Abstract
Autophagy is an important cellular recycling mechanism through self-digestion in responses to cellular stress such as starvation. Studies have shown that autophagy is involved in maintaining the homeostasis of the neural system during stroke. However, molecular mechanisms underlying neuronal autophagy in ischemic stroke remain poorly understood. Previously, we and others have shown that immune-related GTPase M (IRGM; termed IRGM1 in the mouse nomenclature) can regulate the survival of immune cells through autophagy in response to infections and autoimmune conditions. Here, using a permanent middle cerebral artery occlusion (pMCAO) mouse model, we found that IRGM1 was upregulated in the ischemic side of the brain, which was accompanied by a significant autophagic response. In contrast, neuronal autophagy was almost complete lost in Irgm1 knockout (KO) mice after pMCAO induction. In addition, the infarct volume in the Irgm1-KO pMCAO mice was significantly increased as compared to wild-type mice. Histological studies suggested that, at the early stage (within 24 h) of ischemia, the IRGM1-dependent autophagic response is associated with a protection of neurons from necrosis in the ischemic core but a promotion of neuronal apoptosis in the penumbra area. These data demonstrate a novel role of IRGM1 in regulating neuronal autophagy and survival during ischemic stroke.
Keywords: autophagy, IRGM1, pMCAO, necrosis and apoptosis
Introduction
Stroke is one of the most severe neurological diseases threatening millions of lives in the world, which is categorized into ischemic and hemorrhagic stroke.1 Ischemic stroke, accounting for about 87% of the total cases, is usually caused by transient or permanent reduction in cerebral blood flow.2 During ischemia, acute nutrition and oxygen deprivation can lead to neuronal death. Necrosis and apoptosis were known to be two major ways of neuronal death after ischemia.3,4 In a typical lesion of ischemic stroke, neurons within the core area usually die through a form of necrosis rapidly after stroke, while in the penumbra area surrounding the necrotic core, many neurons undergo apoptosis after several hours or days.3,4 Limiting ischemic neurons from further damage has been a big challenge for clinical intervention. In recent decades, great efforts have been made in elucidating the mechanisms promoting neural cell death in ischemia, such as excitotoxicity, acidotoxicity and ionic imbalance, oxidative/nutritive stress and inflammation.5 In contrast, regulations of cell survival by self-protection mechanisms have been less studied.
Recently, autophagy has been recognized as an important cellular balance mechanism that influences the survival of many cell types. During autophagy, cytoplasmic organs sequester inside double-membrane vesicles and then are delivered to the lysosome for degradation. At least in the short term, this process provides energy to keep cell ‘functionally normal.’6 Recent studies highlight the role of autophagy in neuron survival in many neurological diseases.7,8 For example, in the rat permanent middle cerebral artery occlusion (pMCAO), one classic model of human ischemic stroke, it has been shown that microtubule-associated protein 1 light chain 3 type II (MAP1LC3B/LC3-II) and BECN1/Beclin 1, two strong indicative markers for autophagy, are highly upregulated along with increase of neuronal autophagosomes/autolysosomes.9 Nonetheless, the consequence of autophagy in regulating neuron survival is still not clear. In addition, the molecular mechanism regulating neuronal autophagy during stroke remains largely unknown. The differential role of autophagy in regulating different forms of neuron death (apoptosis and necrosis) is also an interesting but unknown question.
IRGM/IRGM1, a member of immunity-related GTPase family, is an interferon-inducible intracellular protein expressed in the Golgi complex, which has been implicated in a wide range of biological functions including cell-mediated immune responses and immunoregulations through autophagy.10-16 Here, we showed that IRGM1 is crucial for the activation of neuronal autophagy and regulation of the neuronal death during ischemic stroke. Our study represents the first report of IRGM1 in regulating neuronal function in response to ischemia.
RESULTS
Autophagy activity was elevated in cortical neurons in the mouse pMCAO model.
