Abstract
The biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway (Carum carvi L.) proceeds from geranyl diphosphate via a three-step pathway. First, geranyl diphosphate is cyclized to (+)-limonene by a monoterpene synthase. Second, this intermediate is stored in the essential oil ducts without further metabolism or is converted by limonene-6-hydroxylase to (+)-trans-carveol. Third, (+)-trans-carveol is oxidized by a dehydrogenase to (+)-carvone. To investigate the regulation of monoterpene formation in caraway, we measured the time course of limonene and carvone accumulation during fruit development and compared it with monoterpene biosynthesis from [U-14C]Suc and the changes in the activities of the three enzymes. The activities of the enzymes explain the profiles of monoterpene accumulation quite well, with limonene-6-hydroxylase playing a pivotal role in controlling the nature of the end product. In the youngest stages, when limonene-6-hydroxylase is undetectable, only limonene was accumulating in appreciable levels. The appearance of limonene-6-hydroxylase correlates closely with the onset of carvone accumulation. At later stages of fruit development, the activities of all three enzymes declined to low levels. Although this correlates closely with a decrease in monoterpene accumulation, the latter may also be the result of competition with other pathways for substrate.
Monoterpenes are 10-carbon members of the isoprenoid family of natural products (Gershenzon and Croteau, 1993). They are widespread in the plant kingdom (Banthorpe and Charlwood, 1980) and are often responsible for the characteristic odors of plants. These substances are believed to function principally in ecological roles, serving as herbivore-feeding deterrents, antifungal defenses, and attractants for pollinators (Langenheim, 1994). The commercial importance of monoterpenes as flavorings, fragrances, and pharmaceuticals has stimulated many efforts to increase their yield in plants.
Caraway (Carum carvi L.), native to Europe, western Asia, and northern Africa, is an important monoterpene-containing herb, which contains (+)-carvone and (+)-limonene as its major monoterpene components. The caraway fruit is a schizocarp, which at harvest is separated into two halves, which are called “seeds.” In this paper we use the agricultural terms “seed” for a half-fruit and “fruit” for the entire fruit (two “seeds”). The seeds and seed oil of caraway are used traditionally in ice cream, candy, baked goods, meat, cheese, pickles, condiments, soft drinks, and alcoholic beverages (Morton, 1976). Recently, (+)-carvone extracted from caraway seeds has been introduced as an effective sprouting inhibitor of potatoes (Oosterhaven et al., 1995). The expanding commercial potential of (+)-carvone has now generated interest in maximizing the yield of this substance from caraway seeds, a goal that requires an understanding of the process of carvone biosynthesis.
More than 30 years ago, Sandermann and Bruns (1965) hypothesized that in dill fruits, which also contain (+)-carvone and (+)-limonene as the main components of its essential oil, limonene is an intermediate in the biosynthesis of carvone. During fruit development the content of carvone (as a percentage of fruit weight) increases at the expense of limonene, providing support for this hypothesis. However, after measuring the changes in the absolute amounts of limonene and carvone in caraway fruits and performing in vivo-radiolabeling experiments, Von Schantz and Ek (1971) and Von Schantz and Huhtikangas (1971) showed that limonene is no longer available as a precursor for carvone biosynthesis once it has been secreted into the essential oil ducts.
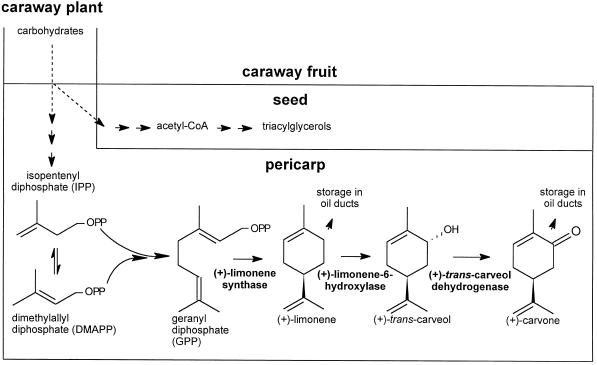
The pathway of (+)-limonene and (+)-carvone biosynthesis in caraway has been assumed (Bouwmeester et al., 1995b) to be analogous to the biosynthesis of (−)-limonene and (−)-carvone in spearmint (Gershenzon et al., 1989) (Fig. 1). In this process, GPP, the ubiquitous precursor of the monoterpenes, is cyclized by a monoterpene synthase to (+)-limonene. The product is either stored in the essential oil ducts or oxidized to (+)-trans-carveol by a Cyt P-450-dependent hydroxylase. Subsequently, a NAD+ or NADP+-utilizing dehydrogenase oxidizes (+)-trans-carveol to (+)-carvone, which is then stored exclusively in the essential oil ducts.
Figure 1.
Biosynthetic pathway of (+)-limonene and (+)-carvone in fruits of caraway.
The annual and biennial forms of caraway have been studied, and show some interesting differences in essential oil formation. Although both forms produce an essential oil that consists predominantly of carvone and limonene, the fruits of annual caraway varieties generally have a lower essential oil content than the fruits of biennial varieties (Bouwmeester and Kuijpers, 1993), probably because of a greater carbon partitioning to essential oil formation in biennial caraway (Bouwmeester et al., 1995a). In addition, biennial caraway usually has a higher carvone-to-limonene ratio.
For both caraway forms, accumulation of limonene and carvone in the fruits is a developmentally regulated process. Whereas limonene accumulation predominates in the early stages of development, carvone accumulation predominates in the later stages such that, when the fruits mature, carvone and limonene contents are approximately equal (Von Schantz and Ek, 1971; Bouwmeester and Kuijpers, 1993; Bouwmeester et al., 1995a). Although it has been suggested that carvone and limonene accumulation continue until fruit maturity (Von Schantz and Ek, 1971; Von Schantz and Huhtikangas, 1971), Bouwmeester et al. (1995a) showed that carvone and limonene accumulation ceases several weeks before.
The pattern of carvone and limonene accumulation in caraway fruits may be explained by the changing levels of biosynthetic enzyme activities during fruit development. In the early stages of development, when only limonene accumulates, the enzyme that converts limonene to trans-carveol may be inactive (Fig. 1). At a later stage, when both limonene and carvone are accumulating, the ratio of limonene to carvone being formed may depend on the relative activities of the enzymes in the pathway. Finally, when limonene and carvone accumulation has ceased, one or more of the enzymes in the pathway before limonene may have been inactivated. Alternatively, the formation of other components of the developing fruit, such as storage proteins, starch, or triacylglycerols, may divert the flow of carbon away from monoterpene biosynthesis.
To better understand the mechanisms regulating the developmental changes of limonene and carvone accumulation in caraway fruits, we investigated the pathway of limonene and carvone biosynthesis from the ubiquitous C10 intermediate GPP. The activities of all three enzymes involved were demonstrated, and their substrate specificities determined. In addition, we studied the appearance of the relevant biosynthetic enzymes during fruit development in annual and biennial caraway in relation to the formation of monoterpenes and the accumulation of triacylglycerols, the major seed reserve.
MATERIALS AND METHODS
Seeds of annual caraway (Carum carvi L. var Karzo) were sown in January, 1995, in potting compost in 5-L plastic containers and after emergence thinned to one plant per pot. Taproots of biennial caraway (C. carvi L. var Bleija) were obtained from an experimental field in Wageningen, The Netherlands, in February, 1995, before regrowth had started, submerged in 2 g/L benomyl for 10 to 15 min to prevent fungal diseases, and planted individually in potting compost in 5-L plastic containers. All plants were placed in a greenhouse at 18/14°C under a cycle of 12-h light/12-h dark and 75% RH. Natural daylight was supplemented with artificial light (Philips, Eindhoven, The Netherlands) during the 12-h high-temperature period, and fertilizer was applied as required. Aphids, thrips, and spider mites were controlled by spraying with pirimicarb, imidacloprid, and methomyl. Powdery mildew was controlled by using a sulfur vaporizer.
