation of ESCs. EBs were cultured in KSR medium for 0-6 days and subjected to analysis. (B) ESCs were cultured in standard ES medium and transfected with control shRNA or AP2γ -specific shRNA lentivirus (shRNA1 & 4) coexpressing GFP. GFP-positive cells were then sorted by FACS and proliferated. These ESCs were cultured as EBs in DMEM containing 10% FBS. The expression of differentiation markers on day 8 was analyzed using qRT-PCR. Relative gene expression levels were normalized to the expression level of Gapdh. (C, D) Double immunostaining of Oct4 (green, artificial color) and Sox (red) proteins in day 8 EBs cultured under the conditions described in B. For EB staining in all of the following experiments, sections from thousands of EB aggregates were stained and statistical analyses were performed. Cells were replated for adherent culture in DMEM containing 10% FBS on Matrigel-coated culture dishes for 1 day and were analyzed by immunostaining for Tuj1 (red), MAP2 (red), CK14 (red) and CK18 (red). Nuclei were stained using DAPI (blue). The percentages of Oct4−/Sox+ NPCs, Tuj1+, MAP2+, CK14+ and CK18+ cells are shown in D. Scale bar, 50 μm.
To test this hypothesis, shRNAs specifically targeted to AP2γ were introduced into ESCs using lentivirus, and two shRNAs (shRNA1 and shRNA4) could efficiently knock down AP2γ expression (Supplementary information, Figure S2A). The control and shRNA1/4-expressing ESCs showed comparable expression levels of pluripotency and differentiation markers (Supplementary information, Figure S2B) and were used for further studies.
Using an unbiased differentiation method, shRNA-expressing ESCs were differentiated as embryoid bodies (EBs) in DMEM containing 10% FBS for 8 days. qRT-PCR analysis showed that AP2γ knockdown upregulated the expression of NPC markers Sox1, Pax6 and Nestin (Figure 1B). Immunostaining of day 8 EBs confirmed that control shRNA-expressing ESCs produced approximately 15% Oct4−/Sox+ NPCs, whereas ESCs with AP2γ shRNAs displayed enhanced neural differentiation, generating 30% Oct4−/Sox+ NPCs (Figure 1C and 1D). Furthermore, the percentages of Tuj1+ and MAP2+ neurons were increased in AP2γ knockdown cells after EB replating (Figure 1C and 1D). EB differentiation was also performed in serum-free KSR medium, which normally generates approximately 80% NPCs at day 6. AP2γ knockdown accelerated neural differentiation as measured by the generation of more Oct4−/Sox+ NPCs at day 4 and more Tuj1+ neurons at day 6 (Supplementary information, Figure S2C-S2F). The examination of the expression of other germ layer markers showed that the expression of the epidermal markers CK1, CK14 and CK18 was downregulated in AP2γ shRNA-expressing cells (Figure 1B), which was also observed in KSR neural differentiation (Supplementary information, Figure S2G and S2H). Consistently, the percentages of CK14+ and CK18+ epidermal cells were reduced by AP2γ knockdown (Figure 1C and 1D). However, the expression of the pluripotency markers Oct4 and Nanog, the mesoderm markers T (also known as Brachyury) and Flk1, and the endoderm markers Gata4 and Gata6 was not affected by AP2γ shRNAs (Figure 1B). Together, these data suggest that AP2γ might be necessary for epidermal commitment and be a negative regulator of neural specification during ESC differentiation.
AP2γ overexpression inhibits neural conversion and promotes epidermal differentiation of ESCs
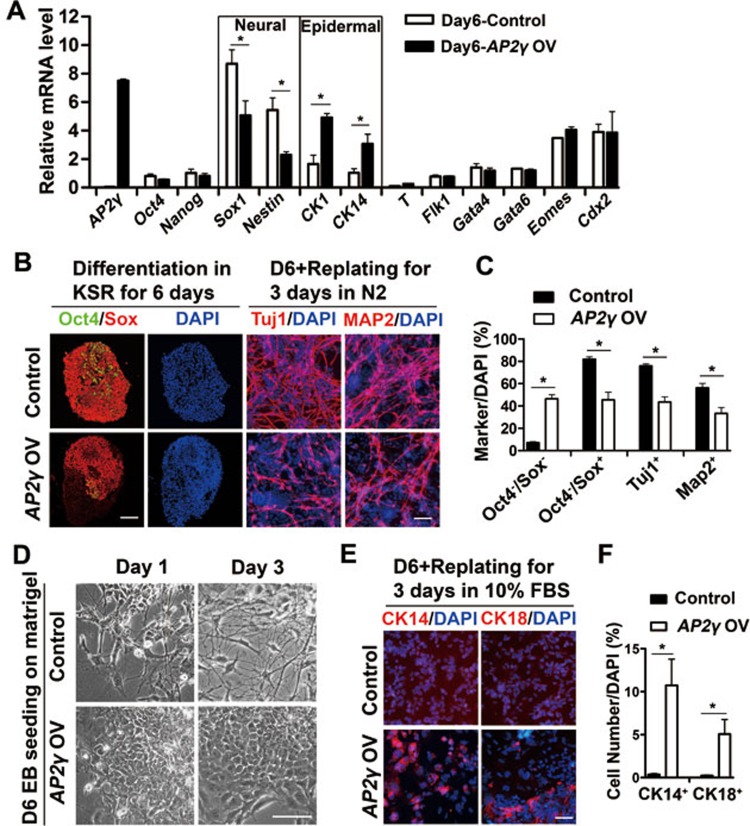
To examine whether AP2γ is sufficient for neural inhibition and epidermal induction, AP2γ was overexpressed in ESCs by lentivirus. Similarly to the AP2γ-knockdown ESCs, AP2γ-overexpressing ESCs showed comparable expression levels of pluripotent markers and proliferation rates compared with those of control cells (Supplementary information, Figure S3). After 6 days of neural differentiation in KSR medium, AP2γ-overexpressing cells showed decreased expression of the NPC markers Sox1 and Nestin (Figure 2A). Immunostaining confirmed that 80% of control ESCs differentiated into Oct4−/Sox+ NPCs, whereas only approximately 40% NPCs were generated from AP2γ-overexpressing cells (Figure 2B and 2C). Subsequent neuronal differentiation was also inhibited by AP2γ overexpression as measured by decreases in the percentages of Tuj1+ and MAP2+ cells (Figure 2B and 2C). In contrast, the expression of epidermal markers (CK1 and CK14) was increased by AP2γ overexpression (Figure 2A). Replated cells from AP2γ-overexpressing EBs showed epithelial-like morphology, whereas control cells grew many neurite-like processes (Figure 2D). It was further confirmed that some epithelial-like cells expressed CK14 and CK18 (Figure 2E and 2F). As in the AP2γ-knockdown cells, the expression of other germ layer markers was not affected by AP2γ overexpression (Figure 2A). Taken together, these results suggest that AP2γ inhibits neural conversion and promotes epidermal differentiation of ESCs.
Figure 2.
AP2γ overexpression suppresses neural commitment and promotes epidermal marker expression during ESC neural differentiation. (A) The expression of neural and epidermal markers was analyzed by qRT-PCR in day 6 EBs derived from control or AP2γ-overexpressing (OV) ESCs in 8% KSR medium. (B, C) Control and AP2γ-overexpressing ESCs were cultured in KSR medium as EBs for 6 days and were replated in N2 medium for 3 days. Double immunostaining of Oct4 (green) and Sox (red) for day 6 EBs and immunostaining of Tuj1 (red) and MAP2 (red) for replated cells were performed. The percentages of Oct4−/Sox+, Tuj1+ and MAP2+ cells are shown in C. (D) Control and AP2γ-overexpressing ESCs were induced in KSR medium as EBs for 6 days, and these EBs were seeded in DMEM containing 10% FBS on Matrigel-coated dishes for 1 or 3 days. The different morphologies are shown in bright field. Scale bar, 100 μm. (E, F) Immunostaining of CK14 (red) and CK18 (red). Day 6 EBs from the conditions described in B were replated in DMEM containing 10% FBS on Matrigel-coated dishes for 3 days. The percentages of CK14+ and CK18+ cells are shown in F. D, day. Scale bar in B and E, 50 μm.