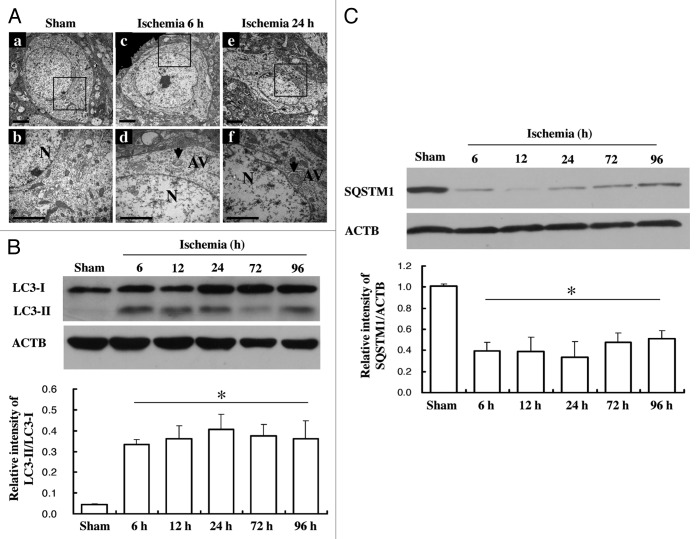
Previous studies using a rat pMCAO model have shown that autophagy activity was significantly increased in the ischemic area of the brain.9 However, this result has not been tested in a mouse model. Using transmission electron microscopy (TEM), we found that, in contrast to sham controls, neurons in the ischemic cortex showed dilated endoplasmic reticulum, swelling mitochondria, darkened lysosomes and increased number of autophagosomes after 6–24 h of ischemic induction (Fig. 1A). In addition, we found that the expression of LC3-II, the key molecular marker of autophagy, started to increase after 6 h of ischemic induction, and the high-level expression was maintained even after 96 h (Fig. 1B). On the other hand, the expression of SQSTM1/p62 decreased at 6 h and slightly recovered at later time points (Fig. 1C), consisting of a well-known reciprocal regulation of these two markers in autophagy. These results confirmed that ischemia can induce neuronal autophagy in the mouse pMCAO model.
Figure 1. Increase of neuron autophagy activity in mice pMCAO model. Samples were collected at different time points after pMCAO from wild-type mice. (A) TEM showed an increase of autophagosomes at 6 h (c and d) or 24 h (e and f) after pMCAO in wild-type mice compare to sham (a and b) (scale bar: 1 µm). Autophagy vacuoles (AV) were indicated by the arrows. (B) LC3-I and LC3-II expression was detected by western blot (upper panel). Comparison was made in terms of their LC3-II/LC3-I ratio (low panel). (C) Expression of SQSTM1/p62 in wild-type mice at different time points after pMCAO. ACTB was used as the loading control. N = 3–4/group; bar: mean ± SEM. *p < 0.05 compared with sham.
IRGM1 is upregulated in the neurons from ischemic side of pMCAO.
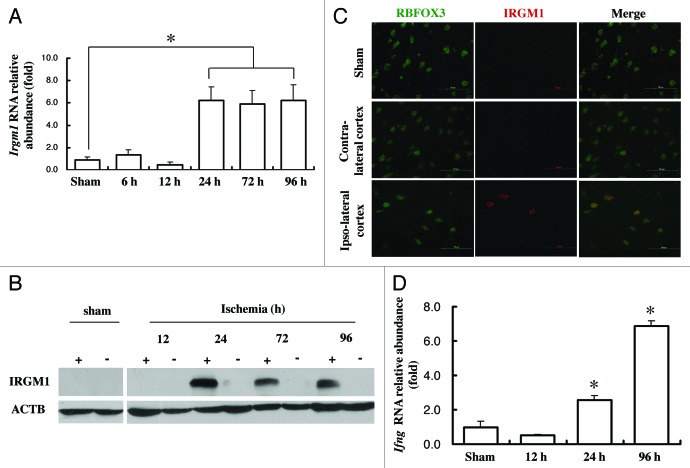
Since IRGM expression is strongly associated with autophagy activity in immune cells during both infectious and autoimmune conditions, we hypothesize that its expression may be altered in a stroke model, which also involves inflammation and immune response. The basal level of IRGM1 expression in brain was quite low (data not shown). However, after pMCAO induction, IRGM1 was strongly upregulated in the ischemic side of the brain at both mRNA and protein levels (Fig. 2A and B). Moreover, immunofluorescence staining indicated that IRGM1 was costained with several neural cell markers. Double staining for IRGM1 and RBFOX3/NeuN, the neuronal marker, confirmed a significantly increased expression of IRGM1 in neurons after pMCAO induction (Fig. 2C). Moreover, the expression of IFNG/IFNγ, which has been suggested to induce IRGM1 expression in immune cells, was also elevated in the ischemic side of the brain after pMCAO (Fig. 2D), indicating that the upregulation of IRGM1 in neurons during cerebral ischemia may be IFNG-dependent as observed in immune cells.
