Dear Editor,
Genomic sequencing of human tumor-derived DNA has identified a vast number of novel tissue-specific genetic mutations occurring at varying frequencies1. Frequently mutated genes are informally referred to as driver alterations (or candidate cancer genes, CAN-genes), whereas those less frequently mutated are considered passenger alterations1,2. With few exceptions, the majority of mutations are loss-of-function alterations and the ones that occur rarely are presumed to be a byproduct of genomic instability or normal mutation rates that do not directly contribute to tumor progression (passengers)3. In addition to computational and biostatistical models for parsing driver from passenger mutations, the utility of biological assays to functionally interrogate cancer genomes is required to determine which of these mutations are able to contribute to a particular tumorigenic phenotype (i.e., anchorage-independent growth, resistance to apoptosis, enhanced invasion through extracellular matrices). Moreover, these assays can also decipher whether less frequently mutated genes are actively involved in cancer progression. Thus, the development of experimental methods to separate putative driver from passenger mutations is critical towards understanding which genes are directly promoting tumorigenesis. Although sequencing of the human colorectal cancer (CRC) genome, in parallel with computational analyses, has detected 140 frequently mutated CAN-genes and up to ∼700 mutations occurring at a lower frequency (passengers)1, it is difficult to distinguish whether these mutations play a causal or incidental role in tumor development. The identification of those mutated genes with tumor-associated roles may therefore lead to novel therapeutic avenues as we transition towards an era of personalized medicine and targeted therapy.
Advances in RNA interference (RNAi) technologies have enabled loss-of-function screens to identify tumor suppressors in mammalian cells, the knockdown of which promotes an assayable cancer-associated phenotype(s)4. Compared to whole-genome screens, RNAi screens focusing on specific gene sets obtained from cancer genome sequencing data are suitable for classifying potential driver from passenger alterations. To identify tumor suppressors of human CRC, we conducted a loss-of-function screen using a small interfering RNA (siRNA) library (Dharmacon, Lafayette, CO) targeting the most frequently mutated genes in CRC (CAN-genes) for the ability to permit cell invasion, a hallmark of cancer. The initial step in the progression of a localized tumor towards invasive and metastatic disease occurs when cells acquire the ability to invade through a basement membrane5. In this screen, we utilized a modified Boyden chamber coated with Matrigel (BD Biosciences, San Jose, CA), a reconstituted extracellular matrix, to recapitulate these events6 using normal migratory, yet non-invasive, epithelial cells. These screens were carried out with non-malignant, Cdk4- and hTERT-immortalized human colonic epithelial cells (HCEC)7 in the background of either TP53-knockdown (1CTP) using short hairpin RNAs (shRNA) or ectopic expression of oncogenic KRASV12 (1CTR)8. The gene sets used in this study (CAN-genes) were derived from the study of Wood et al1. According to this study, APC, KRAS, TP53, and PIK3CA are mutated most often across a panel of sequenced colon tumors.