AP2γ partially mediates BMP4 functions during ESC differentiation
Given that AP2γ phenocopies BMP's effects of promoting epidermal conversion and inhibiting neural commitment during ESC differentiation40, and that AP2γ expression is upregulated by BMP4 (Supplementary information, Figure S2), we speculated that AP2γ mediates BMP functions in neural and epidermal differentiation.
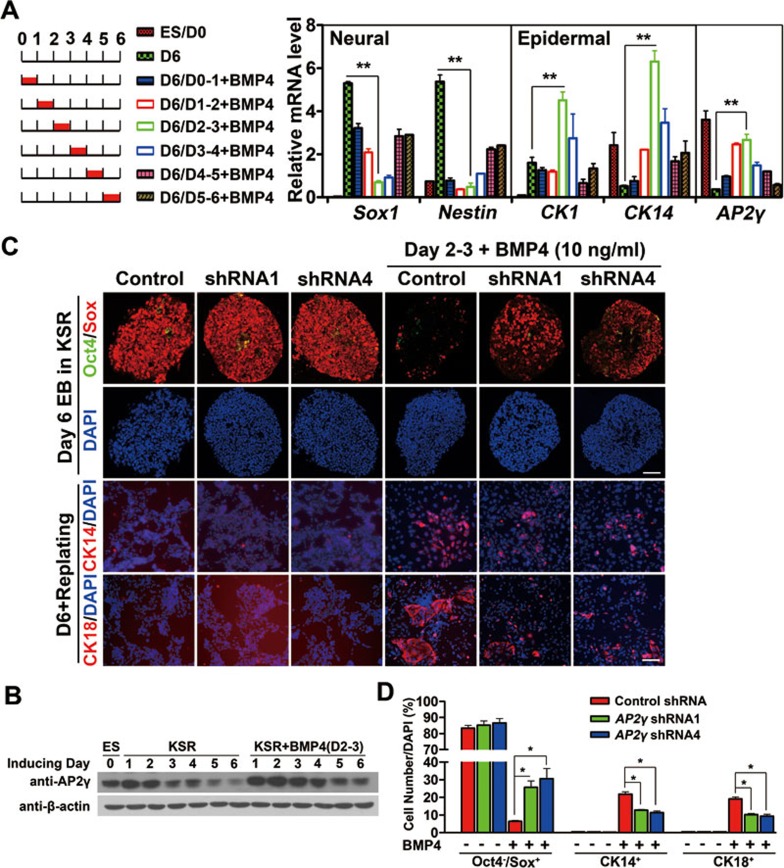
Previously, we showed that BMP4 had different effects at different stages of ESC neural differentiation and that day 2-3 is the most sensitive window of BMP inhibition of neural commitment38. To determine whether AP2γ was most efficiently induced by BMP4 during the same time period, ESCs were cultured in KSR medium supplemented with BMP4 at different time points for 24 h and were analyzed by qRT-PCR at day 6 (Figure 3A, left panel). We found that the highest level of AP2γ mRNA was induced by BMP4 supplementation at day 2-3, during which BMP4 most efficiently induced the expression of CK1 and CK14 and inhibited the expression of Sox1 and Nestin (Figure 3A, right panel). The BMP4-elicited AP2γ upregulation at day 2-3 was confirmed at the protein level by western blot (Figure 3B). Given that AP2γ responsiveness to BMP4 correlates with the neural inhibition and epidermal induction by BMP in ESC differentiation, we further propose that AP2γ might mediate BMP functions during the BMP4-sensitive window.
Figure 3.
AP2γ partially mediates BMP4 functions in ESC neural differentiation. (A) qRT-PCR analysis of ectoderm markers and AP2γ expression in EBs following culture in KSR medium for 6 days with or without BMP4 (10 ng/ml) treatment. BMP4 was added to the medium on the indicated days for 24 h. Undifferentiated ESCs without feeders served as a negative control and normally-induced day 6 EBs served as a positive control. (B) AP2γ expression in ESCs and EBs cultured in KSR medium with or without BMP4 (10 ng/ml) was analyzed by immunoblot from day 1-6. BMP4 was added to the medium at day 2-3 (D2-3) for 24 h. These cells were continuously cultured in KSR medium and harvested on the indicated days. (C, D) ESCs with control or AP2γ shRNAs were cultured in KSR medium as EBs for 6 days without or with BMP4 and were replated in DMEM containing 10% FBS on a Matrigel-coated dish for 2 days. Day 2 EBs were treated with BMP4 (10 ng/ml) for 24 h. Immunostaining of Oct4 (green)/Sox (red) for day 6 EBs and immunostaining of CK14 (red) and CK18 (red) for replated cells were performed, and the percentages of Oct4−/Sox+, CK14+ and CK18+ cells are shown in D. D, day. Scale bar, 50 μm.
To test this hypothesis, we cultured AP2γ shRNA-expressing ESCs in KSR medium with or without BMP4 at day 2-3 and examined marker expression in day 6 EBs using immunostaining. We found that BMP4 reduced the percentage of Oct4−/Sox+ NPCs from 80% to approximately 5% in control shRNA-expressing cells, but this reduction was partially recovered to approximately 25% Oct4−/Sox+ NPCs in AP2γ-knockdown cells (Figure 3C and 3D). This neural inhibition recovery was confirmed by qRT-PCR analysis for Sox1 and Nestin (Supplementary information, Figure S4A). BMP4 also induced approximately 20% of CK14+ and CK18+ cells from control ESCs, but it could induce only 10% of those cells from AP2γ shRNA-expressing cells (Figure 3C and 3D), suggesting that the epidermal promoting activity of BMP4 on the expression of the markers CK1 and CK18 (Supplementary information, Figure S4A) was also impaired by AP2γ knockdown. Moreover, the most efficient induction of the mesoderm marker T and the endoderm markers Gata6 by BMP4 at day 2-3 was observed (Supplementary information, Figure S4B). However, we could not observe the expression change of mesendodermal markers T and Gata6 when AP2γ-knockdown EBs were treated with BMP4 at day 2-3 (Supplementary information, Figure S4C), suggesting that AP2γ may not mediate BMP functions during mesendodermal specification.
Together, these data suggest that AP2γ partially mediates the BMP4 functions of neural inhibition and epidermal induction during the BMP4-sensitive window in ESC neural differentiation.