Flowering of both caraway varieties started around the beginning of May. As an umbelliferous species, caraway has several umbel orders that flower successively in time. The umbels of each umbel order are all in the same developmental stage. Dates of female flowering for 1st- to 4th-order umbels were determined for all individual plants. Because of the inherent variation in flowering time between umbel orders and the variation in flowering time between plants, a range of different developmental stages was obtained. From the beginning of flowering, houseflies were released into the greenhouse at regular intervals to ensure pollination. Because the flies are efficient pollinators of caraway, it was assumed that pollination occurred at the date of female flowering when pistils were receptive.
For the developmental study, nine umbel orders were selected from both caraway varieties on May 22 and June 12 such that the samples ranged in development from 5 to 34 DAP for the biennial form and 7 to 34 DAP for the annual form (Table I). From previous experiments (Bouwmeester and Kuijpers, 1993), it was known that the formation of limonene and carvone level off at about 30 DAP. Representative umbellets of each developmental stage were used for the [14C]Suc-feeding experiment, and fruits were collected from the remaining umbels/umbellets for chemical and biochemical analyses by cutting the stalks with a small pair of scissors just below the fruits. About 0.5 g of fruits was used for the analysis of limonene, carvone, and fatty acid content, whereas about 1 g was employed in the assays of (+)-limonene synthase, (+)-limonene-6-hydroxylase, and (+)-trans-carveol dehydrogenase activities. Fruits were accurately weighed out into plastic vials, frozen in liquid N2, and stored at −80°C until further analysis. Fruit dry matter percentage and mean individual fruit weight were determined by weighing about 0.5 to 1.0 g of fruits before and after drying overnight at 105°C.
Table I.
Dates of pollination (female flowering) and harvest of fruits of annual and biennial caraway and their developmental stage
| Plant and Umbel Order | Date
|
Developmental Stage | |
|---|---|---|---|
| Pollination | Harvest | ||
| DAP | |||
| Annual | |||
| 3 | May 15 | May 22 | 7 |
| 2 | May 12 | May 22 | 10 |
| 2 | May 9 | May 22 | 13 |
| 3 | May 7 | May 22 | 15 |
| 4 | May 27 | Jun 12 | 16 |
| 3 | May 25 | Jun 12 | 18 |
| 4 | May 16 | Jun 12 | 27 |
| 2 | May 15 | Jun 12 | 28 |
| 3 | May 9 | Jun 12 | 34 |
| Biennial | |||
| 3 | May 17 | May 22 | 5 |
| 4 | May 15 | May 22 | 7 |
| 3 | May 12 | May 22 | 10 |
| 3 | May 8 | May 22 | 14 |
| 4 | May 23 | Jun 12 | 20 |
| 4 | May 20 | Jun 12 | 23 |
| 4 | May 16 | Jun 12 | 27 |
| 4 | May 13 | Jun 12 | 30 |
| 3 | May 9 | Jun 12 | 34 |
The demonstration of enzyme activities, optimization of assay conditions, and investigation of substrate and product stereospecificity were carried out using bulk samples of suitable developmental stages, which were collected from plants raised in the greenhouse as described above (biennial caraway) or in the field (annual caraway).
Preparation of Substrates
[1-3H]GPP was synthesized as described by Croteau and Cane (1985). (+)- and (−)-Limonene were from Janssen Chimica (Geel, Belgium) and Merck-Schuchardt (Hohenbrunn, Germany), respectively. trans-Carveol was obtained by Meerwein-Ponndorf-Verley reduction of (+)-carvone using aluminum isopropoxide and dry isopropyl alcohol (Ponndorf, 1926; Johnston and Read, 1934). The resulting mixture of trans- and cis-carveol and a commercial (−)-cis/trans-carveol mixture (Janssen Chimica) were separated using a preparative gas-liquid chromatograph (model 3700, Varian, Sunnyvale, CA) equipped with a glass column (2-m × 4-mm i.d.) packed with 10% (w/w) Carbowax HP on Chromosorb 100–120 (Chrompack International, Middleburg, The Netherlands). The oven temperature was 140°C isothermal, injection port temperature was 200°C, and H2 column flow was 50 mL min−1. The desired product (as judged from the thermal conductivity detector signal) was allowed to condense in a glass capillary, and the compounds were then eluted from the capillary with pentane.
Chiral analysis was performed by GC-MS using a HP 5890 series II gas chromatograph and HP 5972A mass selective detector (Hewlett-Packard) equipped with a fused silica capillary column (25-m × 0.25-mm i.d.) with octakis (6-O-methyl-2, 3-di-O-pentyl)-γ-cyclodextrin (80% [w/w] in OV1707) as the stationary phase (König et al., 1990). The oven was programmed at an initial temperature of 45°C for 1 min, with a ramp of 10°C min−1 to 200°C and a final time of 5 min. The injection port (splitless mode), interface, and MS source temperatures were 175, 290, and 180°C, respectively, and the He inlet pressure was controlled by electronic pressure to achieve a constant column flow of 1.0 mL min−1. Ionization potential was set at 70 eV, and scanning was performed both from 50 to 175 atomic mass units and in the selected ion-monitoring mode: for limonene m/z 68, 93, and 136; for camphor m/z 81, 95, and 152; for carvone m/z 82, 108, and 150; for trans-carveol m/z 84, 109, and 152; and for cis-carveol m/z 84, 109, and 134. Analysis showed that racemization had occurred during the synthesis of the carveols. The mixtures obtained after preparative GC separation were (+)- and (−)-trans-carveol- 1.1:1 and (+)- and (−)-cis-carveol- 1.9:1. Preparative GC separation of the commercial (−)-cis/trans-carveol mixture gave purified samples of (−)-trans-carveol and (−)-cis-carveol, each with <1% of the other geometrical isomer.
Enzyme Isolation
During enzyme isolation and preparation of the assays, all operations were carried out on ice or at 4°C. The extraction procedure and buffer were adjusted principally to optimize hydroxylase activity (H.J. Bouwmeester, M.C.J.M. Konigs, J. Gershenzon, F. Karp, and R. Croteau, unpublished data), which also gave high levels of activity for the other two enzymes. The frozen fruit samples were ground in a prechilled mortar and pestle in prechilled buffer (10 mL/g tissue) containing 50 mm Hepes (pH 7.5), 20% (v/v) glycerol, 50 mm sodium ascorbate, 50 mm NaHSO3, 5 mm MgCl2, 2.5 mm EDTA, 2.5 mm EGTA, 5 mm DTT, 5 μm FAD, 5 μm FMN, 0.5 mm glutathione, 2 mg mL−1 BSA, 5 μg mL−1 leupeptin, and 25 IU mL−1 catalase slurried with 1 g of polyvinylpolypyrrolidone and three spatula tips of purified sea sand. During grinding, additional aliquots of buffer (without polyvinylpolypyrrolidone and sea sand) were added to a total volume of 30 mL/g fruits. The homogenate was transferred to a small beaker containing polystyrene resin (0.5 g/g fruit, Amberlite XAD-4, Serva, Paramus, NJ), sonicated for 4 min in 10-s pulses (on ice), stirred carefully for 12 min, and then filtered through cheesecloth. The filtrate was centrifuged at 20,000g for 20 min (pellet discarded) and then at 150,000g for 90 min. The supernatant was used to assay limonene synthase and trans-carveol dehydrogenase activity, and the 150,000g pellet was used to assay limonene-6-hydroxylase activity. Crude fractions were diluted before assay so that the activities were linear over the time period measured for all developmental stages.
Limonene Synthase
For routine determination of enzyme activity, 5 μL of the 150,000g supernatant was diluted 20-fold in an Eppendorf tube with buffer A (15 mm Tris [pH 7.5], 10% [v/v] glycerol, 50 mm KCl, 1 mm sodium ascorbate, 1 mm MnCl2, and 2 mm DTT), and 35 μm [1-3H]GPP (21 Ci/mol) was added. The reaction mixture was overlaid with 1 mL of hexane to trap volatile products and the contents were mixed. After incubation for 30 min at 30°C, the vials were vigorously mixed and centrifuged briefly to separate phases. A portion of the hexane phase (750 μL) was transferred to a new Eppendorf tube containing 40 mg of silica gel (0.035–0.07 mm, pore diameter 6 nm, Janssen Chimica) to bind terpenols produced by phosphohydrolases, and after mixing and centrifugation 500 μL of the hexane layer was removed for liquid-scintillation counting in 4.5 mL of Ultima Gold cocktail (Packard, Meriden, CT). All assays were performed in duplicate or triplicate; controls that had been boiled for 5 min showed no enzymatic activity.