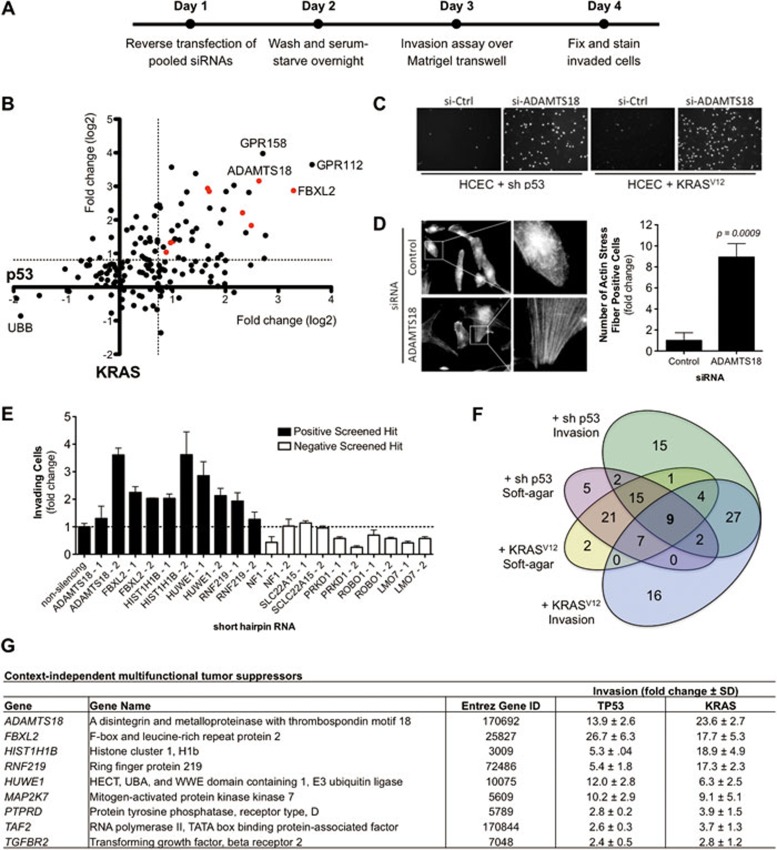
Figure 1A represents a diagram of the screening scheme. Briefly, pooled siRNAs (four siRNAs per gene, sequences listed in Supplementary information, Table S1, methods listed in Supplementary information, Data S1) were reverse-transfected into 1CTP and 1CTR HCECs in 2% serum growth medium for 24 h before cells were washed and serum-starved overnight. Cells transfected for 48 h were then harvested in serum-free medium and plated onto 24-well Matrigel-coated transwell filters (BD Biosciences, San Jose, CA). The bottom chamber is filled with 2% serum medium plus growth factors as a chemoattractant. After overnight incubation, invaded cells were then fixed for Hoechst staining (Invitrogen, Grand Island, NY). Images were taken at 10× with five fields imaged per well. Altogether, pooled siRNAs targeting 159 genes (140 CAN-genes plus 19 additional “passenger” genes that closely interacted with CAN-genes through interaction mapping8) were tested in two cell lines (1CTP and 1CTR) for the ability to enhance invasion in vitro. The overall screening results are shown in Figure 1B as a scatterplot wherein the x- and y-axis represent the fold changes in invasion in the background of 1CTP and 1CTR cells, respectively. siRNAs targeting the essential ubiquitin B (UBB) gene were used to determine transfection efficiency and as a negative control, and a non-targeting control siRNA was used for normalization. A statistical cutoff point of three standard deviations was used to eliminate noise and false positives.
Figure 1.
(A) Overall scheme of focused siRNA screen for tumor suppressors involved in regulating the invasive potential of normal cells. 1CTP (shRNA-TP53) and 1CTR (oncogenic-KRASV12) HCECs were reverse-transfected 48 h with pooled siRNAs prior to re-plating onto Matrigel-coated transwell filters. Invaded cells were Hoechst-stained and data were normalized to non-targeting siRNA control. (B) Scatterplot of the overall screening results. The x-axis represents fold changes in invasion in 1CTP cells, whereas the y-axis represents those for 1CTR cells. Each dot represents a single gene targeted by pooled siRNAs (four siRNAs/gene) and red dots represent multifunctional tumor suppressor genes (listed in G). The dotted line represents the statistical cutoff point and is defined as three standard deviations. (C) Representative images of Hoechst-stained invaded 1CTP and 1CTR cells with control siRNA or siRNA targeting the ADAMTS18 gene. (D) Depletion of ADAMTS18 using siRNAs induces the formation of F-actin stress fibers. (E) Screening results were partially validated in 1CTP cells using shRNA-mediated knockdown of five positive hits and five negative non-hits. Fold change in invasion is normalized to non-silencing shRNA. (F) Venn diagram of overlapping soft-agar growth and invasion suppressing genes in each cell background. Soft-agar hits were obtained from a previously published screen8. Nine genes were identified through comparative analysis of both screens. RNAi knockdown of these genes leads to multiple tumorigenic phenotypes. (G) List of the nine context-independent multifunctional tumor suppressor hits. Knockdown of these nine genes enhances both anchorage-independent growth and invasion in two independent backgrounds in vitro (also shown as red dots in B). Bar graphs are depicted as mean ± SEM.
