Abstract
HER-2 and the vascular endothelial factor receptor (VEGF) represent validated targets for the therapy of multiple tumor types and inhibitors of these receptors have gained increasing importance in the clinic. In this context, novel bioactive agents associated with better therapeutic outcomes and improved safety profile are urgently required. Specifically engineered HER-2- and VEGF-derived peptides in combination with low-dose chemotherapy might provide a substantial impact on tumor metastasis and cancer progression. We tested the antitumor effects of HER-2 and VEGF peptide mimics in combination with metronomic paclitaxel in both PyMT and Balb/c murine model challenged with TUBO cells. The combination of low-dose paclitaxel and HER-2 or VEGF peptide mimics had greater inhibitory effects than either agent alone. Peptide treatment caused virtually no cardiotoxic effects, while paclitaxel and the anti-HER-2 antibody trastuzumab (Herceptin), exerted consistent cardiotoxicity. The combination regimen also promoted significant reductions in tumor burden and prolonged survival rates in both transgenic and transplantable tumor models. Tumor weights were significantly reduced in mice treated with HER-2 peptides alone, and even more in animals that received HER-2 peptide with low-dose paclitaxel, which alone had no significant effects on tumor growth in the transgenic model. Specifically engineered native peptide sequences from HER-2 and VEGF used in combination with metronomic paclitaxel demonstrate enhanced anticancer efficacy and an encouraging safety profile. This novel approach to targeted therapy may offer new avenues for the treatment of breast cancer and other solid tumors that overexpress HER-2 and VEGF.
Keywords: HER-2 peptide mimics, VEGF peptide mimics, angiogenesis, chemoagents epitopes, immunotherapy, monoclonal antibodies, paclitaxel, peptidomimetics, toxicity
Introduction
ERBB2 (best known as HER-2/neu is an oncoprotein that is overexpressed in approximately 20–30% cases of breast cancers and is associated with increased aggressiveness and poor clinical outcome.1 HER-2 is a well-established target for immunotherapy and many different anti-HER-2 strategies have been tested, including several humanized monoclonal antibodies (such as trastuzumab and pertuzumab) and small molecule tyrosine kinase inhibitor (like lapatanib). Pertuzumab has been shown to bind the extracellular domain II of HER-2, thereby interrupting dimerization via a mechanism that differs from that of trastuzumab.2
Most solid tumors cannot grow beyond a size of few millimeters without undergoing the so-called “angiogenic switch,” allowing for neovascularization and the consequent supply of nutrients and oxygen in sufficient amounts.3 Thus, angiogenesis inhibition offers an attractive therapeutic strategy for cancer therapy. The pro-angiogenic factor best known today is the vascular endothelial growth factor (VEGF),4 its overexpression being reported in many different types of cancers. HER-2 upregulation is accompanied by increased expression of VEGF, at both the RNA and protein level in a large panel of cancer cells.5 As VEGF and its receptors are profoundly implicated in different forms of cancer, anti-VEGF antibodies have been developed for use in the clinic, including bevacizumab.6 Many FDA-approved humanized monoclonal antibodies that target HER-2 and VEGF have been associated with undesirable toxic profiles.7 Thus, novel targeted therapies that would to improve clinical outcome at the cost of limited toxicity are urgently required.The main focus of our laboratory has been to develop HER-2-derived peptide vaccines that stimulate the immune system to produce high affinity antibodies exerting antitumor effects. Previously identified and designed B-cell epitopes from the HER-2 protein have successfully been translated into the clinic as candidate vaccines, combined as a chimeric construct with a “promiscuous” T-cell epitope.8 More recently, rather than harnessing the immune system to elicit native-like antitumor antibodies upon vaccination, we have embarked on a different, but related, strategy of interrupting ligand:receptor activation by engineered peptide mimics devoid of a T cell-stimulating moiety. We have validated this hypothesis by successfully demonstrating that VEGF peptide mimics with specific modifications are effective both in vitro and in vivo to block the VEGF:VEGFR2 pathway, thereby inhibiting angiogenesis.9 Similarly, the combination of a HER-2 and a VEGF peptide mimic has been shown to provide enhanced antineoplastic effects in a transplantable BALB/c tumor model.10 To further refine our immunotherapeutic strategies, we recently completed a combination study in which we immunized mice with the MVF-HER-2 (266–296) peptide vaccine, followed by the administration (on a weekly schedule) of VEGF peptide mimics, resulting in enhanced tumor growth prevention in transplantable tumor models.11One of the greatest challenges in anticancer immunotherapy today is to minimize toxicity and maximize efficacy. Thus, combination treatments with low-dose chemotherapy and antiangiogenic/antitumor agents have generated interest in that they are supposed to result in reduced toxicity and prime targeted antitumor activity.12 Antiangiogenic agents cause the normalization of tumor vasculature, thereby increasing the accessibility of drugs to the tumor.13 Many studies have shown greater response rates with the use of a combination approach involving angiogenesis inhibitors in many preclinical settings.14 Paclitaxel is one of the most widely used chemotherapeutic agents for the treatment of various types of solid tumors. Paclitaxel exerts anticancer effects mainly by inhibiting mitosis and hence causing the apoptotic demise of tumor cells. Extensive studies have been performed with paclitaxel alone or in combination with other anticancer agents, in different types of tumors. Most of these studies showed that combining paclitaxel with other anticancer agents improves response rates.15 Due to its usage in many types of cancers and its superior antitumor effects,16 we wanted to gauge the effects of low-dose paclitaxel in combination with HER-2 and/or VEGF peptide mimics, in a transgenic mouse model of human breast cancer.In order to develop a safe, efficient and poorly toxic anticancer strategy, we are extending our overall approach and combining specially engineered peptide VEGF and HER-2 peptide mimics with a low-dose paclitaxel regimen. The rationale for inclusion of chemotherapeutics is further supported by reports in the literature indicating an enhanced efficacy and better therapeutic outcome of this type of approach. Our in vivo results show that the combination of HER-2 and VEGF peptide mimics with low-dose paclitaxel inhibits angiogenesis, causes tumor shrinkage, and produces better response rates than either single agents alone, in both transplantable and transgenic mouse model. Additionally, we show that such peptide therapeutics combined with metronomic paclitaxel are safe, exhibiting no cardiotoxic effects. The most dramatic antitumor effect is observed when paclitaxel is combined with either the HER-2 or VEGF mimics.