For product identification using radio-GLC, 100 μL of the 150,000g supernatant was diluted 10-fold with buffer A in a 9-mL Teflon-lined screw-cap tube. After the addition of 3 μm [1-3H]GPP (250 Ci/mol) and a 1-mL pentane overlay to trap volatile products, the tube was carefully mixed and incubated for 1 h at 30°C. Following the assay, the tube was vigorously mixed and stored at −20°C until further analysis. After thawing, 250 μL of diethyl ether was added to the assay mixture. The organic layer was removed and passed over a short column of silica gel overlaid with anhydrous MgSO4. The assay was extracted with another 1 mL of pentane-diethyl ether (4:1, v/v), which was also passed over the silica column, and the column was washed with 1.5 mL of pentane. After addition of unlabeled α-pinene, sabinene, myrcene, limonene, γ-terpinene, and p-cymene as the carriers, monoterpenes that have all been reported to be constituents of caraway seed essential oil (Wichtmann, 1988), the mixture was slowly concentrated under a stream of N2.
Radio-GLC was performed on a gas chromatograph (series 4160, Cazlo-Erba, Milano, Italy) equipped with a RAGA-90 radioactivity detector (Raytest, Straubenhardt, Germany). Sample components eluting from the column were quantitatively reduced before radioactivity measurement by passage through a conversion reactor filled with platinum chips at 800°C. Two-microliter samples were injected in the cold on-column mode. The fused silica capillary column (30-m × 0.32-mm i.d.) was coated with a film of 0.25 μm of PEG (EconoCap EC-WAX, Alltech Associates, Inc., Deerfield, IL) and operated with a He inlet pressure of 1.35 kg cm−2, giving a flow of 1 mL min−1. The oven temperature was programmed to 70°C for 5 min, followed by a ramp of 5° min−1 to 200°C and a final time of 5 min. To determine retention times and peak identities (by co-elution of radioactivity with reference standards), about 20% of the column effluent was split with an adjustable splitter to a flame-ionization detector (temperature 270°C). The remainder was directed to the conversion reactor and radiodetector. H2 was added prior to the reactor at 3 mL min−1, and CH4 as a quench gas prior to the radioactivity detector (5-mL counting tube) to give a total flow of 36 mL min−1.
Limonene-6-Hydroxylase
Pellets from the 150,000g centrifugation were resuspended in 3.5 mL of buffer B (50 mm Tris [pH 7.2 for the biennial form, pH 7.4 for the annual form], 20% [v/v] glycerol, 1 mm EDTA, 2 mm DTT, 1 μg mL−1 leupeptin, 5 μm FAD, and 5 μm FMN) using a glass rod and a Teflon potter. A 0.5-mL portion of the resuspended pellet was then diluted 2-fold in buffer B in a 9-mL Teflon-lined screw-cap tube, and the reaction was started by the addition of 1 mm NADPH, 5 mm Glc-6-P, 1 IU of Glc-6-P dehydrogenase, and 200 nmol (+)-limonene in 5 μL of hexane. The assays were performed in duplicate. Controls that had been boiled for 5 min showed no enzymatic activity. After incubation for 1 h at 30°C, 1 mL of diethyl ether was added, and the tubes were vigorously mixed and then stored at −20°C until further analysis. For analysis, 25 nmol camphor was added as an internal standard. The reaction mixtures were thawed, vigorously mixed, and briefly centrifugated to separate phases.
The ether phase was then transferred to another vial and the water layer was re-extracted with another 1-mL portion of diethyl ether. Next, the combined diethyl ether extracts were decolorized with activated charcoal, washed with 1 mL of water and, after centrifugation, passed over a short column of silica gel overlaid with anhydrous MgSO4. The water phase was extracted with another 1 mL of diethyl ether, which was also passed over the MgSO4/silica column. After 500 μL of hexane was added, the extracts were concentrated to about 500 μL using a Gyrovap GT centrifugal evaporator (Howe, Banburry, UK) and, subsequently, a stream of N2. Samples were analyzed for camphor, cis- and trans-carveol, and carvone content using GC-MS in the selected ion-monitoring mode as described above, but with an HP-5MS column (30-m × 0.25-mm i.d., 0.25-μm film thickness, 5% [w/w] diphenyl and 95% [w/w] dimethylpolysiloxane stationary phase, Hewlett-Packard) and an oven temperature program of 45°C for 1 min, ramp of 10° min−1 to 220°C, and a final time of 5 min. To assess the levels of carveols initially present, control assays were run in which the reaction was stopped immediately after substrate addition by adding 1 mL of diethyl ether and vigorous mixing.
Carveol Dehydrogenase
Aliquots (50 μL) of the 150,000g supernatant were diluted 20-fold with buffer containing 50 mm Gly (pH 10.5), 10% (v/v) glycerol, and 2 mm DTT in a 9-mL Teflon-lined screw-cap tube. The reaction was started by the addition of 1 mm NAD+ and 240 nmol trans-carveol (a mixture of 130 nmol of the [+]-enantiomer and 110 nmol of the [−]-enantiomer) in 5 μL of pentane. Assays were performed in duplicate. Controls that had been boiled for 5 min showed no enzymatic activity. After incubation for 1 h at 30°C, assays were analyzed as described for limonene-6-hydroxylase. Control assays extracted immediately after substrate addition were used to determine the levels of carvone present at the beginning of the incubation.
Stereoselectivity in Substrate Utilization and Product Formation
To assess the specificity of limonene-6-hydroxylase and carveol dehydrogenase, the relevant enzymes were assayed in duplicate with the following substrates: limonene-6-hydroxylase: (+)- and (−)-limonene at 200 μm; trans-carveol dehydrogenase: (+)/(−)-trans-carveol mixture (130/110 μm), (+)/(−)-cis-carveol mixture (130/70 μm), (−)-trans-carveol (200 μm), and (−)-cis-carveol (170 μm). The product specificity of limonene synthase was assayed with a preparation of annual caraway after chromatography to remove endogenous limonene. Fruits were extracted as described above in a buffer containing 25 mm Mopso (3-[N-morpholino]-2-hydroxypropanesulfonic acid), pH 6.5, 10% (v/v) glycerol, 25 mm sodium ascorbate, 25 mm NaHSO3, 5 mm MgCl2, 2.5 mm EGTA, 2 mm EDTA, 1 mm MnCl2, and 5 mm DTT. The 150,000g supernatant was loaded onto a 15- × 2.5-cm column of DEAE-cellulose (Whatman DE-52) previously equilibrated with buffer containing 15 mm Mes (pH 6.0), 10% (v/v) glycerol, 2 mm NaHSO3, 1 mm MnCl2, and 2 mm DTT. The column was washed with the equilibration buffer and the enzyme was eluted with a 0 to 600 mm KCl gradient. The combined active fractions were desalted to the limonene synthase assay buffer (buffer A), glycerol was added to 30% (v/v), and the material was stored at −80°C. After thawing, seven 200-μL aliquots of the enzyme preparation were diluted 5-fold with buffer A and the assay was started by the addition of 35 μm [1-3H]GPP (21 Ci/mol) to five of the seven vials. The other two vials were used to check endogenous limonene levels. Assays were overlaid with 1 mL of redistilled pentane and were worked up as described above, except that the pentane phases were concentrated using microdistillation to minimize losses of limonene. Enzyme products were analyzed using GC-MS on a machine equipped with a chiral column in the selected ion-monitoring mode as described above.