AP2γ expression shifts from the epiblast to the putative epidermal ectoderm of the early chick embryo
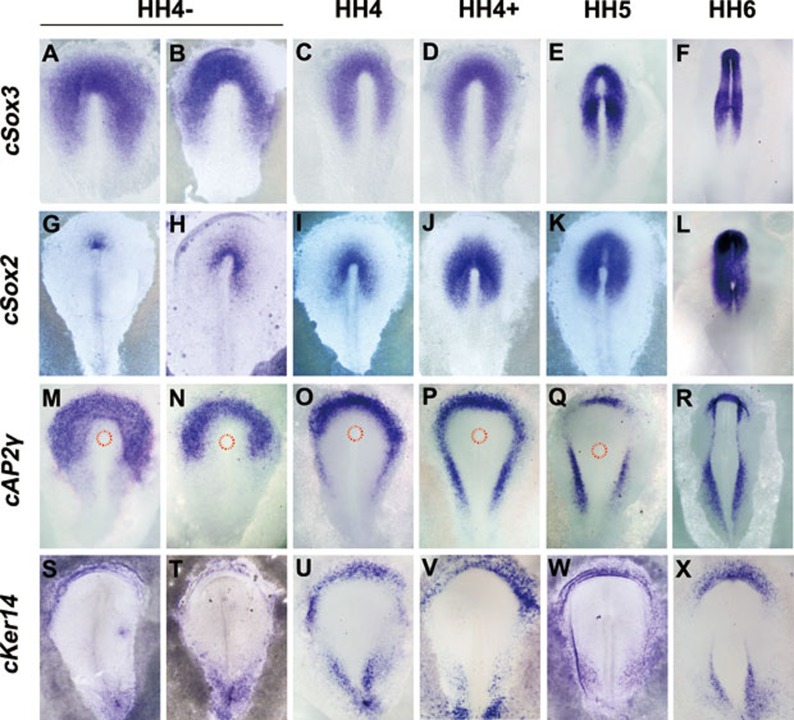
To explore the biological functions of AP2γ in vivo, the spatial-temporal relationship between AP2γ and neural/epidermal markers was examined by whole-mount in situ hybridization (ISH) in early chick embryos. Prior to the detection of the definitive neural plate marker cSox2, cAP2γ was expressed throughout the epiblast marked by the early epiblast marker cSox341 and cAP2γ expression was distributed in the interior of cDlx5-expressing region42 before HH stage 3 (Supplementary information, Figure S5A and S5B).
During neural plate formation accompanying with cSox2 expansion from the anterior tip of the primitive streak and cSox3 shrinking to the neural plate (Figure 4A-4L), cAP2γ expression progressively shifted from the epiblast to the peripheral ectoderm at HH stage 4 (Figure 4M-4P). Double ISH showed that cAP2γ transcripts were complementary to cSox2 expression and partially overlapped with that of cSox3 at the early stage of neural plate formation (HH stage 4−, Supplementary information, Figure S5Ca,c). At HH stage 4+ cAP2γ staining was complementary to cSox3 expression and was separated from cSox2 transcripts with a visible distance (Supplementary information, Figure S5Cb,d). From the full primitive-streak to later stages (HH 5-6), cAP2γ transcripts were restricted to the future epidermal ectoderm (Figure 4Q and 4R). Keratin14 that was previously identified as an epidermal marker24 was first detected at HH stage 4− concurrently with the appearance of cSox2 in the chick embryo. Chick Keratin14 (cKer14) expression was restricted to the presumptive epidermis (Figure 4S-4X), similar to the expression of cAP2γ and the presumptive epidermal markers Gata2/3 at later stages (Supplementary information, Figure S6). Therefore, cKer14 was used as the other epidermal marker in the following studies.
Figure 4.
AP2γ shifts from the epiblast to the peripheral ectoderm concurrent with neural plate expansion in the chick embryo. Expression of cSox3 (A-F), cSox2 (G-L), cAP2γ (M-R) and cKer14 (S-X) is shown by ISH using digoxigenin-labeled riboprobes in HH stage 4-6 chick embryos. The broken red line indicates the area (M-Q) of Hensen's node.
Together, our results show that cAP2γ expression shifts from the epiblast to the putative epidermal ectoderm during neural plate formation at HH stage 4, suggesting that cAP2γ might play roles in neural plate expansion. cAP2γ is also consistently expressed in the epidermal ectoderm, indicating that it might be involved in epidermal development.
AP2γ inhibits neural expansion and promotes epidermal commitment in different regions of epiblast in the chick embryo
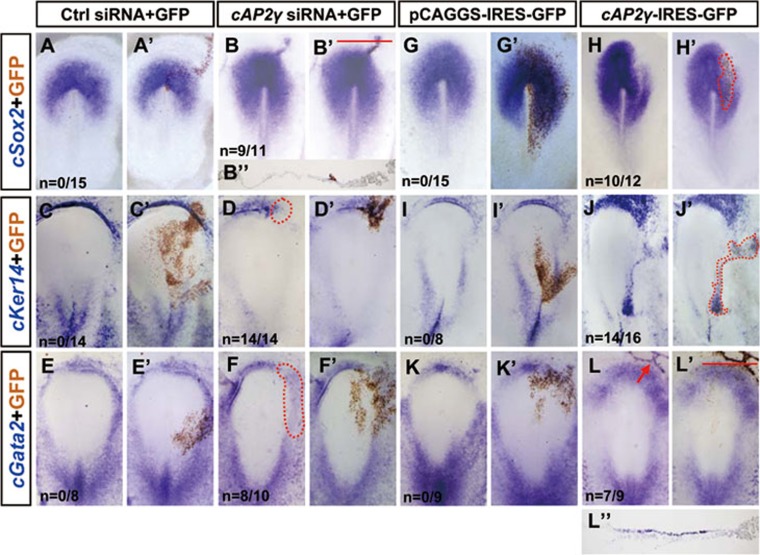
To examine the in vivo function of AP2γ, chemically synthesized siRNAs targeted to cAP2γ were co-electroporated with the electroporation tracer GFP into HH stage 3 chick embryos, and gene expression was analyzed by ISH at later stages (Supplementary information, Figure S7A). We found that AP2γ siRNA could efficiently knockdown cAP2γ expression (Supplementary information, Figure S7B).
In medial epiblast, when AP2γ siRNA was electroporated along a continuous line extending outward from Hensen's node before the onset of cSox2 expression (HH stage 3), we observed a prolonged extension of cSox2 into the peripheral ectoderm (Figure 5A-5B′), which was confirmed by the histological section (Figure 5B″). However, AP2γ siRNA electroporation in a separated region of peripheral ectoderm could not induce cSox2 expression (Supplementary information, Figure S7C). In contrast, AP2γ overexpression in the prospective neural plate suppressed cSox2 expression (Figure 5G-5H′), suggesting that AP2γ can inhibit excessive neural expansion neighboring the cSox2-expressing region at early stages of neural plate formation.
Figure 5.
AP2γ inhibits neural expansion and promotes epidermal commitment at different regions of epiblast. Whole-mount ISH (blue) was performed in chick embryos that had been previously electroporated in the epiblast layer at HH stage 3. The same embryo was stained for GFP expression (brown), which marks the electroporated field (some images are highlighted by broken lines). (A-F′) Control or AP2γ siRNA (50 ng/μl; mixed with GFP, 0.15 μg/μl) electroporated embryos were stained using cSox2, cKer14 and cGata2 probes. Transverse section (red line) of the embryo in B′ is shown in B″. (G-L′) Embryos electroporated with GFP or cAP2γ in the epiblast and stained for cSox2, cKer14 and cGata2. Transverse section (red line) of the embryo in L′ is shown in L″.
In the future epidermal ectoderm cKer14 and cGata2 were suppressed by AP2γ siRNA (Figure 5C-5F′), and the inhibitory effect on cKer14 was fully rescued by co-electroporated mouse AP2γ (mAP2γ) (Supplementary information, Figure S7D). To determine whether AP2γ is sufficient for epidermal induction, we overexpressed cAP2γ in the peripheral epiblast and found that cAP2γ could efficiently induce the ectopic expression of cKer14 and cGata2 (Figure 5I-5L′, 5L″). cSox3 expression was not affected by AP2γ overexpression or knockdown (Supplementary information, Figure S7F), similarly to BMP4 misexpression's effects on the early expression of Sox313. Collectively, AP2γ restricts excessive neural plate expansion during young neural plate generation in the medial epiblast. Furthermore, AP2γ is both sufficient and necessary for epidermal induction in putative epidermal ectoderm.