Results
Selection, design, synthesis and characterization of peptides
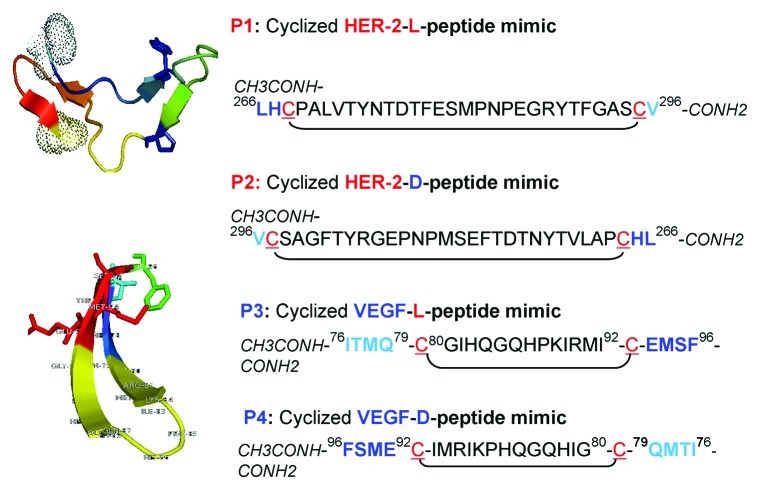
The VEGF peptide mimic residues, corresponding to aa 102–122 (numbered as 76–96 in the crystal structure), correspond to the overlapping binding sites on VEGF for VEGFR2 and avastin. Engineering of this peptide sequence has been described in details elsewhere.9 The sequence and structure of both the HER-2 and VEGF peptide mimics are shown in Figure 1. Briefly, the strategy to create a conformational peptide consisting of an anti-parallel β sheet is described elsewhere,9 where the sequence was modified in a way that the resulting peptide non-cyclized (NC) peptide VEGF-P3 adopted a conformation very similar to the native structure. It also required two artificial cysteines to be introduced between Gln79 and Gly92, and between Ile80 and Glu93. After synthesis and purification of NC VEGF-P3, disulfide bonds were formed by oxidation, enabling the formation of the twisted anti-parallel β-sheet structure in the cyclized (CYC) version of the peptide.17,18 The rationale behind retro-inverso (RI) peptidomimetics is that they should present comparable biological activity but higher bioavailability than their natural counterparts. The RI peptide analog VEGF-RI-P4 was synthesized using D‐amino acids in reverse sequence order, such that the resulting peptide mimic has a reversal of the peptide backbone but a topochemical equivalence to the parent peptide in terms of side‐chain orientation.19
Figure 1. Amino acid sequences and 3-dimensional structure of HER-2 and VEGF peptide mimics. Sequences and 3D model of HER-2 and VEGF peptide mimics. P1 = HER-2 L peptide and P3 = VEGF L peptide; P2 = HER-2-D-retro-inverso (RI)-peptide mimic and P4 = VEGF D- RI-peptide mimic. Peptide sequences of P2 and P4 are inverted and synthesized in the reverse order using D-amino acids.