[U-14C]Suc Feeding
A set of 1.5-mL Eppendorf vials was prepared with 50 μL [U-14C]Suc (25 GBq mmol−1, 7.4 MBq mL−1 in ethanol, Amersham) in each vial. After evaporation of the solvent under a stream of N2, 100 μL of unlabeled Suc in water (30 g L−1) was added to give a final concentration of 88 mm. Three to six representative umbellets of each developmental stage (Table I) were cut under deionized water and their pedicels were placed in the Suc solution through a small hole in the cap of the vial. After incubation at 20°C in a growth cabinet with continuous, fluorescent white light at 60 to 65 μmol m−2 s−1 for 4 to 6 h, the Suc solution was taken up. A second 100-μL aliquot of unlabeled Suc was added for an additional incubation period overnight to ensure maximum uptake of the radiolabel. The second aliquot of Suc had been completely taken up by the next morning, at which time the fruits were collected, weighed, frozen in liquid N2, and stored at −80°C until further analysis. For analysis, the frozen samples were ground to a fine powder in liquid N2 with a mortar and pestle. The liquid N2 and ground fruits were transferred to a 15-mL glass vial and, after evaporation of the N2, 5 mL of pentane was added. The mixture was homogenized for 30 s with an Omni 2000 homogenizer equipped with an Omni 10010 macrogenerator and saw teeth (Omni International, Waterbury, CT). After the debris had settled, 2 mL of the supernatant was transferred to a Pyrex centrifuge tube in which the fatty acids of the triacylglycerols were transesterified to the corresponding methyl esters (Bouwmeester and Kuijpers, 1993). Before and after methylation, radioactivity was determined in aliquots of the pentane phase by liquid-scintillation spectrometry. There was no loss of radioactivity upon methylation. The total amount of pentane-soluble radioactivity per gram of fruits was calculated from the liquid-scintillation counting data.
Before analysis by radio-GLC, the samples were concentrated under a stream of N2. Unlabeled limonene and carvone were added before concentration as carriers to minimize losses of radio-labeled material. However, this was unnecessary in samples from older developmental stages (after 10 DAP) because of the presence of relatively high amounts of (unlabeled) limonene and carvone in the older fruits. Before and after concentration, 1-μL aliquots were taken and diluted 1000- and 10,000-fold with hexane for analysis of the limonene-to-carvone ratio by GC-MS (see below). These ratios were used to estimate the losses of the more-volatile limonene relative to carvone during concentration. Concentrated samples were analyzed by radio-GLC as described above for the limonene synthase assay with the following modifications. A CP-Sil 8 CB capillary column (10-m × 0.53-mm i.d., coated with a 5-μm film of 5% [w/w] phenyl and 95% [w/w] dimethyl polysiloxane, Chrompack) was used with an oven temperature program of 70°C for 1 min, ramp of 25° min−1 to 260°C, and a final time of 16 min. The He inlet pressure was 0.4 kg cm−2, giving a column flow of about 1 mL min−1. Approximately 20% of the column effluent was split to the flame-ionization detector. Before the conversion reactor, H2 was added to the effluent at 2 mL min−1, and, prior to the counting tube, CH4 was added to give a total flow of 30 mL min−1. For precise measurement of radio-peak areas, the splitter was closed so that all of the column effluent was channeled solely to the radioactivity detector.
GC-MS analysis to determine limonene to carvone ratios was performed as described above using an HP-5MS column with an oven temperature program as follows: 45°C for 1 min, ramp of 10° min−1 to 260°C, and a final time of 5 min. The m/z range in the scan mode was set at 50 to 300 atomic mass units.
Analysis of Limonene, Carvone, and Fatty Acid Content
The frozen fruit samples were homogenized with the Omni homogenizer in 10 mL of hexane containing known amounts of isobutylbenzene, camphor, and methyl decanoate as the internal standards. After the residue had settled, 5 mL of the supernatant was transferred to a Pyrex centrifuge tube, 0.2 mL of 2 n KOH in methanol was added, and the contents were vigorously mixed for 20 s to esterify the fatty acids of the triacylglycerols. After addition of 1 mL of water, the contents were mixed again for 20 s and then centrifuged for 2 min at 2000 rpm. The hexane phase was analyzed by GLC (Chrompack CP9000) using a fused-silica CP-Sil 5 CB capillary column (25-m × 0.25-mm i.d.), coated with a film of 0.25 μm of 100% (w/w) dimethyl polysiloxane (Chrompack) operated with He (50 kPa), split injection (1:60), injector temperature of 280°C, flame ionization detector at 280°C, and oven-temperature programming: 110°C for 5 min, 20°C min−1 to 220°C, and 10 min at final time. Carvone and limonene were identified by comparing retention times with authentic standards and were quantified by comparing their detector responses to that of the internal standards.
RESULTS
Demonstration and Characterization of Enzyme Activities
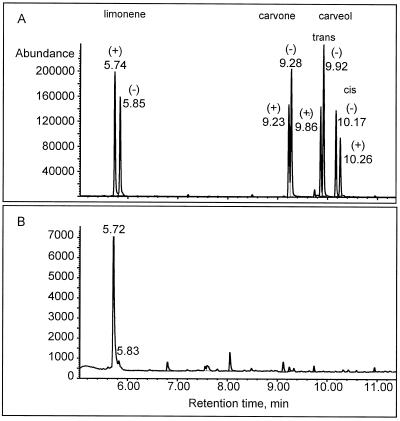
When crude extracts of caraway fruits from different stages of development were assayed for monoterpene synthase activity with GPP as the substrate, limonene was the only monoterpene detected by radio-GLC, with the exception of small amounts of geraniol, a product of phosphohydrolase activity. Thus, the cyclization of GPP to limonene is the first step of carvone biosynthesis in caraway. The activity was operationally soluble (confined to the 150,000g supernatant), displayed a pH optimum of approximately 7.5, and required a divalent metal ion (Mn2+ preferred) for catalysis. The cyclization product was almost exclusively (+)-limonene (Fig. 2). After anion-exchange chromatography to remove the endogenous limonene present in the crude extract, GPP was found to be converted to 98.4% (+)-limonene with only 1.6% of the (−)-enantiomer.
Figure 2.
GC-MS analysis (in selected ion-monitoring mode) of products of limonene synthase assay using octakis-(6-O-methyl-2,3-di-O-pentyl)-γ-cyclodextrin as the chiral stationary phase. Ions monitored: for limonene, m/z 68, 93, and 136; for carvone, m/z 82, 108, and 150; for trans-carveol, m/z 84, 109, and 152; and for cis-carveol, m/z 84, 109, and 134. A, Reference compounds. B, Product of partially purified limonene synthase. For further details, see Methods.
In the second step of the carvone pathway, limonene was converted to trans-carveol by a NADPH-requiring activity from the 150,000g pellet, reminiscent of the microsomal Cyt P-450 hydroxylase from spearmint, previously implicated in the conversion of (−)-limonene to (−)-trans-carveol (Gershenzon et al., 1989; Karp et al., 1990). Hydroxylation of (+)-limonene occurred with high regio- and stereospecificity. (+)-trans-Carveol made up 97% of the total product, with (−)-trans-carveol at 2.5% and (−)-cis-carveol at 0.5% as the minor products. Assays of mixtures of (−)- and (+)-limonene showed that (−)-limonene was also used as a substrate by this enzyme but at only about 10% of the rate of (+)-limonene and with (−)-cis-carveol as the major product.
(+)-trans-Carveol is converted to (+)-carvone in the third and final step of the reaction sequence. The supernatant fraction of caraway fruit extracts possessed a very active trans-carveol dehydrogenase activity. This enzyme exhibited a pH optimum of around 10 and showed an absolute requirement for NAD+. NADP+ could not substitute for NAD+, and could not act synergistically with NAD+. At a pH of 10.5, which was routinely used in experiments to characterize this activity, no general alcohol (ethanol) dehydrogenase activity was detected, as determined spectrophotometrically with ethanol as a substrate (290 mm) and a mixture of NAD+ and NADP+ (at 1 mm each) as cofactors (Sangwan et al., 1993). Substantial ethanol dehydrogenase activity was detected at pH 8.0.