Figure 2. Upregulation of IRGM1 in neuron after pMCAO. RNA and protein sample were isolated either from contra-lateral or lpsi-lateral of the cortex at different time point after pMCAO. (A) Changes of Irgm1 at mRNA level was detected by real-time PCR, (p < 0.05 compare to sham, n = 5/group). (B) Changes of IRGM1 at protein level were measured by western blot. ACTB was used as the loading control, (n = 3/group). “+” means ischemic side; “-” means nonischemic side. (C) The immunofluorescence staining was used to locate IRGM1 (red) expression 24 h after pMCAO. RBFOX3/NeuN (green) was used as the marker for neurons. Data represent 3 independent experiments. (D) mRNA level of Ifng was detected by real-time PCR (p < 0.05, n = 4/group).
Deficiency of neuronal autophagy activity in the Irgm1-KO mice after pMCAO induction.
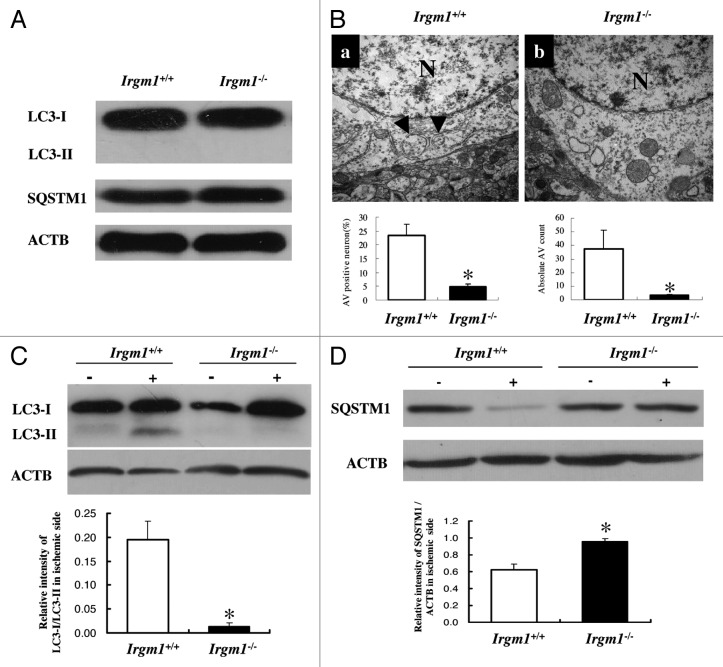
To test whether IRGM1 regulates autophagy in neurons during ischemia, we compared the autophagy activity between wild-type and Irgm1-KO pMCAO mice. As shown in Figure 3, the baseline of autophagy activity was quite low in the brain and was not different between wild-type and Irgm1-KO mice (Fig. 3A). TEM results showed that both the absolute number of autophagosomes and the autophagy vacuole (AV)-positive neurons were significantly lower in the Irgm1-KO as compared to wild-type pMCAO mice (Fig. 3B). Consistently, the reciprocal change of LC3-II or SQSTM1 expression was almost completely lost in the Irgm1-KO pMCAO mice (Fig. 3C and D). These data indicate IRGM1 is an important regulator for neuronal autophagy during ischemic stroke.