Antitumor effects of HER-2 peptide mimics are comparable to that of paclitaxel and trastuzumab
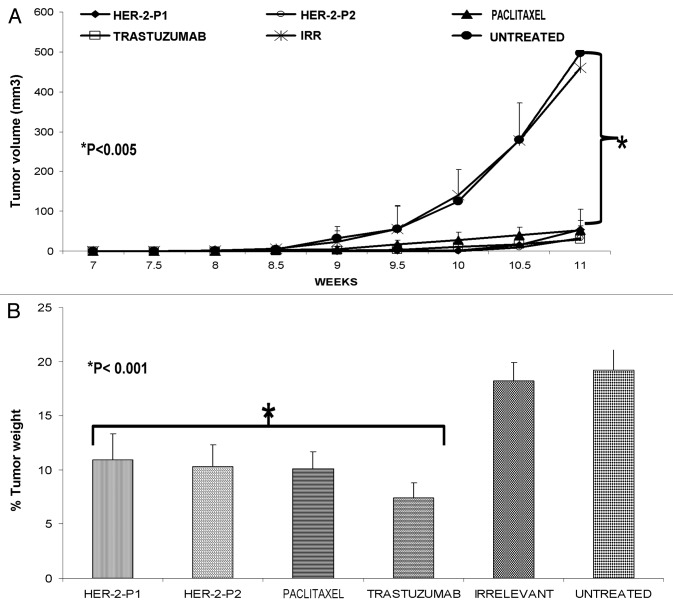
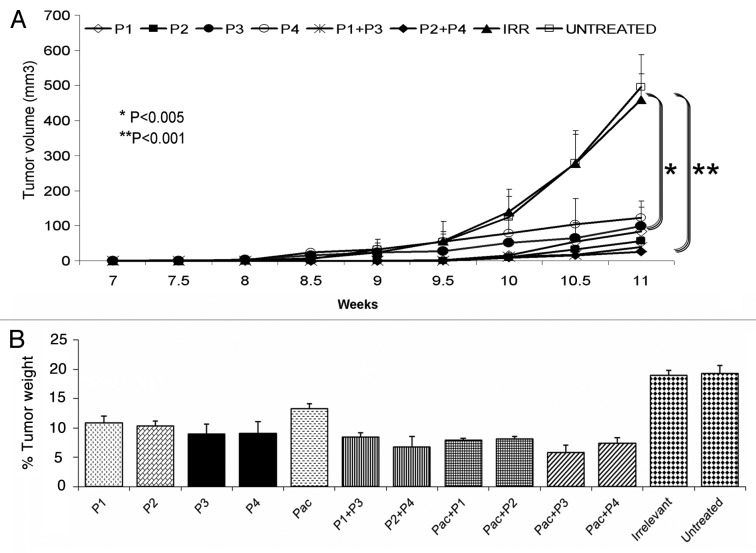
The antitumor efficacy of the HER-2 peptide mimics was compared with that of paclitaxel, by treating female FVB/n mice (which are positive for the PyMT transgene) with 300 µg of the peptides, trastuzumab or paclitaxel. We found that both the L and D-amino acid version of the HER-2 peptide mimics are able to inhibit tumor growth, with an efficacy that is comparable to that of paclitaxel and trastuzumab (Fig. 2A). Since in this model mice develop multiple tumors, we measured only the size of the largest one (Fig. 2A) and we determined the percentage tumor weights (all tumors) after individual treatments (Fig. 2B). Our results show that HER-2 peptides (P1 and P2) are able to cause a significant reduction in tumor weight (*p < 0.001) when compared with irrelevant peptides (Fig. 2B) or to no therapy. The administration of trastuzumab and paclitaxel reduced tumor weight comparably to HER peptide mimics.
Figure 2. Antitumor effects of HER-2 peptide mimics are comparable to that of paclitaxel and trastuzumab. Female Fvbn mice (n = 5) were treated intravenously with HER-2 peptide mimics, paclitaxel or trastuzumab. Mice were treated weekly from week 4 to 10 and tumor volume was measured twice weekly. All mice were sacrificed at week 11 and tumors extracted and weighed. All treatments significantly inhibited tumor growth with a p value of < 0.001 (A) and the effect was also evident on normalized tumor weight (B). Error bars represent standard deviations from the mean.
Cardiotoxic effects of HER-2 peptide treatment in comparison with trastuzumab and taxol
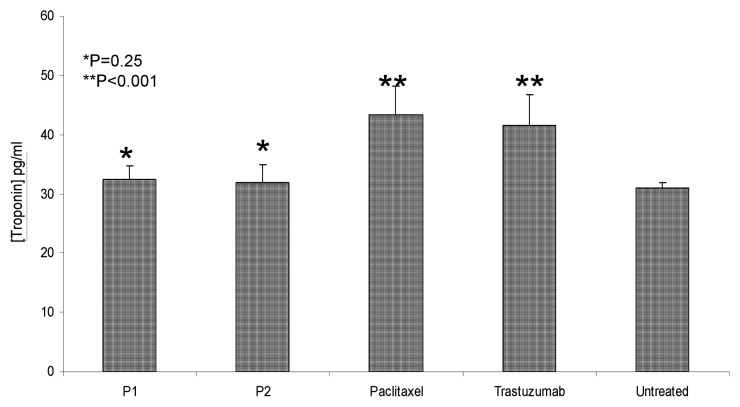
One of the major drawbacks of current chemotherapeutic regimens is cardiotoxicity. Trastuzumab has been shown to cause cardiotoxic effects in a proportion of cancer patients.20,21 Along similar lines, studies have demonstrated the need to optimize the treatment regimen for paclitaxel due to numerous side effects as a result of its limited specificity. We therefore investigated the cardiotoxic effects of our peptide mimics and compared them with those of trastuzumab and paclitaxel. We measured the levels of cardiac troponin I in the serum, as collected by retro-orbital bleeding. Our findings indicate that trastuzumab and paclitaxel cause a significant increase in serum levels of cardiac troponin I (*p < 0.001) while HER-2 peptide mimics do not (*p = 0.25), as compared with no therapy (Fig. 3).
Figure 3. Cardiotoxic effects of HER-2 peptide treatment in comparison with trastuzumab and paclitaxel. Blood was collected via retro-orbital bleeding from mice (n = 5) treated with HER-2 peptides, paclitaxel or trastuzumab before being euthanized at the end of treatment. Serum was purified from the blood and levels of cardiac troponin I were measured using ELISA. HER-2 peptide mimics had no significant effects on cardiotoxicity (*p = 0.25) while paclitaxel and trastuzumab caused significant cardiotoxic effects (**p < 0.001). Error bars represent standard errors of the mean.