AP2γ partially mediates BMP functions in ectodermal patterning of the chick embryo
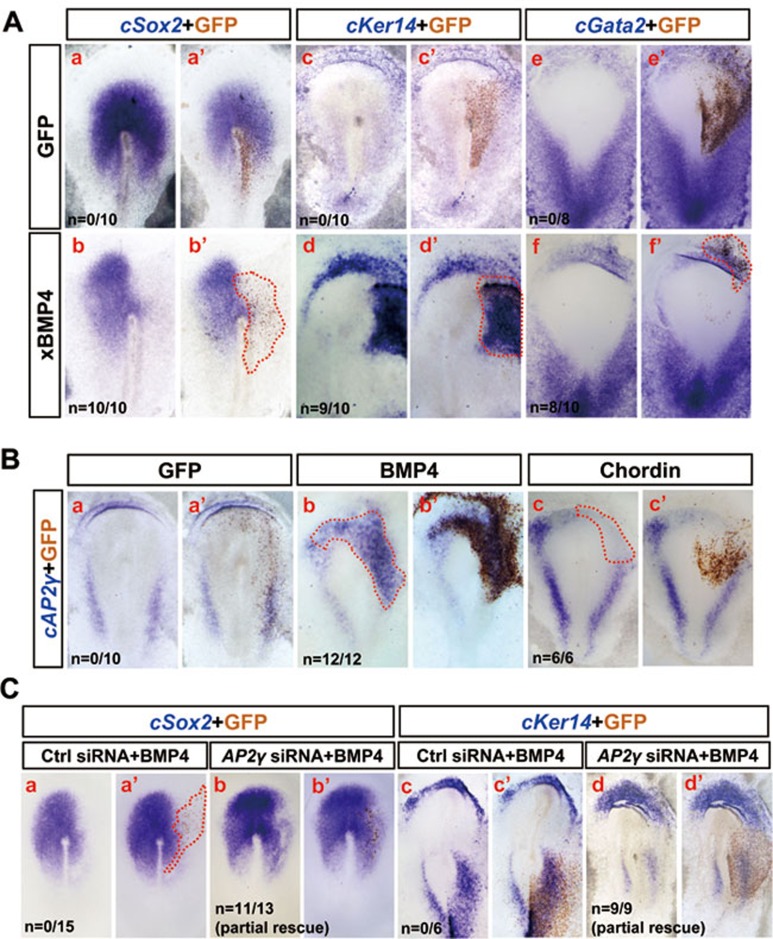
Given that AP2γ can partially mediate the BMP4 functions of neural inhibition and epidermal conversion during ESC differentiation (Figure 3), we asked whether AP2γ has similar functions in vivo. We first electroporated Xenopus BMP4 (xBMP4) into HH stage 3 chick embryos and found that forced expression of xBMP4 completely blocked cSox2 expression (Figure 6Aa-b′) and induced the ectopic expression of the epidermal markers (cKer14 and cGata2) in non-neural ectoderm (Figure 6Ac-f′). cAP2γ expression could be induced by xBMP4 and inhibited by BMP inhibitor chordin (Figure 6B), suggesting that the dynamic expression pattern of cAP2γ (Figure 4) might be controlled by BMP signaling activity.
Figure 6.
AP2γ partially mediates BMP4 functions in ectodermal patterning. (A) Whole-mount ISH analysis of cSox2, cKer14 and cGata2 in control GFP- or xBMP4-electroporated chick embryos at HH stage 5. (B) Whole-mount ISH analysis of cAP2γ in control GFP-, xBMP4- or Chordin-electroporated chick embryos. (C) Control or AP2γ siRNA (50 ng/μl) and xBMP4 (0.3 μg/μl) were co-electroporated into the chick embryo epiblast. Embryos were subsequently stained for cSox2 and cKer14.
To explore whether AP2γ is necessary for BMP4 functions, we co-electroporated AP2γ siRNA and xBMP4 into the prospective neural plate of HH stage 3 embryos and found that xBMP4 with control siRNA strongly blocked cSox2 expression (Figure 6Ca,a′), whereas AP2γ siRNA partially rescued xBMP4 inhibition of cSox2 expression (Figure 6Cb,b′). Similarly, xBMP4-induced cKer14 expression was weakened by AP2γ siRNA (Figure 6Cc-d′). qRT-PCR analysis confirmed that the BMP effects of neural inhibition and epidermal induction were partially impaired by AP2γ siRNA (Supplementary information, Figure S8). Together, these results suggest that AP2γ partially mediates BMP functions in ectodermal patterning of the chick embryo.
AP2γ is a direct downstream target of BMP signaling
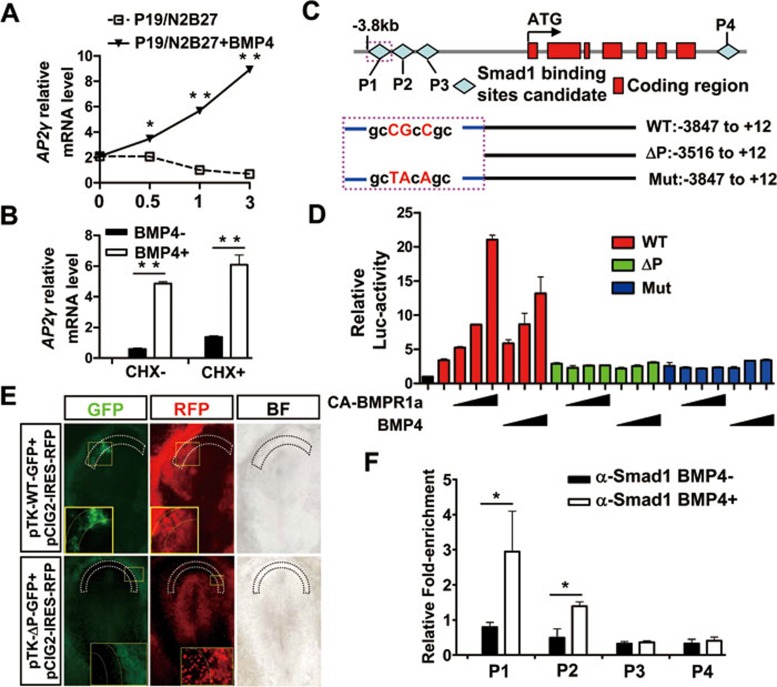
To test whether AP2γ is directly regulated by BMP, we cultured P19 cells in N2B27 serum-free medium and added BMP4 for 0.5-3 h. We found that AP2γ transcription was elevated within 0.5 h of BMP4 treatment (Figure 7A), suggesting that AP2γ is an early BMP4 responsive gene. We also found that AP2γ expression was suppressed in a dose-dependent manner by the BMPR1a inhibitor dorsomorphin43, similar to the known direct BMP targets Msx2 and Id144,45 (Supplementary information, Figure S9A). Moreover, Msx2, Id1 and AP2γ were induced by BMP4 in the presence of the protein synthesis inhibitor cycloheximide (CHX) (Figure 7B and Supplementary information, Figure S9B), whereas the indirect target Wnt346 was not responsive to BMP4 with CHX pre-treatment (Supplementary information, Figure S9B).
Figure 7.