Figure 3. Deficiency of autophagy in Irgm1 knockout mice. (A) The basal level of LC3-I, LC3-II and SQSTM1/p62 was measured by western blot. (B) TEM showed lower autophagy vacuoles were found in Irgm1–/– mice after pMCAO (upper panel). Neuron autophagy observed by TEM was quantified by either percentage of AV positive neurons (left) or the total number of AV (right). Data represent 4 mice per group, 3 section per mice; 20 fields/section. p < 0.05. (C) LC3-I and LC3-II expression was detected by western blot (upper panel). Comparison was made between Irgm1+/+ and Irgm1–/– mice in terms of their LC3-II/LC3-I ratio (low panel, p < 0.05, n = 4/group). (D) Expression of SQSTM1/p62 in Irgm1+/+ and Irgm1–/– mice 24 h after pMCAO. ACTB was used as the loading control. Bar represents mean ± SEM from 4 mice in each group.*p < 0.05 compared with Irgm1+/+ group. ‘+’: ischemic side; ‘-’: nonischemic side.
IRGM1-mediated autophagy activation is associated with decreased neuronal necrosis in the core ischemic lesion but increased apoptosis in the penumbra area.
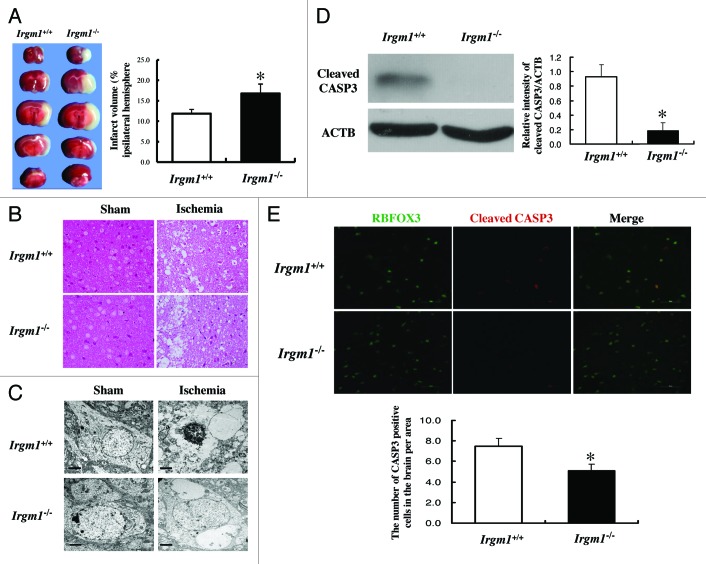
We then asked how IRGM1-induced autophagy influences the neuronal survival after ischemic induction. As shown in Figure 4A, 2,3,5-triphenyltetrazolium chloride (TTC) staining indicated that the volume of the infarct area in Irgm1-KO mice was significantly larger than in the wild-type mice after pMCAO induction. Both histochemical (Fig. 4B) and TEM (Fig. 4C) studies showed a necrotic morphology of neurons in the core ischemic lesion, and that the number of dead neurons was significantly higher in Irgm1-KO mice than in wild-type mice. However, apoptotic neurons in the penumbra area within the first 24 h of ischemia were much less in the KO mice than in wild-type mice, as indicated by decreased expression of cleaved CASP3/Caspase 3 and lower numbers of CASP3-positive neurons in this area (Fig. 4D and E). These data collectively suggest that the IRGM1-mediated early activation of autophagy protects neurons from death in the core ischemic lesion but promotes apoptosis in the penumbra zone.