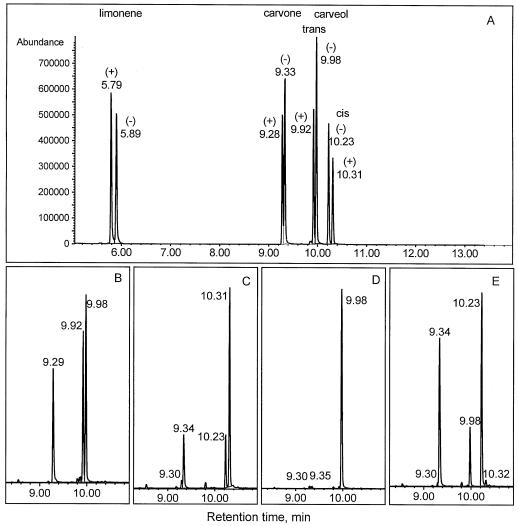
Carveol dehydrogenase exhibited moderate preference for its natural substrate, (+)-trans-carveol (Table II). Of the substrates tested, the mixture of (+)/(−)-trans-carveol gave the highest activity. (−)-cis-Carveol also exhibited significant activity, giving approximately 65% of the activity of the (+)/(−)-trans-carveol mixture. The very low activity with (−)-trans-carveol as a substrate (5% of the activity observed with the [+]/[−]-trans-carveol mixture) suggested that the enzyme is very sensitive to substrate chirality, a supposition confirmed by GC-MS analysis on a chiral stationary phase of the products and the unreacted substrates (Fig. 3). Whereas the mixture of (+)- and (−)-trans-carveols was converted to (+)-carvone (Fig. 3B), the pure (−)-enantiomer did not yield detectable products (Fig. 3D). In contrast, for the cis-carveols, both the (+)/(−) mixture (Fig. 3C) and the pure (−)-enantiomer (Fig. 3E) were readily converted to (−)-carvone. The assay with (−)-cis-carveol (Fig. 3E) provides further evidence of the unreactivity of (−)-trans-carveol with this enzyme preparation, since the (−)-trans-isomer is a trace impurity (0.8%) of the (−)-cis-carveol used as a substrate. (−)-trans-Carveol made up less than 1% of the substrate before reaction, but represented nearly 25% of the unconverted carveols after reaction.
Table II.
Dehydrogenase activity with various carveol isomers as substrates in 50 mm Gly buffer, pH 10.5, with 10% (v/v) glycerol, 2 mm DTT, and 1 mm each of NAD+ and NADP+
| Substrate | Activity | Maximum |
|---|---|---|
| μm | nmol h−1 g−1 dry wt | % |
| (+)/(−)-trans-Carveol (37/33) | 45.2 | 100 |
| (+)/(−)-cis-Carveol (16/8) | 8.09 | 18 |
| (−)-trans-Carveol (50) | 2.03 | 5 |
| (−)-cis-Carveol (40) | 30.30 | 67 |
Figure 3.
GC-MS analyses (in selected ion-monitoring mode) of products of carveol dehydrogenase assays using octakis-(6-O-methyl-2,3-di-O-pentyl)-γ-cyclodextrin as the chiral stationary phase. Ions monitored: for limonene, m/z 68, 93, and 136; for carvone, m/z 82, 108, and 150; for trans-carveol, m/z 84, 109, and 152; for cis-carveol, m/z 84, 109, and 134. A, Reference compounds. B through E, Products of carveol dehydrogenase activity with a (+)/(−)-trans-carveol mixture (B), a (+)/(−)-cis-carveol mixture (C), (−)-trans-carveol (D), and (−)-cis-carveol (E) as the substrates. For further details, see Methods.
Developmental Changes in Monoterpene and Fatty Acid Content and the Rate of Biosynthesis
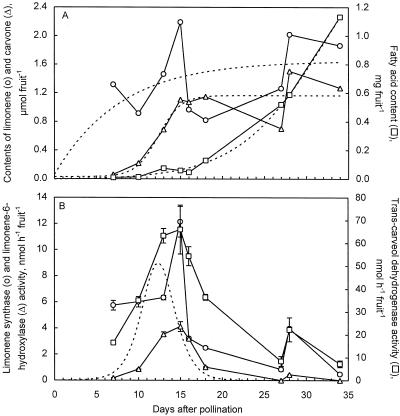
The pattern of accumulation of the monoterpenes limonene and carvone in developing caraway fruits was investigated by extracting fruits of nine different developmental stages in hexane and by analyzing the extracts with GC. The levels of fatty acids in these extracts (both free and bound as triacylglycerols) were also measured by quantifying the fatty acid methyl esters formed after hydrolysis and transesterification in methanolic KOH. The patterns of accumulation of limonene, carvone, and fatty acids and the activities of the three enzymes assayed were similar for annual and biennial caraway. Therefore, only data for annual caraway are shown. Young fruits contained very low levels of carvone and fatty acids, but high concentrations of limonene, up to 1.2 μmol fruit−1 (Fig. 4A), equivalent to approximately 130 mg g−1 fruit dry weight. From about 10 DAP, carvone content increased rapidly until about 15 DAP, at which time it became approximately the same as that of limonene. From about 15 DAP, the concentration of both monoterpenes followed a similar pattern until the end of the experiment at 35 DAP. At this stage, the monoterpene concentration of both caraway forms was similar, about 54 mg g−1 fruit dry weight, but the biennial form had a higher carvone-to-limonene ratio than the annual form (1.2 versus 0.7; data not shown). Figure 4A shows clearly that the accumulation of monoterpenes is confined to the early stages of fruit development and that limonene accumulation precedes carvone accumulation by a period of 5 to 10 d.
Figure 4.
Accumulation of limonene (○), carvone (▵), and fatty acids (□) (A), and changes in activities of limonene synthase (○), limonene-6-hydroxylase (▵), and trans-carveol dehydrogenase (□) (B) during development of fruits of annual caraway. Dotted lines in A indicate sigmoidal curves of the equation: y = a + b/(1 + exp[−(x − c)/d]). r2 values are 0.52 for limonene, 0.86 for carvone, and 0.99 for fatty acids. The dotted line in B indicates the carvone accumulation rate, which was calculated by taking the 1st-order derivative of the sigmoidal-curve fit to the carvone content data as shown in A. Data for A were obtained from pooled samples of 0.5 g of fruits of each developmental stage containing 40 to 130 individual fruits. Data for B were obtained from pooled samples of 1.0 g of fruits. Enzyme assays were carried out in triplicate (limonene synthase) or in duplicate (other activities) under linear conditions. Error bars indicate se.
The accumulation of fatty acids occurred late in fruit development, beginning at 15 to 25 DAP, and increased steadily until the end of the experiment to about 1 to 1.2 mg fruit−1 (Fig. 4A). Fatty acid composition in the late stages of fruit development was 1% stearic acid, 4% palmitic acid, 35% linoleic acid, and 60% petroselinic and oleic acids (not separated in our analysis) for annual caraway and 0.5% stearic, 5% palmitic, 38% linoleic, and 57% petroselinic/oleic for the biennial form.
Experiments to determine the rate of monoterpene and fatty acid biosynthesis by measuring the incorporation of [U-14C]Suc into pentane-soluble compounds showed similar trends. Overall incorporation of radioactivity was significantly higher in the younger than in the older stages, decreasing from 3 to 6% of administered Suc at 5 to 15 DAP to less than 1% after 16 to 20 DAP (Table III). In the youngest stages (5–7 DAP), the majority of label was incorporated into limonene, with less incorporation into carvone and little or no incorporation into fatty acids. No radiolabel was observed in the monoterpene intermediate trans-carveol at any developmental stage. The rate of radiolabel incorporation into limonene declined rapidly with development and was not detectable after 15 DAP. The incorporation of [U-14C]Suc into carvone increased as the rate of incorporation into limonene declined, but was also undetectable after 15 DAP. From this period onward, the only pentane-soluble compounds to incorporate [U-14C]Suc were the fatty acids.
Table III.
14C-radiolabel incorporation from [U-14C]Suc into pentane-soluble compounds of caraway fruits at different developmental stages
| Developmental Stage | Incorporation of Label Administered |
14C Incorporation
|
||
|---|---|---|---|---|
| Limonene | Carvone | Fatty acids | ||
| DAP | % | % of total | ||
| 7 | 3.24 | 71 | 29 | 0 |
| 10 | 4.17 | 34 | 48 | 18 |
| 13 | 3.62 | 24 | 48 | 28 |
| 15 | 4.36 | 21 | 59 | 20 |
| 16 | 1.24 | 0 | 0 | 100 |
| 18 | 0.77 | 0 | 0 | 100 |
| 27 | 0.63 | 0 | 0 | 100 |
| 28 | 1.06 | 0 | 0 | 100 |
| 34 | 0.62 | 0 | 0 | 100 |
For further details, see Methods.