AP2γ is directly regulated by BMP4. (A) AP2γ expression was examined using qRT-PCR in N2B27 serum-free medium-induced P19 cells with or without BMP4 (10 ng/ml) treatment for 0.5, 1 or 3 h. (B) qRT-PCR analysis of AP2γ expression with or without BMP4 (10 ng/ml) in the presence of CHX (10 ng/ml). P19 cells were cultured in standard medium and induced by N2B27 medium for 3.5 h. CHX was added to the medium 30 min prior to BMP4 treatment (for 3 h) to block new protein synthesis. (C) Diagram of predicted Smad1-binding site candidates (P1-P4) in the upstream and downstream of the AP2γ transcribed region. The 3.8-kb (WT: −3 847 to +12) fragment, 3.5-kb (ΔP: −3 516 to +12) fragment and mutation (Mut: sites mutation in the putative Smad binding site from −3 602 to −3 595) fragment were constructed into a pGL3-basic vector. (D) Luciferase (Luc) reporter assay was performed by co-transfection of pRL-TK (10 ng) with WT-Luc (50 ng), ΔP-Luc (50 ng) or Mut-Luc (50 ng) with increasing amounts of CA-BMPR1a (0.1, 0.2 or 0.4 μg) or with increasing concentrations of BMP4 (5, 10 or 20 ng/ml). P19 cells were starved in N2B27 serum-free medium for 12 h and transfected with the indicated plasmids. After 12 h of transfection the cells were treated by BMP4 for 24 h and the relative luciferase activity was analyzed using the dual luciferase reporter (DLR) system. (E) The WT fragment or ΔP fragment was constructed into the pTK-GFP vectors and co-electroporated with pCIG2-IRES-RFP into HH stage 3 embryos. The presumptive AP2γ-expressing region was indicated by broken lines, and the specified regions were enlarged. BF, bright field. (F) ChIP was performed using a Smad1 antibody versus normal IgG as a control. N2B27-induced P19 cells were treated with or without BMP4 for 3 h and then subjected to the ChIP assay. qRT-PCR was performed for regions within the possible Smad-binding sites P1-P4, and values were normalized to the input.
To further show that AP2γ is a direct target of BMP4, we screened the upstream and downstream of the mAP2γ transcribed region and found four putative Smad-binding sites (P1-P4) (Figure 7C). We then cloned a 3.8-kb (WT), a 3.5-kb (ΔP) and a mutation fragment (Mut: site mutation at the possible Smad-binding site) of the upstream of the mAP2γ transcribed region (Figure 7C), and constructed reporter plasmids to perform luciferase assays in P19 cells. We found that BMP4 and constitutively active (CA)-BMPR1a increased WT fragment driven-luciferase activity in a dose-dependent manner, but the ΔP and mutation fragments showed no responsive activity (Figure 7D), suggesting that AP2γ expression can be induced by BMP/Smad signaling that is dependent on the responsive element within this −3.8- to −3.5-kb fragment. Furthermore, WT fragment electroporated into the chick epiblast drove GFP expression in the presumptive AP2γ-expressing area, whereas the ΔP fragment had no such activity (Figure 7E, WT: 6/9, ΔP: 0/10). Chromatin immunoprecipitation (ChIP) assays with Smad1 antibody showed that Smad1 was mainly enriched at the putative site P1 under BMP4 stimulation and that the neighboring P2 site showed weak binding activity (Figure 7F). Smad1 was also enriched in the Id1 and Msx2 promoter regions as a positive control (Supplementary information, Figure S9C). Together, these results indicate that AP2γ is a direct downstream target of BMP signaling and is upregulated by BMP4 through the recruitment of Smad1-containing complex to the binding site in the AP2γ promoter.
Discussion
AP2γ is expressed in the inner cell mass (ICM) and trophectoderm of mouse embryo32,47, and previous studies have focused on its functions in extraembryonic development. However, the functions of AP2γ during development from ICM to neural/epidermal ectoderm are not fully understood. Using ESC differentiation as an in vitro model, we found that AP2γ knockdown facilitates neural conversion and impairs epidermal commitment, while AP2γ overexpression inhibits neural conversion and promotes epidermal differentiation (Figures 1 and 2). Our finding suggests that AP2γ might be involved in ectodermal patterning, but its exact in vivo function required investigation in an animal model.
Early chick embryo was chosen as the in vivo model. Detailed spatiotemporal expression analysis showed that AP2γ is expressed in the entire epiblast before HH stage 3 and gradually shifts outwards to the putative epidermal ectoderm at HH stage 4, accompanying with complementary cSox2 expansion. Similarly, mouse AP2γ might have expression changes from ICM at E3.547 to non-neural ectoderm at the late gastrulation stage48. The dynamic expression pattern of cAP2γ suggests that AP2γ might play multiple roles in ectodermal patterning in different regions of the epiblast.
During the early phase of neural plate generation (HH 4− and 4) the expression patterns of cAP2γ and cSox2 are complementary to each other, and cAP2γ acts as a gatekeeper to restrict excessive neural expansion. When cAP2γ was knocked down along a continuous trail in the nascent neural plate, cSox2 escaped from the gate into the outer territory of the ectoderm (Figure 5), similar to Gata2/3 morpholino-induced neural extension by inhibiting BMP signaling49. The continuity requirement is consistent with the notion that cell communication within the neural plate is necessary for the induction of neural markers49 and that BMP inhibition was not sufficient for neural induction13. Moreover, the ectopic expression of cAP2γ restricts normal neural expansion (Figure 5). These results indicate that cAP2γ is sufficient and necessary for the inhibition of excessive neural plate expansion during early neuralization. This raises the question of how cAP2γ inhibits cSox2 extension. There are two possibilities: (1) AP2γ functions as a transcriptional repressor through direct binding to the cSox2 enhancer N2, which possesses a characterized AP2-binding site22,50,51; (2) AP2γ biases the epiblast to differentiate into non-neural tissues52. At HH stage 4+, the expression of cAP2γ and cSox2 was separated and confined to their predisposed regions, which implies that neural expansion is a multi-step process13 under the strict restriction of AP2γ in the nascent neural plate.
cAP2γ is also expressed in the surface ectoderm throughout HH stage 4 and might play important roles in epidermal development. Epidermal development undergoes epidermal progenitor commitment from the surface ectoderm. Previously, epidermal progenitors were thought to be determined around E8-E12 in mouse embryos53, which is later than neural induction. Keratin (K5 and K14) expression is first detected at E9.5 in the surface ectoderm and is recognized as the hallmark of the stratified epithelia24. Here, we found that cKer14 begins to be expressed at the onset of neural induction and can be ectopically induced by xBMP4 in non-neural ectoderm of the chick embryo (Figures 4 and 6), implying that epidermal and neural commitment might occur simultaneously in different regions. AP2γ has been implicated in regulating keratin expression in terminal epidermal development34,54, and AP2-binding sites exist in the epidermis-specific enhancer of the K14 promoter55,56. However, it is unknown whether AP2γ is involved in the initial epidermal commitment. We show that cKer14 expression generally overlapped with cAP2γ (Figure 4) and that AP2γ was sufficient and necessary for the expression of epidermal markers in the future epidermis (Figures 2 and 5). cKer14 may be one of the earliest markers for primarily committed epidermal progenitors in chick ectodermal patterning and AP2γ might act upstream of cKer14 to initiate epidermal differentiation in the surface ectoderm. cGata2 was detected at early stages and was ectopically induced by xBMP4 and cAP2γ (Figures 4, 5, 6). It is possible that Gata2 induction occurs in parallel with primary epidermal commitment. TAp63α is required for the stratification program of epidermal morphogenesis57,58, whereas its transcripts appear later at HH stage 6 in the chick embryo59. We propose that AP2γ triggers the initial step of epidermal development in the surface ectoderm. Thereafter when p63 and AP2γ are expressed in the committed surface ectoderm after HH stage 6, AP2γ might act downstream of p63 in the stratification program52 and combine with other epidermal factors such as p63, Msx1 and AP2α to regulate the subsequent epidermal maturation at later stages19,26,34,54,60,61.