Combination treatment with low-dose paclitaxel and HER-2 or VEGF peptide mimics exerts superior antitumor effects in a transgenic mouse model
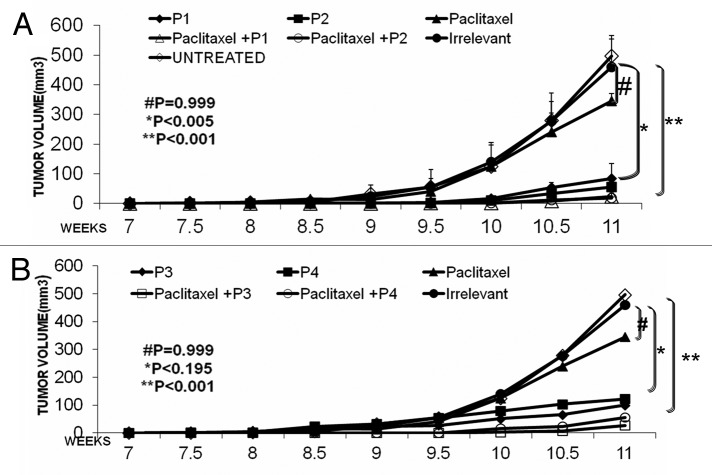
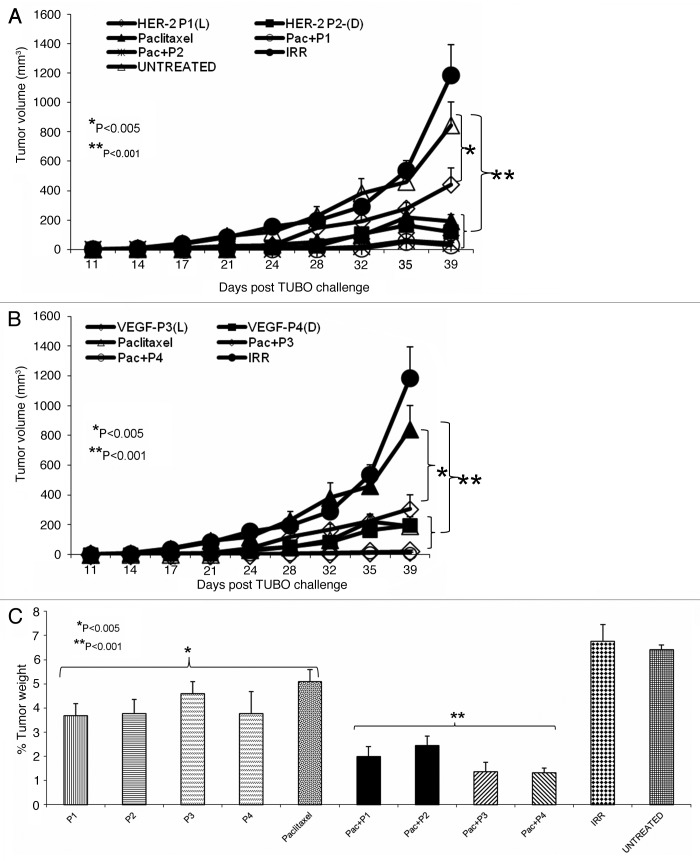
After confirming the cardiotoxic effects of paclitaxel and trastuzumab at the usual dose of 300 µg/mouse, we reduced the amount to a non-toxic dose of 60 µg/mouse (personal communication from Dr. Ginoula Clement). We hypothesized that combination regimens employing low-dose paclitaxel and peptide mimics would yield synergistic/additive antineoplastic effects in vivo in the absence of significant cardiotoxicity. In order to test this hypothesis, we used 60 µg of paclitaxel in combination with 300 µg of HER-2 or VEGF peptide mimics in the PyMT transgenic mouse model of human breast cancer.22 Our results show that the treatment with individual HER-2 peptide mimics (P1 and P2) produced a significant reduction in tumor growth (p < 0.005). When the HER-2 peptide mimic was combined with low-dose paclitaxel, antitumor effect were further ameliorated (p < 0.001) (Fig. 4A) pointing to an additive interaction. On the other hand, VEGF peptide mimics (P3 and P4) alone were able to inhibit tumor growth but the effects were not statistically significant (*p < 0.195). However, when combined with low-dose paclitaxel, an improved antitumor effect was observed (p < 0.001) (Fig. 4B). These results show that low-dose paclitaxel and VEGF peptide mimics alone had no significant effect on tumor growth, while significant anticancer activity was observed when the peptide mimics were combined with paclitaxel. We were also interested in examining the antitumor effects of the combination of HER-2 and VEGF peptide mimics. As shown in Figure 4C, treatment with individual HER-2 peptides (P1 and P2) had significant effects on tumor growth (*p < 0.005) while the VEGF peptide mimics (P3 and P4) showed no significant activity, though there was a delay in tumor development when compared to no therapy. The combination of HER-2 and VEGF peptide mimics (P1+P3 and P2+P4) produced greater antitumor effects as compared with individual HER-2 or VEGF peptide mimics (**p < 0.001). There was also a significant delay in the onset of tumor development in response to the combination therapy (around week 10) as compared with either monotherrapies (around week 8.5). Next, we looked at the overall effects of the combination treatment on the tumor burden, by measuring tumor weight. Mice receiving both HER-2 and VEGF peptides showed tumor weight reduced to less than 10% of the control value, while mice treated with either peptide alone exhibited tumor weight greater than 10% of the control value in the cases (Fig. 4D). The best result was obtained with the RI HER-2 peptide and VEGF peptide mimics.