The level of activity of the three enzymes of monoterpene biosynthesis varied considerably over the period of fruit development (Fig. 4B). In the youngest stages, both limonene synthase and trans-carveol dehydrogenase were active but limonene-6-hydroxylase was not; however, the maximum in all three activities occurred at about 15 DAP. From this stage, the activities of all three enzymes declined with similar kinetics until they were virtually undetectable. At all stages of development, trans-carveol dehydrogenase activity was 2 to 15 times greater than limonene synthase activity, and both were considerably higher than limonene-6-hydroxylase activity. The dotted line in Figure 4B depicts the carvone accumulation rate, and represents the first derivative of the sigmoidal curve describing carvone accumulation in Figure 4A. In general, there is a good correlation between the mathematically derived rate of carvone accumulation and the limonene-6-hydroxylase activity measured in vitro, although the measured enzyme activity often underestimated the carvone accumulation rate in planta, especially during the first 15 DAP.
DISCUSSION
The Pathway of Monoterpene Biosynthesis in Caraway
The formation of (+)-carvone in caraway fruits proceeds via a three-step pathway from GPP completely analogous to the formation of (−)-carvone in spearmint (Gershenzon et al., 1989). In the first step, GPP is cyclized to (+)-limonene by a monoterpene synthase that is very similar in its basic properties to many monoterpene synthases previously characterized from angiosperms (Alonso and Croteau, 1993; Gershenzon and Croteau, 1993). This enzyme also produces minor amounts (1.6%) of the opposite enantiomer, (−)-limonene (Fig. 2), which is also found in trace levels (0.6%) in the essential oil extracted from caraway fruits (Bouwmeester et al., 1995b).
In the second step, after cyclization (+)-limonene is hydroxylated to (+)-trans-carveol by a particulate, NADPH-utilizing activity, the properties of which resemble those of other Cyt P-450 monoterpene hydroxylases (Mihaliak et al., 1993). Caraway (+)-limonene-6-hydroxylase also proved capable of oxygenating the opposite enantiomer, (−)-limonene, as has been reported for other monoterpene hydroxylases (Karp et al., 1990). Further characteristics of the caraway (+)-limonene-6-hydroxylase will be described in a subsequent paper (H.J. Bouwmeester, M.C.J.M. Konings, J. Gershenzon, F. Karp, and R. Croteau, unpublished data).
The third and final step of monoterpene formation in caraway fruit is the oxidation of (+)-trans-carveol to (+)-carvone. The properties of this enzyme resemble those of other monoterpenol dehydrogenases described in the literature. As a group, the monoterpenol dehydrogenases possess a wide range of pH optima, varying from around 8.0 (Croteau and Felton, 1980; Sangwan et al., 1993) to 9.0 to 10.0 (Potty and Bruemmer, 1970; Kjonaas et al., 1985; Hallahan et al., 1995). The trans-carveol dehydrogenase studied here also has a high pH optimum, around 10.0. This finding is consistent with a trend noted earlier by Kjonaas et al. (1985) that monoterpenol dehydrogenases utilizing α,β-unsaturated alcohols have higher pH optima than monoterpenol dehydrogenases utilizing saturated alcohols. The high pH optimum for caraway (+)-trans-carveol dehydrogenase seems to have little physiological relevance because the enzyme is probably localized in the cytoplasm, where a pH of 7.0 to 7.5 can be assumed. Among other monoterpenol dehydrogenases, some require either NAD+ or NADP+, whereas some show substantial catalytic activity with both cofactors (Potty and Bruemmer, 1970; Croteau and Felton, 1980; Kjonaas et al., 1985; Sangwan et al., 1993; Hallahan et al., 1995). Caraway trans-carveol dehydrogenase shares its requirement for NAD+ with another α,β-unsaturated monoterpenol dehydrogenase, trans-isopiperitenol dehydrogenase from peppermint (Kjonaas et al., 1985).
The substrate specificity of (+)-trans-carveol dehydrogenase, like that of other monoterpenol dehydrogenases, is not very strict. Nevertheless, the oxidation of monoterpenols in other plant species has been found to be catalyzed by a dehydrogenase activity other than the general alcohol (ethanol) dehydrogenase (Croteau and Felton, 1980). This was confirmed for caraway by the absence of ethanol dehydrogenase activity at pH 10.5, near the pH optimum for trans-carveol dehydrogenase activity, and by the fact that only two of the four isomeric carveols [(+)-trans- and (−)-cis-carveol] were oxidized (Fig. 3; Table II). Caraway (+)-trans-carveol dehydrogenase exhibits only moderate substrate specificity but high enantioselectivity, in contrast to peppermint isopiperitenol dehydrogenase, which oxidizes both enantiomers of trans-isopiperitenol but does not catalyze reaction with cis-isopiperitenol (Kjonaas et al., 1985). Further purification of trans-carveol dehydrogenase is necessary to determine whether a single activity is capable of oxidizing both (+)-trans- and (−)-cis-carveol. The low substrate specificity of both (+)-trans-carveol dehydrogenase and the second enzyme in the pathway, (+)-limonene-6-hydroxylase, stand in contrast to the high enantiomeric purity of the carvone that accumulates in caraway fruit. The enantiomeric purity of carvone thus results from the high product specificity of the first enzyme, (+)-limonene synthase, which ensures that only a single stereoisomer is made available to the later, less-specific enzymes.
Regulation of Monoterpene Formation in Caraway
Enzyme Control
The three enzymes of monoterpene biosynthesis in caraway, limonene synthase, limonene-6-hydroxylase, and trans-carveol dehydrogenase, undergo dramatic changes in activity during fruit development (Fig. 4B), which appears to explain much of the pattern of limonene and carvone accumulation (Fig. 4, A and B). During the early stages of fruit development (5–10 DAP) there is an abundant accumulation of limonene, but very little carvone is produced. At this time, both the first and third enzymes of the pathway, limonene synthase and trans-carveol dehydrogenase, exhibit high levels of activity. However, only trace levels of the second enzyme, limonene-6-hydroxylase, were observed. The absence of significant amounts of limonene-6-hydroxylase apparently prevents the oxidation of limonene to trans-carveol. This blocks the formation of carvone, despite the consistently high levels of trans-carveol dehydrogenase activity, leading to a buildup of limonene. From 10 DAP, there is a rise in limonene-6-hydroxylase activity that coincides with the appearance of substantial amounts of carvone.
Similar changes in terpenoid accumulation patterns occur during plant development in many other plant species, including dill (Porter et al., 1983) and peppermint (Voirin and Bayet, 1996). However, to our knowledge, the enzymatic bases of such shifts in accumulation have been unexamined until now. In contrast, the rapid switches in terpenoid metabolism that occur upon pathogen infection have been extensively investigated. For example, when cell cultures of tobacco or potato are treated with fungal elicitors, there is an induction of sesquiterpenoid phytoalexin biosynthesis and a repression of sterol formation (Brindle et al., 1988; Vögeli and Chappell, 1988; Chappell, 1995). Enzymatic analyses have revealed that fungal elicitors activate farnesyl diphosphate-utilizing enzymes in phytoalexin biosynthesis while reducing the activity of squalene synthase, a branchpoint enzyme in sterol formation that also competes for farnesyl diphosphate.