Depletion of 42 out of 159 (26.4%) screened genes promoted invasion in both cell backgrounds in a context-independent manner. The overall distribution of all siRNA pools screened in both backgrounds (calculated as changes in cell invasion) is depicted graphically in Supplementary information, Figure S1 and numerically in Supplementary information, Tables S2 and S3. The results of the twenty most potent suppressors of invasion in each context are shown in Supplementary information, Figure S2. Among these, knockdown of two G-protein coupled receptors (GPR112 and GPR158) with previously unidentified roles in cancer progression was among the most effective in inducing cell invasion. Moreover, Figure 1C shows representative screening images of Hoechst-stained invaded cells on a transwell filter where depletion of ADAMTS18 levels by pooled siRNAs induces context-independent cell invasion. Deconvolution of pooled ADAMTS18 siRNAs revealed that three out of four siRNAs tested positive for increased cell invasion, strongly suggesting “on-target” effects of RNAi (Supplementary information, Figure S3). Since stress fiber formation has been associated with cell migration and focal adhesion assembly that may promote cell invasion9, as a proof of principle experiment, we next determined whether ADAMTS18 depletion could lead to rearrangements in the actin cytoskeleton. siRNA-mediated knockdown of ADAMTS18 in 1CTP HCECs induced an approximate nine-fold increase in the number of cells positive for actin stress fiber formation compared to a non-targeting control siRNA (Figure 1D).
Next, we sought to validate several of the identified hits using stable shRNA knockdowns (pGIPZ library, Open Biosystems, Lafayette, CO). We chose to retest five genes among each group that scored positive in the siRNA screen and five random genes that scored negative using two shRNAs per gene in 1CTP cells. A non-targeting shRNA was used for normalization. On average, shRNAs targeting genes that scored positive from the screen (black bars) resulted in a 2.3 ± 0.8-fold change in invasion compared to 0.6 ± 0.3-fold change using shRNAs against negatively scored genes (white bars) (Figure 1E). Quantitative real-time PCR were performed on four of the positive hits (FBXL2, HIST1H1B, HUWE1, and RNF219) to determine shRNA knockdown efficiency (primer sequences listed in Supplementary information, Table S4). Seven out of eight hairpins caused marked reduction in target gene mRNA levels (Supplementary information, Figure S4), with the exception of one shRNA targeting HIST1H1B.
We have previously completed similar loss-of-function screens for tumor suppressors, the knockdown of which allows for bypass of anchorage-dependent proliferation restraints (i.e., growth in soft-agar)8. From those studies, we identified 52 context-independent regulators of soft-agar growth representing ∼33% of all CAN-genes, an unexpectedly high percentage. Cross-analysis with this previously completed screen for soft-agar growth revealed that nine out of the 42 invasion-suppressing genes identified in the current study also permitted anchorage-independent growth upon knockdown (Figure 1F and Supplementary information, Table S5). These nine overlapping genes represent those mutated at a high frequency in human CRC with multiple tumorigenic phenotypes when depleted in vitro in a context-independent manner (Figure 1G). Furthermore, the effect of these siRNAs on HCEC proliferation is shown in Supplementary information, Figure S5.
One of the most potent suppressors of invasion that also conferred soft-agar growth upon RNAi-mediated knockdown is the ADAMTS18 gene. ADAMTS18 (a disintegrin and metalloproteinase with thrombospondin motifs 18) belongs to a family of ADAMTS peptidases with well-studied roles in extracellular matrix degradation and reorganization. Mutations in ADAMTS18 have previously been linked to melanoma progression10. ADAMTS18 is mutated in 6% of colorectal carcinomas out of 193 sequenced tumors (The Cancer Genome Atlas), representing a large number of patients out of the ∼140 000 annually diagnosed colon cancer cases in the United States alone (American Cancer Society).
In summary, these results allow for deconstructing driver and passenger alterations within a given tumor context characterized by cancer-associated phenotypes. Loss-of-function screening using RNAi provides a robust method to rapidly analyze the cellular effects of depleting specific genes identified from cancer genome sequencing efforts. While not comprehensive of all the genomic changes that occur in CRC, these studies are an initial step in validating and complementing bioinformatic approaches in selecting the most critical genes to pursue for future therapeutic discovery. The other multifunctional tumor suppressors identified from comparative analysis of the two independent screens include a group of ubiquitin ligases (FBXL2, RNF219, HUWE1), a histone protein (HIST1H1B), cell signaling components (MAP2K7, PTPRD, TGFBR2), and a subunit of transcription factor II D (TAF2). Future studies will involve screening candidate driver mutations for the ability to bypass CRC chemotherapy-induced apoptosis (i.e., mutations promoting resistance to 5-fluorouracil treatment).