Figure 4. Combination treatment produces superior antitumor effects in a transgenic mouse model. Female FVB/n mice (n = 5) were treated intravenously with HER-2 peptide mimics, VEGF peptide mimics, paclitaxel alone or in combination with the peptides. Mice were treated weekly from week 4 to 10 and tumor volume was measured twice weekly. All mice were sacrificed at week 11 and tumors extracted and weighed. Treatment with paclitaxel alone had no significant effect on tumor growth (#p = 0.999) while the HER-2 peptide mimics (P1 and P2) (A) and VEGF peptide mimics (P3 and P4) (B) in combination with low-dose paclitaxel caused a significant inhibition of tumor growth over time (**p < 0.001).
Antitumor effects of peptide mimics and paclitaxel in a transplantable mouse model
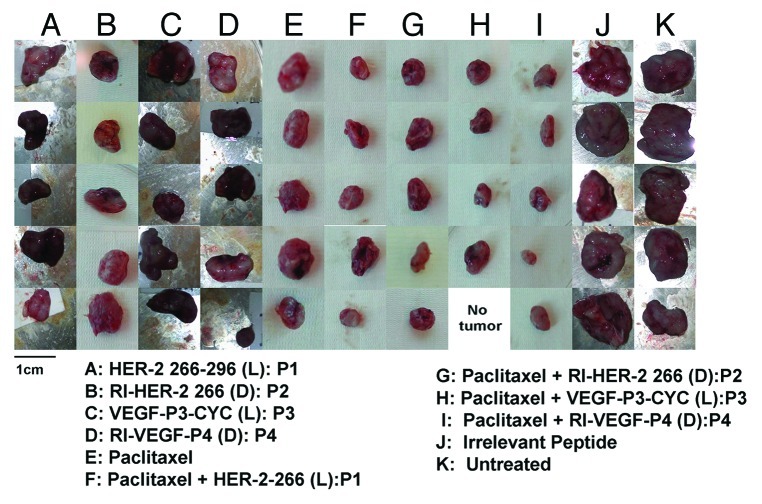
We evaluated the antitumor and antiangiogenic activities of the peptides alone or in combination with paclitaxel in a transplantable tumor model. To this aim, we used wild-type Balb/c mice that were challenged with TUBO cells, very aggressive tumor cells established from the Balb-neuT transgenic mice.23 Peptides alone were able to cause a delay in the onset of tumor development (*p < 0.001) (Figs. 5A and B), a delay that was increased when peptides were combined with paclitaxel. The degree of inhibition observed with the combination treatment was greater than that observed with either peptide or paclitaxel alone. We also examined the effects of these treatments on tumor weight, finding that single treatments (peptides or paclitaxel) caused a significant reduction of tumor burden (*p < 0.005), which was largely exacerbated in the case of the combination regimen (**p < 0.001) (Fig. 6). Mice treated with the combination of HER-2 and VEGF peptides or paclitaxel and either peptide showed a greater reduction in size (Fig. 7).
Figure 5. Antitumor effects of combination treatment with HER-2 and VEGF peptide mimics in a transgenic mouse model. Combination treatment with both HER-2 and VEGF peptide mimics (P1 +P3 and P2+P4) cause a greater inhibition of tumor growth (**p < 0.001) while treatment with HER-2 peptides alone was associated with a less significant effect (*p < 0.005) when compared with no therapy (A). All treatments caused a significant reduction in percentage tumor weight (*p < 0.001) as shown (B). Error bars represent standard deviation of the mean.
Figure 6. Antitumor effects of peptide mimics and paclitaxel in a transplantable mouse model. Wild type BALB/c mice (n = 5) at the age of 5–7 weeks were challenged with TUBO cells. Mice were then treated intravenously with paclitaxel, HER-2 peptides, VEGF peptides or a combination of paclitaxel and the peptides. Results (A and B) indicate a delay in tumor growth and development in the treated groups and the effect was more evident with the combination of paclitaxel and the peptides (*p < 0.001). Mice that were treated with both peptides and paclitaxel showed a significant reduction in tumor weight a (p < 0.001) as compared with control animals, while mice treated with either peptides alone or paclitaxel alone showed a significant difference with p < 0.005. Tumor measurement was performed using calipers and error bars represent standard deviations from the mean.
Figure 7. Combination treatment causes a reduction in tumor size at the end of treatment. Tumors from mice treated with single or combination agents were excised and photos taken using a Nikon Camera. Photos show a great reduction in size which is most evident in the case of combination treatment with low-dose paclitaxel and HER-2 or VEGF peptide mimics.