In caraway limonene-6-hydroxylase may serve as an important rate-controlling step in carvone formation, not only because of its limited temporal occurrence, but also as a result of its kinetics. The hydroxylation of limonene to trans-carveol appears to be a much slower transformation than the subsequent dehydrogenation to carvone, as indicated by the lack of any accumulation of the intermediate, trans-carveol. No 14C-labeled trans-carveol was detected after [U-14C]Suc feeding in this study. In addition, analysis of the essential oil of caraway fruits has revealed only minute amounts of carveols: trans, 0.3 to 0.5%; and cis, 0.2% (Bouwmeester et al., 1995b). Monoterpene hydroxylation may also possess regulatory importance in the formation of monoterpenes in peppermint. In this species, (−)-limonene, an olefin precursor of oxygenated monoterpenes, makes up a much higher percentage of the total monoterpene pool in younger than in older tissue. As peppermint leaves mature, the percentage of limonene drops and the percentage of the oxygenated products menthone and menthol increases (Brun et al., 1991; Voirin and Bayet, 1995), suggesting that the 3-hydroxylation of (−)-limonene to (−)-trans-isopiperitenol, the next intermediate in menthone/menthol biosynthesis, is a regulatory step in monoterpene formation. The enzymatic changes responsible for this metabolic switch are currently under investigation (M. Rufener, J. Gershenzon, and R. Croteau, unpublished results).
The correlation between limonene-6-hydroxylase activity and the profile of carvone accumulation during fruit development is not exact (Fig. 4B). For example, in vitro activity is frequently insufficient to account for carvone accumulation, probably because of difficulties in quantitatively extracting and assaying this enzyme. Limonene-6-hydroxylase is a Cyt P-450-dependent oxygenase (Bolwell et al., 1994) which is found in the light membrane (microsomal) fraction of the cell (H.J. Bouwmeester, M.C.J.M. Konings, J. Gershenzon, F. Karp, and R. Croteau, unpublished data). The activities of enzymes of this class are often underestimated as a result of inefficient extraction and poor stability (Mihaliak et al., 1993; Funk et al., 1994). After extraction from caraway fruits, limonene-6-hydroxylase activity is gradually lost even at 4°C in the presence of protective agents (H.J. Bouwmeester, M.C.J.M. .Konings, J. Gershenzon, F. Karp, and R. Croteau, unpublished data).
Substrate Limitation
The formation of monoterpenes in caraway fruit may also be controlled by enzymes acting prior to limonene synthase, which control flux entering the monoterpene pathway (Gershenzon and Croteau, 1990). At 15 to 20 DAP, the biosynthesis of limonene and carvone in developing fruits appears to cease, based on the lack of further [14C]Suc incorporation (Table III) and the leveling off of the accumulation curves (Fig. 4A). Nevertheless, ample activities of all three enzymes of the pathway are still present, judging by the results of the in vitro assays (Fig. 4B). At this stage of development, the formation of monoterpenes could be limited by substrate partitioning to competing pathways, such as triacylglycerol synthesis. The rate of fatty acid formation in developing fruits increases dramatically at this stage (Table III; Fig. 4A). The biosynthesis of structural carbohydrates, which has been found to slightly precede triacylglycerol accumulation in caraway fruits (Luyendijk, 1956), may also compete for the supply of fixed carbon.
The accumulation patterns of monoterpenes and fatty acids during caraway fruit development and the profiles of enzyme activities involved in monoterpene biosynthesis were quite similar for the two caraway forms (data not shown). However, in annual caraway, accumulation of limonene and carvone started earlier in development, reached higher levels per fruit, and ceased earlier than in biennial caraway. These differences were partially reflected in the time courses of enzyme activity for limonene synthase and limonene-6-hydroxylase. Both activities were higher in annual than in biennial caraway at early stages of development. However, the biennial form continued to accumulate limonene and carvone after the monoterpene content of annual caraway had already stabilized. The continued formation of monoterpenes in the later stages of development of biennial caraway may also explain the higher carvone-to-limonene ratio of this form. If competition for substrate is important in controlling the rate of monoterpene biosynthesis in caraway fruits, this phenomenon appears to be less important, or of later onset, in the biennial form.
Other Modes of Regulation
The formation of monoterpenes in developing caraway fruits may be controlled by subcellular compartmentation of the various enzymes. The first enzyme of the pathway, limonene synthase, appears to be localized in the leukoplasts, colorless plastids with few internal membranes (Gleizes et al., 1983; McCaskill and Croteau, 1995). The presence of this activity in the 150,000g supernatant is likely due to the fragility of the leukoplasts during enzyme isolation (Gleizes et al., 1983). However, limonene-6-hydroxylase, like many other Cyt P-450 oxygenases (Bolwell et al., 1994), is probably found in the ER. Hence, for the operation of the monoterpene pathway, the limonene formed in the leukoplasts must be transferred into the ER. If no limonene-6-hydroxylase activity is present in the ER, as during early fruit development, the limonene will move directly into the oil ducts, possibly via the ER network. However, when limonene-6-hydroxylase is present, limonene is hydroxylated in the ER and then oxidized to carvone. A role for the ER in transport of monoterpenes from leukoplasts to essential oil ducts is supported by the ultrastructural study of Bosabalidis (1996) with celery petioles in which the monoterpene hydrocarbon 1,3,8-menthatriene accumulates. At the stage of essential oil formation, ER elements were shown to associate with leukoplasts containing osmiophilic secretory droplets. These droplets were transferred into the ER and then transported to the essential oil ducts. The trans-carveol dehydrogenase is probably cytosolic, judging by its recovery in the 150,000g supernatant. It is unclear whether for oxidation the trans-carveol has to be released from the ER into the cytoplasm or whether the dehydrogenase, though it is cytoplasmic, operates in close association with the ER. The close correlation between limonene-6-hydroxylase activity and carvone accumulation (Fig. 4B), and the fact that the increase in the rate of carvone accumulation during fruit development coincides with the decrease in the rate of limonene accumulation (Fig. 4A), seem to confirm that both of these monoterpenes are produced from the same pathway, and not by separate pathways localized at different cellular sites.
In conclusion, the patterns of limonene and carvone accumulation during the early development of caraway fruit can be attributed largely to changes in the time course of monoterpene biosynthesis caused by alterations in the levels of limonene synthase and limonene-6-hydroxylase activities. However, at later stages of development, competition with other pathways for substrate may serve to limit monoterpene accumulation. At the enzyme level, the hydroxylation of limonene to trans-carveol seems to be a critical, rate-limiting step in carvone biosynthesis. We are currently characterizing this enzyme in more detail and plan to isolate the corresponding gene from a caraway cDNA library. Overexpression of this gene in caraway fruit should provide a rigorous evaluation of its regulatory importance in carvone biosynthesis.
ACKNOWLEDGMENTS
The authors wish to thank Tom Savage and Frank Karp for their helpful suggestions, Jeroen Wilmer for his help in keeping the radio-gas-liquid chromatograph operating, Jacques Davies for help with radio-GLC and GC-MS measurements, and Adrie Kooijman, Joop van Westeneng, Herman Mersbergen, and Greg Wichelns for raising the plants.
Abbreviations:
- DAP
days after pollination
- GPP
geranyl diphosphate
Footnotes
This work was supported by the Commissie Herstructurering Akkerbouw Noorden des Lands of the Dutch Ministry of Agriculture, Nature Management, and Fisheries, the U.S. Department of Energy (grant no. DE-FG03-96ER20212), and the Washington State University Agricultural Research Center (project no. 0268).