During the AP2γ expression shift from the epiblast to the surface ectoderm, AP2γ transitionally occupies the gap between the peripheral epidermis and the neural plate named pre-placodal region. We observed that BMP4 and AP2γ could inhibit the expression of pre-pladocal markers cEya2 and cSix1 (data not shown), suggesting that AP2γ might be also involved in pre-placode development62. Furthermore, AP2γ has redundant activities with AP2α in neural crest induction and non-neural ectoderm derivative development26. Other factors such as Pax7, Msx1, Zic1, Dlx5 and AP2α were shown as neural border specifiers30,63,64,65,66,67,68. During early neuralization of the chick embryo, some factors are mainly expressed in the peripheral (Pax7, Dlx5 and AP2α) or caudal (Msx1) ectoderm and the expressions of others (Zic1) partially overlaps with cSox2 expression68. However, there are no reports of gene expression complementary to early cSox2-expressing territory. We show that during the expression shift of AP2γ, its expression consistently surrounds the expanding neural plate in the anterior and lateral region. At the onset of neuralization, AP2γ-expressing cells in the future neural plate have the competence to differentiate into neural cells and the excessive expansion of cSox2 is restricted by AP2γ. Only when AP2γ is cleared from neighboring cells around the cSox2-expressing region can cSox2 expand moderately. AP2γ might function as one of the earliest neural border genes, and its expressing territory might represent the “pre-border” state proposed by Streit and Stern63. The AP2γ-expressing epiblast at HH stage 3+/4− may differentiate into one of three directions: neural plate (the epiblast around Hensen's node gradually loses AP2γ expression and expresses cSox2), neural crest or pre-placode (AP2γ might collaborate with some neural border factors to specify the neural crest or pre-placodal ectoderm26,62) or epidermis (AP2γ combines with epidermal factors to determine epidermal fate in the surface ectoderm34,52,69).
BMPs are the most important morphogens involved in ectodermal patterning, neural border establishment and maintenance and neural crest specification13,42,63,70. However, the intracellular effectors of BMP signaling during these processes are not well known. Here, we identified AP2γ as a novel BMP downstream target that is directly regulated by BMP4 through Smad1 binding to the AP2γ promoter (Figure 7). BMPR1a-knockout mice die at E8.0 while BMPR1b-null mice are viable71,72, implying that BMPR1a was the possible receptor mediating BMP functions during ectodermal patterning. In the early chick embryo, cAP2γ can be ectopically induced by BMP4 and suppressed by chordin (Figure 6), suggesting that clearance of AP2γ transcripts from the medial epiblast might be elicited by BMP antagonists secreted from the organizer with gradually enhanced expression63,73. It is also possible that AP2γ is concurrently regulated by other signals, such as FGF and Wnt74. BMP is proved to inhibit prematuration of neural tissues in the mouse embryo12. AP2γ might be the executor of BMP signaling in the nucleus to guarantee the normal progress of neural development by suppressing excessive cSox2 expansion in the young neural plate. Moreover, AP2γ partially mediates the BMP4 function of epidermal induction in the peripheral ectoderm. Further investigation is needed to determine whether AP2γ mediates BMP functions in pre-placodal development and neural crest specification, during which AP2α might play important roles downstream of BMP signaling29,30.
To summarize, we propose a model for AP2γ function in ectodermal patterning of the chick embryo (Supplementary information, Figure S10). During neural induction, BMPs are expressed mainly in the non-neural ectoderm, and the BMP inhibitors chordin and noggin are sequentially expressed in node or notochord63,73, resulting in medial to lateral clearance of BMP activity. AP2γ expression shifts progressively from the entire epiblast to the non-neural ectoderm under the regulation of BMP and its antagonists (Figure 6B). During that process, AP2γ might mediate dual roles of BMP in ectodermal patterning: AP2γ inhibits excessive neural expansion in the young neural plate to prevent precocious neuralization and also initiates epidermal commitment in the surface ectoderm.
Materials and Methods
Cell culture and treatment
P19 cells were cultured as previously described75. Mouse ESC lines R1 was used. ESCs were maintained on mitomycin C-treated mouse embryonic fibroblasts (feeders) in standard medium. P19 cells and ESC neural differentiation was performed as described previously37,39. The factors and inhibitors, such as BMP4 (10 ng/ml; R&D Systems, Minneapolis, MN, USA), Noggin (100 ng/ml; Peprotech, Rocky Hill, NJ, USA), SB431542 (10 μM, Sigma), Dorsomorphin (0.5-20 μM, Enzo life sciences) were used.
Gene overexpression and knockdown in ESCs
Mouse AP2γ was cloned from ESC/R1 cDNA and constructed into lentiviral vector pFUGW-IRES-EGFP for overexpression in ES cells. The empty lentiviral expression vector pFUGW-EGFP was used as a negative control. Lentiviral vector pLentiLox 3.7 expressing shRNA was used for AP2γ knockdown in ESCs. The control and AP2γ shRNA sequences were listed in Supplementary information, Table S1. Lentiviral packaging and lentiviral transfection were performed as described76. GFP-positive cells were sorted with FACS Aria cell sorter (BD Biosciences) and were used for analysis according to the diagram in Supplementary information, Figure S11.
Immunostaining
Immunostaining was performed as described previously77. The following primary antibodies were used. Mouse monoclonal antibodies included: anti-GFP (1:400, MP Biomedicals), anti-Oct4 (1:200, Santa Cruz Biotechnology), anti-nestin (1:200, Upstate), anti-Tuj1 (1:500, Sigma), anti-MAP2 (1:500, Sigma), anti-AP2γ (1:500, Abcam), anti-cytokeratin18 (1:200, Abcam) and anti-cytokeratin14 (1:50, Abcam). Rabbit polyclonal antibodies were anti-GFP (1:400, Santa Cruz Biotechnology), an anti-Sox1/(2)/3 (1:100) that has a preference for Sox1 and Sox3 over Sox278,79. Fluorescein isothiocyanate- and Cy3-conjugated secondary antibodies were used (1:200 and 1:500, Jackson ImmunoResearch).
Early chick embryo electroporation
Fertilized eggs (Shanghai Academy of Agricultural Sciences, Shanghai, China) were incubated at 38 °C to the desired stages. Plasmids (0.1-2 μg/μl) or siRNA reagents with Fast Green and Sucrose were electroporated at Hamburger and Hamilton (HH) 3 as previously described80. cAP2γ (NCBI: XM_417497) that displays 72% identity to mAP2γ was cloned. The C-terminal region of isolated cDNA was subcloned for probe preparation. The primers used for cloning were listed in Supplementary information, Table S2.
Whole-mount ISH and sectioning of embryos following ISH
Whole-mount ISH of the early chick embryo, double ISH and sectioning of embryos following whole-mount ISH were performed as described previously41,81,82,83. Detailed protocols are available upon request. The following probes were used: cAP2γ, cKeratin14, cSox2, cSox3, cGata2, cGata3 and cDlx5.
RNA preparation and qRT-PCR analysis
Total RNA was extracted from cultured cells or chick embryos using TRIzol reagent. Reverse transcription and qRT-PCR were performed as described previously75,84. The primers are listed in Supplementary information, Table S3.
RNA interference in chick embryo
The siRNAs used for knockdown experiments in chick embryo were chemically synthesized by Shanghai GeneChem Co., Ltd. cAP2γ and control siRNA sequences were listed in Supplementary information, Table S1. siRNAs (0.5 μg/μl) were electroporated into the epiblast of early chick embryo and 0.15 μg/μl GFP plasmid was co-electroporated as a tracer.
Luciferase assay
The luciferase assay was performed as described previously77,84 according to the manufacturer's instructions (Promega). During N2B27 aggregation induction, P19 cells were transiently transfected with pRL-TK and pGL3-basic-AP2γ promoter plasmids. CA-BMPR1a was co-transfected or BMP4 was added to the medium after transfection for 12 h. Luciferase activity was determined after treatment for 24 h.
ChIP
ChIP assay was performed according to the method described75,84. Mouse monoclonal anti-Smad1 antibody (10 μg per sample, CST) and normal mouse IgG in control groups were used for immunoprecipitation. Primers for ChIP assay are listed in Supplementary information, Table S4.