Combination treatment decreases the number of actively dividing cells in both transplantable and transgenic mouse model of breast cancer
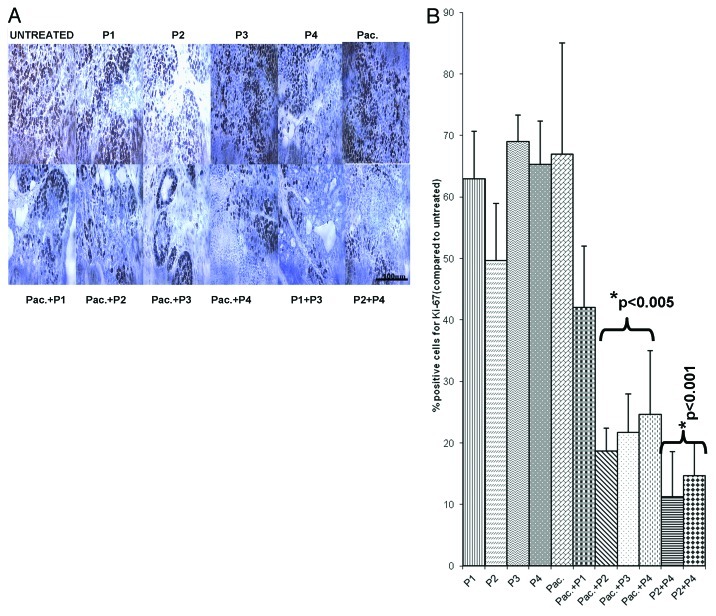
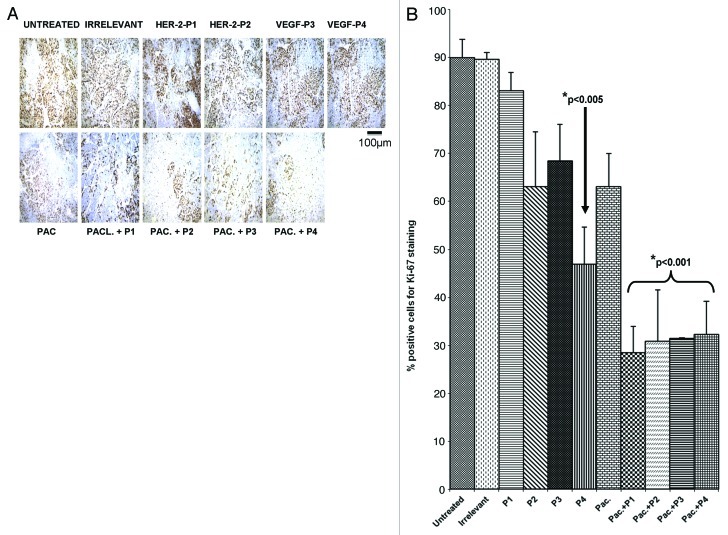
Paclitaxel mainly operates as a mitotic inhibitor.24,25 In order to clarify the antitumor mechanisms elicited by the HER-2 and VEGF peptide mimics and compare them with those of paclitaxel, we examined the effects of the different treatments on the number of actively dividing cells. Figures 8 and 9 show the amount of dividing cells in transgenic and transplantable models respectively, using Ki-67 staining. The number of cells greatly decreased in the case of single agent treatment, a decrease that was largely exacerbated by the combination regimen (p < 0.001).
Figure 8. Combination treatment decreases the number of actively dividing cells in a transgenic mouse model of Breast cancer. Quantification of the number of actively dividing cells in tumor sections using Ki-67 staining. (A) Tissue sections show the amount of positive cells. The stain is specific for cells that are actively dividing. (B) Quantification of the staining using Image J software. Data represent mean values from three different fields and error bars represent standard deviations from the mean.
Figure 9. Combination treatment decreases the number of actively dividing cells in a transplantable mouse model of breast cancer. Quantification of the number of actively dividing cells in tumor sections using Ki-67 staining. (A) Tissue sections show the amount of positive cells. The stain is specific for cells that are actively dividing. (B) Quantification of the staining using Image J software. Data represent mean values from three different fields and error bars represent standard deviations from the mean.
Combination treatment significantly decreases the microvascular density
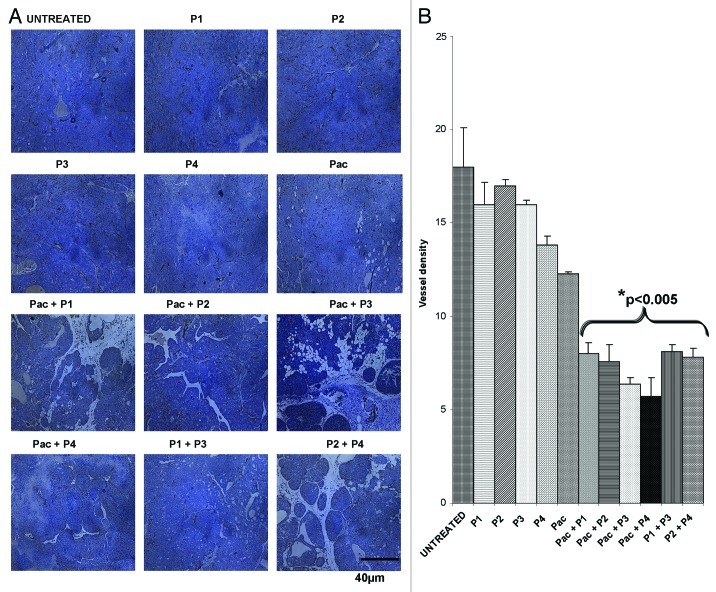
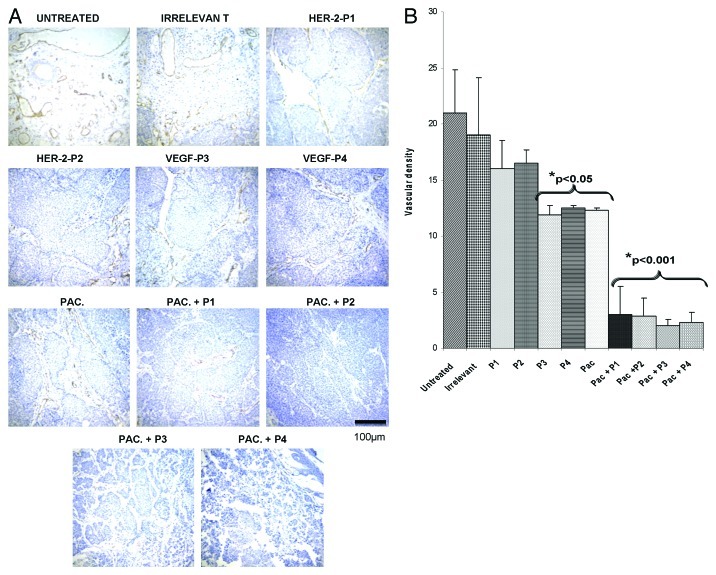
To further clarify the mechanisms of action of HER-2 and VEGF peptide mimics, we examined vessel density in tumors using an anti-CD31 antibody. The number of microvessels positive for anti-CD31 staining in the tumors resected from mice treated with the peptides alone or in combination with paclitaxel was lower than that observed in control untreated tumors, in both models used in this study (p < 0.001) (Figs. 10 and 11).