LITERATURE CITED
- Alonso WR, Croteau R. Prenyltransferases and cyclases. In: Lea PJ, editor. Methods in Plant Biochemistry, Vol 9: Enzymes of Secondary Metabolism. London: Academic Press; 1993. pp. 239–260. [Google Scholar]
- Banthorpe DV, Charlwood BV. The terpenoids. In: Bell EA, Charlwood BV, editors. Encyclopedia of Plant Physiology, New Series, Vol 8: Secondary Plant Products. Berlin: Springer-Verlag; 1980. pp. 185–220. [Google Scholar]
- Bolwell GP, Bozak K, Zimmerlin A. Plant cytochrome P450. Phytochemistry. 1994;37:1491–1506. doi: 10.1016/s0031-9422(00)89567-9. [DOI] [PubMed] [Google Scholar]
- Bosabalidis AM. Ontogenesis, ultrastructure and morphometry of the petiole oil ducts of celery (Apium graveolens L.) Flavour Fragrance J. 1996;11:269–274. [Google Scholar]
- Bouwmeester HJ, Davies JAR, Smid HG, Welten RSA. Physiological limitations to carvone yield in caraway (Carum carvi L.) Ind Crops Prod. 1995a;4:39–51. [Google Scholar]
- Bouwmeester HJ, Davies JAR, Toxopeus H. Enantiomeric composition of carvone, limonene and carveols in seeds of dill, and annual and biennial caraway varieties. J Agr Food Chem. 1995b;43:3057–3064. [Google Scholar]
- Bouwmeester HJ, Kuijpers A-M. Relationship between assimilate supply and essential oil accumulation in annual and biennial caraway (Carum carvi L.) J Essent Oil Res. 1993;5:143–152. [Google Scholar]
- Brindle PA, Kuhn PJ, Threlfall DR. Biosynthesis and metabolism of sesquiterpenoid phytoalexins and triterpenoids in potato cell suspension cultures. Phytochemistry. 1988;27:133–150. [Google Scholar]
- Brun N, Colson M, Perrin A, Voirin B. Chemical and morphological studies of the effects of ageing on monoterpene composition in Mentha × piperita leaves. Can J Bot. 1991;69:2271–2278. [Google Scholar]
- Chappell J. Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu Rev Plant Mol Biol. 1995;46:521–547. [Google Scholar]
- Croteau R, Cane DE. Monoterpene and sesquiterpene cyclases. Methods Enzymol. 1985;110:383–405. [Google Scholar]
- Croteau R, Felton NM. Substrate specificity of monoterpenol dehydrogenases from Foeniculum vulgare and Tanacetum vulgare. Phytochemistry. 1980;19:1343–1347. [Google Scholar]
- Funk C, Lewinsohn E, Vogel BS, Steele CL, Croteau R. Regulation of oleoresinosis in grand fir (Abies grandis). Coordinate induction of monoterpene and diterpene cyclases and two cytochrome P450-dependent diterpenoid hydroxylases by stem wounding. Plant Physiol. 1994;106:999–1005. doi: 10.1104/pp.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, Croteau R (1990) Regulation of monoterpene biosynthesis in higher plants. In GHN Towers, HA Stafford, eds, Biochemistry of the Mevalonic Acid Pathway to Terpenoids, Recent Advances in Phytochemistry, Vol 24. Plenum Press, New York, pp 99–160
- Gershenzon J, Croteau R (1993) Terpenoid biosynthesis: the basic pathway and formation of monoterpenes, sesquiterpenes and diterpenes. In TS Moore Jr, ed, Lipid Metabolism in Plants. CRC Press, Boca Raton, FL pp 339–388
- Gershenzon J, Maffei M, Croteau R. Biochemical and histochemical localization of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata) Plant Physiol. 1989;89:1351–1357. doi: 10.1104/pp.89.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleizes M, Pauly G, Carde J-P, Marpeau A, Bernard-Dagan C. Monoterpene hydrocarbon biosynthesis by isolated leucoplasts of Citrofortunella mitis. Planta. 1983;159:373–381. doi: 10.1007/BF00393177. [DOI] [PubMed] [Google Scholar]
- Hallahan DL, West JM, Wallsgrove RM, Smiley DWM, Dawson GW, Pickett JA, Hamilton JGC. Purification and characterization of an acyclic monoterpene primary alcohol:NADP+ oxidoreductase from catmint (Nepeta racemosa) Arch Biochem Biophys. 1995;318:105–112. doi: 10.1006/abbi.1995.1210. [DOI] [PubMed] [Google Scholar]
- Johnston RG, Read J. Researches in the carvone series. Part II. Some unsaturated alcohols. J Chem Soc. 1934;1934:233–237. [Google Scholar]
- Karp F, Mihaliak CA, Harris JL, Croteau R. Monoterpene biosynthesis: specificity of the hydroxylation of (−)-limonene by enzyme preparations from peppermint (Mentha piperita), spearmint (Mentha spicata) and perilla (Perilla frutescens) Arch Biochem Biophys. 1990;276:219–226. doi: 10.1016/0003-9861(90)90029-x. [DOI] [PubMed] [Google Scholar]
- Kjonaas RB, Venkatachalam KV, Croteau R. Metabolism of monoterpenes: oxidation of isopiperitenol to isopiperitenone and subsequent isomerization to piperitenone by soluble enzyme preparations from peppermint (Mentha piperita) leaves. Arch Biochem Biophys. 1985;238:49–60. doi: 10.1016/0003-9861(85)90139-0. [DOI] [PubMed] [Google Scholar]
- König WA, Icheln D, Runge T, Pforr I, Krebs A. Cyclodextrins as chiral stationary phases in capillary gas chromatography. Part VII. Cyclodextrins with an inverse substitution pattern: synthesis and enantioselectivity. J High Res Chrom. 1990;13:702–707. [Google Scholar]
- Langenheim JH. Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol. 1994;20:1223–1280. doi: 10.1007/BF02059809. [DOI] [PubMed] [Google Scholar]
- Luyendijk EN (1956) Over de vorming van vluchtige olie in de vruchten van enkele Umbelliferen. PhD thesis. Rijksuniversiteit Leiden, The Netherlands
- McCaskill D, Croteau R. Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha × piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta. 1995;197:49–56. [Google Scholar]
- Mihaliak C, Karp F, Croteau R. Cytochrome P-450 terpene hydroxylases. In: Lea PJ, editor. Methods in Plant Biochemistry, Vol 9: Enzymes of Secondary Metabolism. London: Academic Press; 1993. pp. 261–279. [Google Scholar]
- Morton JF (1976) Herbs and Spices. Golden Press, New York
- Oosterhaven K, Poolman B, Smid EJ. S-Carvone as a natural potato sprout inhibiting, fungistatic and bacteriostatic compound. Ind Crops Prod. 1995;4:23–31. [Google Scholar]
- Ponndorf W. Der reversibele Austausch der Oxydationsstufen zwischen Aldehyden oder Ketonen einerseits und primären oder sekundären Alkoholen anderseits. Z Angew Chem. 1926;39:138–143. [Google Scholar]
- Porter NG, Shaw ML, Shaw GJ, Ellingham PJ. Content and composition of dill herb oil in the whole plant and the different plant parts during crop development. New Zealand J Agr Res. 1983;26:119–127. [Google Scholar]
- Potty VH, Bruemmer JH. Oxidation of geraniol by an enzyme system from orange. Phytochemistry. 1970;9:1003–1007. [Google Scholar]
- Sandermann W, Bruns K. Biogenese von carvon in Anethum graveolens L. Planta Medica. 1965;13:364–368. [Google Scholar]
- Sangwan RS, Singh-Sangwan N, Luthra R. Metabolism of acyclic monoterpenes: partial purification and properties of geraniol dehydrogenase from lemongrass (Cymbopogon flexuosus Stapf) leaves. J Plant Physiol. 1993;142:129–134. [Google Scholar]
- Vögeli U, Chappell J. Induction of sesquiterpene cyclase and suppression of squalene synthetase activities in plant cell cultures treated with fungal elicitor. Plant Physiol. 1988;88:1291–1296. doi: 10.1104/pp.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voirin B, Bayet C. Developmental changes in the monoterpene composition of Mentha × piperita leaves from individual peltate trichomes. Phytochemistry. 1996;43:573–580. [Google Scholar]
- Von Schantz M, Ek BS. Über die Bildung von Ätherischem Öl in Kümmel. Carum carvi L. Sci Pharm. 1971;39:81–101. [Google Scholar]
- Von Schantz M, Huhtikangas A. Über die Bildung von limonen und carvon in Kümmel. Carum carvi. Phytochemistry. 1971;10:1787–1793. [Google Scholar]
- Wichtmann E-M (1988) Die ätherischen Öle von Kümmel (Carum carvi L.), Gemüsefenchel (Foeniculum vulgare Miller subsp. capillaceum (Gilib.) Holmboe var. azóricum (Miller) Thellung), Pastinak (Pastinaca sativa L.) und Liebstöckl (Levisticum officinale Koch) im Keimpflanzenstadium und ihre Veränderung im Verlauf der Ontogenese in Korrelation zur Anatomie der Exkretgänge. PhD thesis. University of Hamburg, Germany