Acknowledgments
This work is supported by CPRIT Training Grant (RP101496) to PL, NASA Grants (NNX09AU95G, NNX11AC15G and NNX11AC54G) to JWS, and NCI SPORE (CA70907) to JWS.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Material
Overall distribution of all pooled siRNAs screened in (A) 1CTP and (B) 1CTR human colonic epithelial cells (HCECs) for changes in cell invasion.
The top twenty most potent enhancers of invasion in (A) 1CTP and (B) 1CTR HCECs upon depletion by pooled siRNAs.
Deconvolution of pooled ADAMTS18 siRNAs in (A) 1CTP and (B) 1CTR HCECs for enhancements in cell invasion.
shRNA knockdown efficiency as assessed by quantitative real-time PCR.
Transfection of pooled siRNAs targeting the context-independent multifunctional tumor suppressors do not drastically change proliferation of (A) 1CTP and (B) 1CTR HCECs after 96hr transfection.
Screened siRNA Sequences
Fold change in invasion relative to control (TP53-Knockdown)
Fold change in invasion relative to control (Oncogenic KRAS)
Quantitative PCR Primer Sequences
Cross-analysis of invasion and soft-agar suppressors
Materials and Methods
References
- Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Stratton MR. Exploring the genomes of cancer cells: progress and promise. Science. 2011;331:1553–1558. doi: 10.1126/science.1204040. [DOI] [PubMed] [Google Scholar]
- Carter H, Chen S, Isik L, et al. Cancer-specific high-throughput annotation of somatic mutations: computational prediction of driver missense mutations. Cancer Res. 2009;69:6660–6667. doi: 10.1158/0008-5472.CAN-09-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabach MR, Luo J, Solimini NL, et al. Cancer proliferation gene discovery through functional genomics. Science. 2008;319:620–624. doi: 10.1126/science.1149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N, Kang Y. Unravelling the complexity of metastasis-molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11:735–748. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Iwamoto Y, Kleinman HK, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- Roig AI, Eskiocak U, Hight SK, et al. Immortalized epithelial cells derived from human colon biopsies express stem cell markers and differentiate in vitro. Gastroenterology. 2010;138:1012–1021. doi: 10.1053/j.gastro.2009.11.052. [DOI] [PubMed] [Google Scholar]
- Eskiocak U, Kim SB, Ly P, et al. Functional parsing of driver mutations in the colorectal cancer genome reveals numerous suppressors of anchorage-independent growth. Cancer Res. 2011;71:4359–4365. doi: 10.1158/0008-5472.CAN-11-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Prickett TD, Viloria CG, et al. Mutational and functional analysis reveals ADAMTS18 metalloproteinase as a novel driver in melanoma. Mol Cancer Res. 2010;8:1513–1525. doi: 10.1158/1541-7786.MCR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overall distribution of all pooled siRNAs screened in (A) 1CTP and (B) 1CTR human colonic epithelial cells (HCECs) for changes in cell invasion.
The top twenty most potent enhancers of invasion in (A) 1CTP and (B) 1CTR HCECs upon depletion by pooled siRNAs.
Deconvolution of pooled ADAMTS18 siRNAs in (A) 1CTP and (B) 1CTR HCECs for enhancements in cell invasion.
shRNA knockdown efficiency as assessed by quantitative real-time PCR.
Transfection of pooled siRNAs targeting the context-independent multifunctional tumor suppressors do not drastically change proliferation of (A) 1CTP and (B) 1CTR HCECs after 96hr transfection.
Screened siRNA Sequences
Fold change in invasion relative to control (TP53-Knockdown)
Fold change in invasion relative to control (Oncogenic KRAS)
Quantitative PCR Primer Sequences
Cross-analysis of invasion and soft-agar suppressors
Materials and Methods