Figure 10. Combination treatment decreases microvascular density in tumors in transgenic mouse model. Evaluation of vessel density in tumor sections. (A) Vascular staining using anti-CD31 antibody. (B) Effects of combination treatment on the tumor vessel density after quantification with the Image J software. Data represents mean values and error bars represents mean standard deviations.
Figure 11. Combination treatment decreases microvascular density in tumors in Balb/c transplantable mouse model. Evaluation of vessel density in tumor sections. (A) Vascular staining using anti-CD31 antibody. (B) Effects of combination treatment on the tumor vessel density after quantification with the Image J software. Data represents mean values and error bars represents mean standard deviations.
Discussion
A plethora of anticancer agents have been developed to target tyrosine kinase receptors (RTKs), including antibodies against RTKs or their ligands and small molecule inhibitors that target RK intracellular kinase domain.26-28 Several FDA-approved therapies targeting both HER-2 (e.g., trastuzumab) and VEGF (e.g., bevacizumab) promoted significant toxic effects including cardiac dysfunction and congestive heart failure.6,7,29 Moreover, many patients treated with these drugs undergo disease progression due to development of resistance. The clinical application of humanized monoclonal antibodies is generally limited by a great number of concerns including the frequency of treatments, their costs, the limited duration of their effects, an undesirable immunogenicity, the development of acquired resistance, and substantial risks of cardiotoxic episodes.30 Similarly, small molecule RTK inhibitors such as sunitinib, which have entered clinical trials alone or in combination with radiotherapy or conventional chemotherapy, are overshadowed by problems of efficacy, development of resistance and undesirable safety profiles, which altogether impede their clinical progress.7 Despite the relative success of these drugs, there is an unmet need for novel molecular cancer therapeutics. Innovative therapies that target molecular pathways aberrantly activated only in cancer cells (thus exerting potent antineoplastic effects but low toxicity) are urgently needed.
The development of peptides that specifically block receptor-ligand interactions, thanks to structure-based design, constitute a promising avenue for the development of highly targeted anticancer therapeutics. The main objective of this strategy is to preserve the conformational integrity of the bioactive surface while retaining sufficient flexibility to cooperatively bind a given receptor. Such peptide mimics are water soluble, usually devoid of immunogenic potential and have the ability to easily cross tissue barriers.31 One of the major drawbacks of using peptides as therapeutic agents is that they are quickly degraded by proteosomal enzymes.32 This obstacle can be overcome by the use of modified peptides (Fig. 1). The RI modification consists in utilizing D-amino acids (which have reversed chirality) and inverting the amino acid sequence (from N-to-C) (resulting in the reversal of the peptide backbone). The product is a topographical equivalent of the parent peptide with the amino side chain in a similar orientation. Because RI peptides are synthesized with D-amino acids and proteases usually recognize L-amino acids, they are more resistant to proteosomal degradation, and therefore are characterized by improved bioavailability in vivo.32-34
In the present study, we have focused our efforts on the combination of HER-2 and VEGF peptide mimics accompanied by low doses of chemotherapy. First, we evaluated the antitumor effects of HER-2 peptides (P1 and P2) in comparison to that of paclitaxel and trastuzumab. We found that these treatments exert comparable antitumor effects (Figs. 2A and B). However, both paclitaxel and trastuzumab are associated with elevated cardiotoxicity, as demonstrated by the amounts of cardiac troponin I released in the serum upon treatment (Fig. 3). The HER-2 peptide was associated with the lowest toxicity profile.