Figure 4. Increase of neuron necrosis in Irgm1 KO mice after pMCAO. (A) TTC staining was used to measure the volume of the infarct area (5–6 mice/group. p < 0.05). (B) Semi-thin sections were stained with basic fuchsin to show cell structure after pMCAO in both wild-type and Irgm1 KO mice (scale bar: 50 µm). (C) Example data of TEM shows neuron apoptosis and necrosis after pMCAO in wild-type and Irgm1 KO mice. (D) Cleaved CASP3 expression at 24 h after pMCAO in either Irgm1+/+ or Irgm1–/– mice was determined by western blot (p < 0.05, n = 4). (E) Immuno-fluorescence staining to quantify and compare CASP3-positive neurons between Irgm1+/+ and Irgm1–/– mice (p < 0.05, n = 4).
Discussion
In this study, we have revealed a novel role of IRGM1 in regulating neuronal autophagy and apoptosis in a mouse ischemic stroke model. Possibly through induction by IFNG, IRGM1 was strongly upregulated in neurons after acute ischemia, which, in turn, promotes the activation of neuronal autophagy. In the early stage (within 24 h) of ischemia, the IRGM1-dependent autophagy activation is associated with a protection of neurons from necrosis in the ischemic core, and an increased neuronal apoptosis in the penumbra area.
Several mechanisms have been proposed to explain how IRGM regulate autophagy in myeloid cells. These include accelerating the conversion of endogenous LC3-I to LC3-II,14 inducing the fusion of autophagosomes with lysosomes by activating class III PtdIns3K,17 and interacting with other immunity-related GTPase (IRG) family members such as IIGP1, which indirectly regulates autophagy.18 Human IRGM is also important in controlling cell autophagy. It has been shown that during infection human IRGM can translocate to mitochondria, affect mitochondria fission and induce autophagy.19 In addition, a very recent study showed that human IRGM can physically interact with several autophagy-associated proteins including ATG5, ATG10, SH3GLB1/Bif-1 and MAP1LC3C/LC3 gamma, which are involved in initiation/elongation of the phases of autophagy.20 Our data showed that the conversion from LC3-I to LC3-II was completely suppressed in neurons of Irgm1-KO mice, indicating a similar mechanism underlying which IRGM regulates the neuronal autophagy in the initiation stage in the pMCAO model.
The role of autophagy in regulating cell death remains controversial. Depending on the context and intensity, autophagy may either protect cell from death or induce cell death. At the early stage, autophagy provides materials and energy to support cell survival and resist stress. However, after consuming excessive vital cellular organs via autophagy, cells may be vulnerable to death.21 During stroke, autophagy was thought to ameliorate the acute shortage of energy and oxygen supply. Pretreatment with autophagy-inducers such as rapamycin protects neurons from death, while pre-treatment with autophagy-inhibitors enhances necrosis.22 In contrast, post-treatment with 3-methyladenine (3-MA), an autophagy-inhibitor, reduced the lesion volume in rat.23 The distinct results from pre- and post-treatment targeting autophagy may reflect the different effects of autophagy at early and late stages. Consistent with this notion, our current study showed that, in the early phase of ischemia (within 24 hrs), IRGM1 induced autophagy in neurons and protected neurons from necrosis in the core ischemic area. However, it is unclear why the IRGM-induced autophagy was also associated with increased apoptosis in the penumbra area, and what the consequences are of this effect on the early stage of stroke. Since the overall infarct size in the Irgm1-KO was significantly increased as compared with the wild-type mice, it is reasonable to assume that the protective effect by IRGM-dependent autophagy on neurons dominates this stage.
The direct mechanisms underlying the regulation of cellular apoptosis by IRGM have been reported in the recent years. Singh et al. suggest that overexpression of certain isoform of human IRGM can lead to BAX-BAK1/bak-dependent cell death.19 IRGM can also bind to SH3GLB1,20 a pro-apoptotic protein that can induce BAX-BAK1-dependent apoptosis.24 Moreover, our previous study shows that deficiency of Irgm1 promotes the apoptosis of activated autoreactive CD4 T cells in an experimental autoimmune encephalomyelitis (EAE) model, possibly through a negative regulation of the IFNG-dependent pathway.25 These reports collectively indicate that IRGM can regulate cellular apoptosis by different molecular mechanisms in different settings. It is, therefore, important to unravel the IRGM-dependent regulation of neuronal apoptosis in future studies.