Statistics
In each experiment, 50-100 cell aggregates were examined. Each experiment was repeated at least three times, and similar results were obtained. Data were presented as mean ± SD. Student's t tests were used to compare the effects of all treatments. Differences were considered statistically significant at * P < 0.05, ** P < 0.05. For statistic analysis in chick embryo, the successfully electroporated embryos (GFP-expressing embryos) were calculated.
Acknowledgments
We thank Dr Claudio Stern (University College London, UK) for chick probes cSox2, cSox3, cDlx5, cGata2, cGata3 and Xenopus BMP4 expression plasmid, and thank Dr Andrea Streit (King's College London, UK) for critical reading of the manuscript as well as for cEya2, cSix1 probes. This work was supported in part by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01010201), the National Basic Research Program of China (973 Program; 2009CB941100), the National Natural Science Foundation of China (30830034, 90919046), Shanghai Key Project of Basic Science Research (08DJ1400501) and Council of Shanghai Municipal Government for Science and Technology (088014199).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
AP2γ is induced by BMP4 dose-dependently in P19 cell neural differentiation.
AP2γ knockdown doesn't affect the pluripotency of ESCs and the expression of other germ layers markers during differentiation.
AP2γ overexpression has no impact on the pluripotency and the proliferation rates of ESCs.
AP2γ dosen't mediate mesendoderm induction by BMP4 during ESC neural differentiation.
The section of cAP2γ stained chick embryos.
Comparative expression pattern of epidermal markers.
The effects of cAP2γ knockdown or overexpression on the expression of cSox2, cKer14 and cSox3.
BMP4 effects on ectodermal patterning were partially impaired by AP2γ siRNA.
AP2γ might be directly regulated by BMP4.
The model for AP2γ functions in ectodermal patterning of chick embryo.
The diagram of AP2γ functional analysis in ESCs.
ShRNA sepuences
Primer sequences (5′→3′) for cloning
Primer sequences (5′→3′) for Q-PCR and RT-PCR
Primer sequences (5′→3′) for ChIP assay
References
- Wilson SI, Edlund T. Neural induction: toward a unifying mechanism. Nat Neurosci. 2001;4 Suppl:1161–1168. doi: 10.1038/nn747. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Dias MS, Schoenwolf GC. Formation of ectopic neurepithelium in chick blastoderms: age-related capacities for induction and self-differentiation following transplantation of quail Hensen's nodes. Anat Rec. 1990;228:437–448. doi: 10.1002/ar.1092280410. [DOI] [PubMed] [Google Scholar]
- Streit A, Sockanathan S, Perez L, et al. Preventing the loss of competence for neural induction: HGF/SF, L5 and Sox-2. Development. 1997;124:1191–1202. doi: 10.1242/dev.124.6.1191. [DOI] [PubMed] [Google Scholar]
- Storey KG, Crossley JM, De Robertis EM, Norris WE, Stern CD. Neural induction and regionalisation in the chick embryo. Development. 1992;114:729–741. doi: 10.1242/dev.114.3.729. [DOI] [PubMed] [Google Scholar]
- Storey KG, Selleck MA, Stern CD. Neural induction and regionalisation by different subpopulations of cells in Hensen's node. Development. 1995;121:417–428. doi: 10.1242/dev.121.2.417. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Neave B, Holder N, Patient R. A graded response to BMP-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech Dev. 1997;62:183–195. doi: 10.1016/s0925-4773(97)00659-x. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997;88:13–17. doi: 10.1016/s0092-8674(00)81853-x. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Hemmati-Brivanlou A. Neural induction. Annu Rev Cell Dev Biol. 1999;15:411–433. doi: 10.1146/annurev.cellbio.15.1.411. [DOI] [PubMed] [Google Scholar]
- Di-Gregorio A, Sancho M, Stuckey DW, et al. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development. 2007;134:3359–3369. doi: 10.1242/dev.005967. [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–5681. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T, et al. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Launay C, Fromentoux V, Shi DL, Boucaut JC. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996;122:869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Miyama K, Yamada G, Yamamoto TS, et al. A BMP-inducible gene, dlx5, regulates osteoblast differentiation and mesoderm induction. Dev Biol. 1999;208:123–133. doi: 10.1006/dbio.1998.9197. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ueno N, Hemmati-Brivanlou A. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development. 1997;124:3037–3044. doi: 10.1242/dev.124.16.3037. [DOI] [PubMed] [Google Scholar]
- Bakkers J, Hild M, Kramer C, Furutani-Seiki M, Hammerschmidt M. Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev Cell. 2002;2:617–627. doi: 10.1016/s1534-5807(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Eckert D, Buhl S, Weber S, Jager R, Schorle H. The AP-2 family of transcription factors. Genome Biol. 2005;6:246. doi: 10.1186/gb-2005-6-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger-Eversheim K, Moser M, Schorle H, Buettner R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene. 2000;260:1–12. doi: 10.1016/s0378-1119(00)00454-6. [DOI] [PubMed] [Google Scholar]
- Imagawa M, Chiu R, Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987;51:251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Byrne C, Tainsky M, Fuchs E. Programming gene expression in developing epidermis. Development. 1994;120:2369–2383. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Huber L, Majdazari A, Schutz G, Williams T, Rohrer H. The transcription factors AP-2beta and AP-2alpha are required for survival of sympathetic progenitors and differentiated sympathetic neurons. Dev Biol. 2011;355:89–100. doi: 10.1016/j.ydbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Li W, Cornell RA. Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Dev Biol. 2007;304:338–354. doi: 10.1016/j.ydbio.2006.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman TL, Javier AL, Campeau SA, Knight RD, Schilling TF. Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. J Exp Zool B Mol Dev Evol. 2007;308:679–691. doi: 10.1002/jez.b.21189. [DOI] [PubMed] [Google Scholar]
- Luo T, Lee YH, Saint-Jeannet JP, Sargent TD. Induction of neural crest in Xenopus by transcription factor AP2alpha. Proc Natl Acad Sci USA. 2003;100:532–537. doi: 10.1073/pnas.0237226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Deng CX, Wang Q, Jiao K. Disruption of Smad4 in neural crest cells leads to mid-gestation death with pharyngeal arch, craniofacial and cardiac defects. Dev Biol. 2008;316:417–430. doi: 10.1016/j.ydbio.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Croze N, Maczkowiak F, Monsoro-Burq AH. Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc Natl Acad Sci USA. 2011;108:155–160. doi: 10.1073/pnas.1010740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Dissecting early regulatory relationships in the lamprey neural crest gene network. Proc Natl Acad Sci USA. 2008;105:20083–20088. doi: 10.1073/pnas.0806009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckenberg P, Buhl S, Woynecki T, et al. The transcription factor TCFAP2C/AP-2gamma cooperates with CDX2 to maintain trophectoderm formation. Mol Cell Biol. 2010;30:3310–3320. doi: 10.1128/MCB.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud C, Oulad-Abdelghani M, Bouillet P, Decimo D, Chambon P, Dolle P. AP-2.2, a novel gene related to AP-2, is expressed in the forebrain, limbs and face during mouse embryogenesis. Mech Dev. 1996;54:83–94. doi: 10.1016/0925-4773(95)00463-7. [DOI] [PubMed] [Google Scholar]
- Guttormsen J, Koster MI, Stevens JR, Roop DR, Williams T, Winger QA. Disruption of epidermal specific gene expression and delayed skin development in AP-2 gamma mutant mice. Dev Biol. 2008;317:187–195. doi: 10.1016/j.ydbio.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Eckert D, Nettersheim D, et al. Critical function of AP-2 gamma/TCFAP2C in mouse embryonic germ cell maintenance. Biol Reprod. 2009;82:214–223. doi: 10.1095/biolreprod.109.078717. [DOI] [PubMed] [Google Scholar]
- Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- Xia C, Wang C, Zhang K, Qian C, Jing N. Induction of a high population of neural stem cells with anterior neuroectoderm characters from epiblast-like P19 embryonic carcinoma cells. Differentiation. 2007;75:912–927. doi: 10.1111/j.1432-0436.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li L, Huang C, et al. Distinct functions of BMP4 during different stages of mouse ES cell neural commitment. Development. 2010;137:2095–2105. doi: 10.1242/dev.049494. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Rex M, Orme A, Uwanogho D, et al. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn. 1997;209:323–332. doi: 10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Pera E, Stein S, Kessel M. Ectodermal patterning in the avian embryo: epidermis versus neural plate. Development. 1999;126:63–73. doi: 10.1242/dev.126.1.63. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazzi G, Wang Y, Sassoon D. Msx2 is a transcriptional regulator in the BMP4-mediated programmed cell death pathway. Dev Biol. 1997;186:127–138. doi: 10.1006/dbio.1997.8576. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells. 2002;7:949–960. doi: 10.1046/j.1365-2443.2002.00573.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li J, Tan Z, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- Winger Q, Huang J, Auman HJ, Lewandoski M, Williams T. Analysis of transcription factor AP-2 expression and function during mouse preimplantation development. Biol Reprod. 2006;75:324–333. doi: 10.1095/biolreprod.106.052407. [DOI] [PubMed] [Google Scholar]
- Cajal M, Lawson KA, Hill B, et al. Clonal and molecular analysis of the prospective anterior neural boundary in the mouse embryo. Development. 2012;139:423–436. doi: 10.1242/dev.075499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C, De Almeida I, Papanayotou C, et al. Cell communication with the neural plate is required for induction of neural markers by BMP inhibition: evidence for homeogenetic induction and implications for Xenopus animal cap and chick explant assays. Dev Biol. 2009;327:478–486. doi: 10.1016/j.ydbio.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohibullah N, Donner A, Ippolito JA, Williams T. SELEX and missing phosphate contact analyses reveal flexibility within the AP-2[alpha] protein: DNA binding complex. Nucleic Acids Res. 1999;27:2760–2769. doi: 10.1093/nar/27.13.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Leung YK, Ho SM. AP-2 regulates the transcription of estrogen receptor (ER)-beta by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Oncogene. 2007;26:7346–7354. doi: 10.1038/sj.onc.1210537. [DOI] [PubMed] [Google Scholar]
- Koster MI, Kim S, Huang J, Williams T, Roop DR. TAp63alpha induces AP-2gamma as an early event in epidermal morphogenesis. Dev Biol. 2006;289:253–261. doi: 10.1016/j.ydbio.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Epidermal cell lineage. Biochem Cell Biol. 1998;76:889–898. doi: 10.1139/bcb-76-6-889. [DOI] [PubMed] [Google Scholar]
- Wang X, Pasolli HA, Williams T, Fuchs E. AP-2 factors act in concert with Notch to orchestrate terminal differentiation in skin epidermis. J Cell Biol. 2008;183:37–48. doi: 10.1083/jcb.200804030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Degenstein L, Copenhaver C, Fuchs E. Defining the regulatory factors required for epidermal gene expression. Mol Cell Biol. 2000;20:2543–2555. doi: 10.1128/mcb.20.7.2543-2555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Byrne C, Fuchs E. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci USA. 1991;88:7948–7952. doi: 10.1073/pnas.88.18.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Roop DR. The role of p63 in development and differentiation of the epidermis. J Dermatol Sci. 2004;34:3–9. doi: 10.1016/j.jdermsci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Yasue A, Tao H, Nohno T, Moriyama K, Noji S, Ohuchi H. Cloning and expression of the chick p63 gene. Mech Dev. 2001;100:105–108. doi: 10.1016/s0925-4773(00)00504-9. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Wanner R, Zhang J, Henz BM, Rosenbach T. AP-2 gene expression and modulation by retinoic acid during keratinocyte differentiation. Biochem Biophys Res Commun. 1996;223:666–669. doi: 10.1006/bbrc.1996.0952. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 2010;6:pii, e 1001133. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- McLarren KW, Litsiou A, Streit A. DLX5 positions the neural crest and preplacode region at the border of the neural plate. Dev Biol. 2003;259:34–47. doi: 10.1016/s0012-1606(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Sato T, Sasai N, Sasai Y. Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development. 2005;132:2355–2363. doi: 10.1242/dev.01823. [DOI] [PubMed] [Google Scholar]
- Khudyakov J, Bronner-Fraser M. Comprehensive spatiotemporal analysis of early chick neural crest network genes. Dev Dyn. 2009;238:716–723. doi: 10.1002/dvdy.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Thomas ML, Weeks DL, Sargent TD. Transcription factor AP-2 is an essential and direct regulator of epidermal development in Xenopus. Dev Biol. 2002;245:136–144. doi: 10.1006/dbio.2002.0621. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Starbuck MW, Gentile MA, et al. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem. 2004;279:27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Luo T, Sargent TD. Expression of TFAP2beta and TFAP2gamma genes in Xenopus laevis. Gene Expr Patterns. 2006;6:589–595. doi: 10.1016/j.modgep.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Jin Z, Liu L, Bian W, et al. Different transcription factors regulate nestin gene expression during P19 cell neural differentiation and central nervous system development. J Biol Chem. 2009;284:8160–8173. doi: 10.1074/jbc.M805632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- Gao X, Bian W, Yang J, et al. A role of N-cadherin in neuronal differentiation of embryonic carcinoma P19 cells. Biochem Biophys Res Commun. 2001;284:1098–1103. doi: 10.1006/bbrc.2001.5089. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kamachi Y, Tanouchi A, Hamada H, Jing N, Kondoh H. Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol. 2004;24:8834–8846. doi: 10.1128/MCB.24.20.8834-8846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Shimazaki T, Sobue G, Okano H. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol. 2004;275:124–142. doi: 10.1016/j.ydbio.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Papanayotou C, Stern CD. Spatially and temporally controlled electroporation of early chick embryos. Nat Protoc. 2008;3:419–426. doi: 10.1038/nprot.2008.10. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Combined whole-mount in situ hybridization and immunohistochemistry in avian embryos. Methods. 2001;23:339–344. doi: 10.1006/meth.2000.1146. [DOI] [PubMed] [Google Scholar]
- Huang C, Chen J, Zhang T, et al. The dual histone demethylase KDM7A promotes neural induction in early chick embryos. Dev Dyn. 2010;239:3350–3357. doi: 10.1002/dvdy.22465. [DOI] [PubMed] [Google Scholar]
- Peng G, Han M, Du Y, et al. SIP30 is regulated by ERK in peripheral nerve injury-induced neuropathic pain. J Biol Chem. 2009;284:30138–30147. doi: 10.1074/jbc.M109.036756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AP2γ is induced by BMP4 dose-dependently in P19 cell neural differentiation.
AP2γ knockdown doesn't affect the pluripotency of ESCs and the expression of other germ layers markers during differentiation.
AP2γ overexpression has no impact on the pluripotency and the proliferation rates of ESCs.
AP2γ dosen't mediate mesendoderm induction by BMP4 during ESC neural differentiation.
The section of cAP2γ stained chick embryos.
Comparative expression pattern of epidermal markers.
The effects of cAP2γ knockdown or overexpression on the expression of cSox2, cKer14 and cSox3.
BMP4 effects on ectodermal patterning were partially impaired by AP2γ siRNA.
AP2γ might be directly regulated by BMP4.
The model for AP2γ functions in ectodermal patterning of chick embryo.
The diagram of AP2γ functional analysis in ESCs.
ShRNA sepuences
Primer sequences (5′→3′) for cloning
Primer sequences (5′→3′) for Q-PCR and RT-PCR
Primer sequences (5′→3′) for ChIP assay