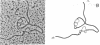Abstract
Recombinant bacteriophage (from a library of chicken chromosomal DNA fragments inserted into lambda Charon 4A) have been isolated which contain the coding information for both of the adult chicken alpha-globin genes, alpha A and alpha D. One of these recombinant phage also contains an as yet unidentified embryonic alpha-like globin gene sequence. The two adult genes are encoded on the same DNA strand and are separated by approximately 2.4 kilobase pairs, with the arrangement of the genes relative to the direction of transcription being 5'-alpha D-alpha A-3'. Electron microscopic R-loop visualization experiments demonstrate that both alpha-globin genes contain two intervening sequences of similar size in a manner analogous to the structure observed in the mouse alpha-globin gene. The linkage of the two highly divergent chicken adult alpha-globin genes further underscores the principle that chromosomal clustering of families of developmentally related genes may be a general phenomenon in higher eukaryotic gene sequence arrangement.
Full text
PDF



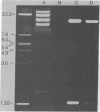
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Ingram V. M. Structural studies on chick embryonic hemoglobins. J Biol Chem. 1974 Jun 25;249(12):3960–3972. [PubMed] [Google Scholar]
- Bruns G. A., Ingram V. M. Erythropoiesis in the developing chick embryo. Dev Biol. 1973 Feb;30(2):455–459. doi: 10.1016/0012-1606(73)90102-4. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Analysis of the adult and embryonic chicken globin genes in chromosomal DNA. J Biol Chem. 1978 Nov 25;253(22):8239–8246. [PubMed] [Google Scholar]
- Flamm U., Best J. S., Braunitzer G. Die Primärstruktur der -Ketten des Kaninchenhämoglobins. Hoppe Seylers Z Physiol Chem. 1971 Jul;352(7):885–895. [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Characterisation of deletions which affect the expression of fetal globin genes in man. Nature. 1979 Jun 14;279(5714):598–603. doi: 10.1038/279598a0. [DOI] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Stubblefield E., Payvar F., Engel J. D., Dodgson J. B., Spector D., Cordell B., Schimke R. T., Varmus H. E. Gene localization by chromosome fractionation: globin genes are on at least two chromosomes and three estrogen-inducible genes are on three chromosomes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1348–1352. doi: 10.1073/pnas.76.3.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy E., Hardison R. C., Quon D., Maniatis T. The linkage arrangement of four rabbit beta-like globin genes. Cell. 1979 Dec;18(4):1273–1283. doi: 10.1016/0092-8674(79)90238-1. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Leder A., Miller H. I., Hamer D. H., Seidman J. G., Norman B., Sullivan M., Leder P. Comparison of cloned mouse alpha- and beta-globin genes: conservation of intervening sequence locations and extragenic homology. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6187–6191. doi: 10.1073/pnas.75.12.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Takei H., Wu K. C., Shiozawa T. The primary structure of the polypeptide chain of A II component of adult chicken hemoglobin. Int J Protein Res. 1971;3(3):173–174. doi: 10.1111/j.1399-3011.1971.tb01708.x. [DOI] [PubMed] [Google Scholar]
- Old J. M., Clegg J. B., Weatherall D. J., Booth P. B. Haemoglobin J Tongariki is associated with alpha thalassaemia. Nature. 1978 May 25;273(5660):319–320. doi: 10.1038/273319a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. The duplicated human alpha globin genes lie close together in cellular DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5950–5954. doi: 10.1073/pnas.75.12.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]