In conclusion, our work suggests a novel and crucial role of IRGM1 in regulating neuronal autophagy and apoptosis at the early stage of ischemia, which is important to dictate the fate of neurons and the overall outcomes of ischemic cerebral infarct. Further studies are needed to unravel the detailed mechanisms underlying these regulations and their effects on later stages of stroke.
Materials and Methods
Mice
Male C57BL/6 (B6) mice were purchased from HFK Bioscience. Irgm1 knock-out mice (Irgm1–/–) were a gift by Dr. Gregory A. Taylor (Geriatric Research, Education, and Clinical Center, VA Medical Center) and were backcrossed to B6 background for 10 generations. Male Irgm1–/– mice were used for the experiments. All mice were housed in the animal facilities of the Harbin Medical University and the Animal Center of the Institute of Genetics and Developmental Biology (Chinese Academy of Sciences). All experimental procedures involved were performed according to protocols approved by the Institutional Animal Care and Use Committee at the Institute of Genetics and Developmental Biology.
Mouse models of permanent middle cerebral artery occlusion (pMCAO).
Mice were anesthetized with intraperitoneal injection of 4% choral hydrate (350 mg/kg). Through a ventral midline incision, the right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA) were isolated and ligated. A 10 mm length of monofilament nylon suture (0.12–0.14 mm) was inserted from the right CCA to the internal carotid artery (ICA) through a small incision in the common carotid artery and then advanced to the Circle of Willis to occlude the origin of the right middle cerebral artery. The suture remained there until the mice were sacrificed. Sham-operated mice underwent the same procedures except for the pMCAO. To observe the time course for IRGM expression following pMCAO, animals were sacrificed at 0 (sham), 6, 12, 24, 72 and 96 h, post-pMCAO. Irgm1–/– mice were sacrificed at 6 and 24 h post-pMCAO.
Determination of infarct size
Mice were sacrificed 24 h after pMCAO. Brains were cut in five 2-mm thick coronal sections. The slices were stained for 30 min at 37°C with 2% 2,3,5-triphenyltrazolium chloride (Sigma-Aldrich, TTC 17779) in PBS to visualize the infarctions. Infarct volume was analyzed by NIS-Elements BR40000, Nikon DS-U2/L2-Ri1 and calculated following the formula:
Vinfarct (mm3) = [Area TTC section1 (mm2) × 2 mm] + [Area TTC section2 (mm2) × 2 mm] + … [Area TTC section5 (mm2) × 2 mm]
Pinfarct (%) = Vinfarct / Vtotal
Quantitative real-time PCR
Total RNA was extracted from cerebral cortex of pMCAO mice in different time points using TRIzol reagent (Invitrogen, 15596-026) according to the manufacturer’s protocol. cDNA synthesis was performed using random hexamer primers and the TaqMan reverse transcription kit (Applied Biosystems, 4366596). Samples were subjected to real-time PCR analysis on an ABI Stepone Sequence Detection System under standard conditions. The primers and probe for mouse Irgm1 were designed using Primer Express software (Applied Biosystems) based on GeneBank accession no. U19119 forward primer (5′-TGGCAATGGCATGTCATCTT-3′, reverse primer 5′-AGTACTCAGTCCGCGTCTTCGT-3′ and probe ACTTCGAGTCATCGGC). Relative mRNA abundance was normalized against 18S RNA as the endogenous control (Applied Biosystems, AM1718). For expression of Ifng, the SYBR Green mixture (Roche, 04913914001) with 3 pmol primers in a final reaction volume of 20 µl was used. The primer sequences for Ifng were forward 5′-TGAACGCTACACACTGCATCTTGG-3′ and reverse 5′-CGACTCCTTTTCCGCTTCCTGAG-3′. For quantification of the changes in gene expression, we used the comparative Ct method to calculate the relative-fold changes normalized by GAPDH transcript levels using the primers, forward 5′-CGGCCGCATCTTCTTGTGCA-3′ and reverse 5′-GCCGTGAGTGGAGTCATACT-3′ (Invitrogen). Each sample was assayed in triplicate.
Immuno-fluorescence staining
Frozen sections were stained to assess IRGM1, cleaved CASP3 and RBFOX3/NeuN expression. Cryopreserved brain sections were fixed with cold acetone for 10 min. Slides were permeabilized and blocked in PBS containing 5% BSA and 0.2% saponin for 60 min. They were then incubated with rabbit anti-IRGM Ab (HuaAn Biotechnology Company, 09HAP0401), in combination with either mouse anti-NeuN monoclonal Ab (Millipore, MAB377) or rabbit anti-cleaved CASP3 Ab (Beyotime, AC033), followed by secondary antibodies: donkey anti-rabbit IgG (Abcam, Dylight@549, ab98489), goat anti-mouse IgG (Abcam, DyLight@488, ab96879). Sections were examined by fluorescence microscopy (Nikon, ECLIPSE 80i). H&E staining of an adjacent section was performed to identify the core and penumbra area. The infarction and its border were identified by neuronal pyknosis, karyolysis and decreased eosin staining, while the penumbra area was identified from the edge of the infarction to 1 mm toward adjacent cerebral tissue (Fig. S1), as described previously.26,27 CASP3 positive neurons were counted at the penumbra region.
Western blotting
Western blotting was used to assess IRGM1, LC3 and SQSTM1/p62 expression in cerebral cortex. Briefly, whole protein lysates were separated by SDS-PolyAcrylamide Gel Electrophoresis (SDS-PAGE) and blotted onto a nitrocellulose blotting membrane. Antibodies used for western blotting include anti-IRGM monoclonal Ab (AbMart, 1C11), anti-LC3 Ab (Cell Signaling, 2775), anti-SQSTM1/p62 (Abcam, ab56416), anti-β-actin (Sigma, A3854), anti-mouse IgG (Cell Signaling, 7076) and anti-rabbit IgG (Beyotime, A0208). Results were normalized based on ACTB expression.
Transmission electron microscopy
The cortex was cut into 1-mm3 size cubes and fixed in 1% freshly made paraformaldehyde with 2% glutaraldehyde for 24 h. Samples were fixed for 2 h in 1% osmium tetroxide and dehydrated in graded ethanol and embedded in araldite. Semi-thin sections were cut and stained with basic fuchsin. Penumbra area of ischemic cortex was located by light microscope. Ultrathin sections were cut and stained with uranylacetate and lead citrate, and then observed with transmission electron microscope (Hitachi, H-765 Japan).
Statistical analyses
The statistical analyses were performed using SPSS program. All of the values were presented as the mean ± SEM, and were analyzed using t-test or a one-way analysis of variance (ANOVA). Statistical significance was defined as p < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Gregory A. Taylor for providing the Irgm1–/– mice, Dr. Zhiheng Xu for assistance with animal breeding and Prof. Hulun Li for project discussion and technical support. These studies were funded by overseas scholars of research projects, Department of Education, Heilongjiang Province Funding (1153h08) to Dr. Hongwei Xu; and National Natural Science Foundation Project (No.30870885), Jiangxi Provincial Natural Science Foundation (No. 2007GQY2521), Jiangxi Provincial Department of Science & Technology (2007) to Dr. Chaodong Wang.
Glossary
Abbreviation:
- IRGM1
immune-related GTPase M
- KO
knockout
- pMCAO
permanent middle cerebral artery occlusion
- IFNG
interferon gamma
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/autophagy/article/21561
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/21561
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des. 2008;14:3565–73. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- 4.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–9. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 5.Auriel E, Bornstein NM. Neuroprotection in acute ischemic stroke--current status. J Cell Mol Med. 2010;14:2200–2. doi: 10.1111/j.1582-4934.2010.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xilouri M, Stefanis L. Autophagy in the central nervous system: implications for neurodegenerative disorders. CNS Neurol Disord Drug Targets. 2010;9:701–19. doi: 10.2174/187152710793237421. [DOI] [PubMed] [Google Scholar]
- 8.Smith CM, Chen Y, Sullivan ML, Kochanek PM, Clark RS. Autophagy in acute brain injury: feast, famine, or folly? Neurobiol Dis. 2011;43:52–9. doi: 10.1016/j.nbd.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, et al. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–9. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- 10.Bekpen C, Hunn JP, Rohde C, Parvanova I, Guethlein L, Dunn DM, et al. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6:R92. doi: 10.1186/gb-2005-6-11-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–9. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 12.Santiago HC, Feng CG, Bafica A, Roffe E, Arantes RM, Cheever A, et al. Mice deficient in LRG-47 display enhanced susceptibility to Trypanosoma cruzi infection associated with defective hemopoiesis and intracellular control of parasite growth. J Immunol. 2005;175:8165–72. doi: 10.4049/jimmunol.175.12.8165. [DOI] [PubMed] [Google Scholar]
- 14.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–41. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- 15.King KY, Baldridge MT, Weksberg DC, Chambers SM, Lukov GL, Wu S, et al. Irgm1 protects hematopoietic stem cells by negative regulation of IFN signaling. Blood. 2011;118:1525–33. doi: 10.1182/blood-2011-01-328682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bafica A, Feng CG, Santiago HC, Aliberti J, Cheever A, Thomas KE, et al. The IFN-inducible GTPase LRG47 (Irgm1) negatively regulates TLR4-triggered proinflammatory cytokine production and prevents endotoxemia. J Immunol. 2007;179:5514–22. doi: 10.4049/jimmunol.179.8.5514. [DOI] [PubMed] [Google Scholar]
- 17.Tiwari S, Choi HP, Matsuzawa T, Pypaert M, MacMicking JD. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P(2) and PtdIns(3,4,5)P(3) promotes immunity to mycobacteria. Nat Immunol. 2009;10:907–17. doi: 10.1038/ni.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry SC, Daniell XG, Burroughs AR, Indaram M, Howell DN, Coers J, et al. Balance of Irgm protein activities determines IFN-gamma-induced host defense. J Leukoc Biol. 2009;85:877–85. doi: 10.1189/jlb.1008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SB, Ornatowski W, Vergne I, Naylor J, Delgado M, Roberts E, et al. Human IRGM regulates autophagy and cell-autonomous immunity functions through mitochondria. Nat Cell Biol. 2010;12:1154–65. doi: 10.1038/ncb2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grégoire IP, Richetta C, Meyniel-Schicklin L, Borel S, Pradezynski F, Diaz O, et al. IRGM is a common target of RNA viruses that subvert the autophagy network. PLoS Pathog. 2011;7:e1002422. doi: 10.1371/journal.ppat.1002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–10. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–39. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66:378–89. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, Karbowski M, Yamaguchi H, Kazi A, Wu J, Sebti SM, et al. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol. 2005;25:9369–82. doi: 10.1128/MCB.25.21.9369-9382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Wu ZY, Fang F, Guo L, Chen D, Chen JX, et al. Genetic deficiency of Irgm1 (LRG-47) suppresses induction of experimental autoimmune encephalomyelitis by promoting apoptosis of activated CD4+ T cells. FASEB J. 2010;24:1583–92. doi: 10.1096/fj.09-137323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai DX, Kong QF, Xu WS, Bai SS, Peng HS, Zhao K, et al. RAGE expression is up-regulated in human cerebral ischemia and pMCAO rats. Neurosci Lett. 2008;445:117–21. doi: 10.1016/j.neulet.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 27.Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, et al. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–76. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.