We postulated that combining low-dose paclitaxel with peptide mimics may yield additive/and or synergistic effects in vivo with little or no cardiotoxicity. Many studies have also shown that low doses of chemotherapy are relatively non-toxic and yields greater antitumor effects when combined with other anticancer interventions, such as radiation therapy.35-37 Results obtained with the combination of low-dose paclitaxel with either HER-2 or VEGF peptide mimics point to increase antitumor effects in vivo as compared with single treatments (Fig. 4A and B). Low doses of paclitaxel alone or in combination with peptide mimics did not increase cardiotoxicity (results not shown). These results illustrate the validity of using minimal doses of chemotherapy in combination with other anticancer agents.
In addition to the Balb/c transplantable model, which develop tumors approximately at day 21 after a challenge with TUBO cells,38 we used the PyMT transgenic model, which starts to develop mammary tumors at around five weeks of age.22 The PyMT (polyoma middle T oncoprotein) transgenic mouse is an excellent model for understanding breast cancer progression due to its strict similarities with human breast cancer, on both morphological characteristics and biochemical phenotype. Tumor growth and development in this model results from the overexpression of HER-2 and VEGF, eventually promoting metastasis. In the other model, we used TUBO cells, which overexpress HER-2/neu. Tumor growth and metastasis in this model also depends on the elevated expression of HER-2 and VEGF. Therefore, both these models are adequate to test the validity and the efficacy of the HER-2 and VEGF peptide mimics.
In order to assess any side effects due to treatment, we examined cardiotoxic effects as induced by the HER-2 peptides (in comparison with paclitaxel and trastuzumab) by measuring serum levels of cardiac troponin I after treatment. The administration of paclitaxel or trastuzumab, but not that of HER peptide mimics, induced significant cardiotoxicity, We next evaluated the effects of individual HER-2 and VEGF peptide mimics vs. combination regimens based on equivalent peptide amounts and we obtained significant inhibition of tumor growth in vivo (**p < 0.001) as compared with individual treatment with HER-2 peptides (*p < 0.005) (Fig. 5A). We also compared peptide mimics, individually taken, with paclitaxel and our results were similar in all cases (*p < 0.01) (Fig. 5B). Of note, the combination of HER-2 and VEGF peptide mimics exerts no significant cardiotoxicity. Actually, the best results were observed when paclitaxel was combined with the VEGF, rather than with HER-2, peptide mimics. The D-amino VEGF peptide in combination with paclitaxel showed the greatest antitumor effects, with 20% tumor-free animals at the end of the experiment (Figs. 6 and 7). Tumor growth was relatively slower in mice subjected to the combination treatment than in mice receiving peptide mimics as single agents (Fig. 6). Tumors extracted from mice treated receiving the combination therapy also showed decreased vascularization (Fig. 7). Thus, our data indicate that the combination of paclitaxel and HER-2 or VEGF peptide mimics treatment causes a consistent decrease in tumor burden.
The effect of such a combination treatment in a transplantable tumor model was similar to that observed in the transgenic model, further validating our strategy. Low-dose paclitaxel in combination with HER-2 or VEGF peptide mimics caused a greater inhibition of tumor growth and development than either of these interventions alone (Figs. 6 and 7). Immunohistochemical studies showed a consistent reduction in the amount of actively dividing cells and microvascular density in mice receiving the combination treatment as compared with animals receiving monotherapy, in both transgenic and transplantable models (Figs. 8 and 9). These results illustrate that minimal doses of paclitaxel in combination with HER-2 and VEGF peptide mimics additively inhibits cancer cell growth. Similar additive effects were evident on the inhibition of microvessel density, as determined by immunohistochemistry upon staining with an anti-CD31 antibody. Tumor growth and metastasis is highly dependent on increased microvascular density (for the supply of oxygen and nutrient) and the reduction of blood vessels is very important for antitumor responses. In both the transgenic and transplantable models, microvascular density was greatly decreased when the combination treatment was used (Figs. 10 and 11), indicating that the inhibitors, when used in combination, were able to prevent tumor growth by affecting vascularization..
In conclusion, our results illustrate that HER-2 and VEGF peptide mimics exert potent antitumor effects when combined with low-dose paclitaxel. The advantages of our strategies are that these peptides are generally safe, and this safety profile remains unchanged even when peptides are combine with low-dose paclitaxel. In the case of combining HER-2 with VEGF peptide mimics, D-amino acid peptides had better efficacy than their L-amino acid counterparts. These results are consistent with our hypothesis that that the D-amino acid derivatives should be more effective than their L counterparts due to their greater stability in vivo. We conclude that combining peptide mimics with low-dose chemotherapy may offer a beneficial alternative to standard regimens in the clinical practice. Combination treatments targeting angiogenesis and metronomic chemotherapy may result in better patient survival and little toxicity. There is an urgent need for safe combination approaches that enhance antitumor immunity by targeting tumor-associated antigens and molecules involved in tumor angiogenesis. To circumvent the development of resistance in targeted therapy, strategies aimed at combining inhibition of compensatory pathways may offer substantial advantages in the future. Our work in that respect is aimed at evaluating the combinatorial inhibition of HER-1, HER-3 and the insulin growth factor receptor (IGFR) for the therapy of intractable cancers.
Materials and Methods
Drugs
Paclitaxel was purchased from the Ohio State University Pharmacy and trastuzumab was a kind gift from Dr. William Carson.
Synthesis and characterization of conformational peptides
Peptide synthesis was performed on a Milligen/Biosearch 9600 peptide solid phase synthesizer (Bedford, MA) using Fmoc/t-But chemistry. Preloaded Fmoc-Val-Clear acid resin (0.35 mmol/g) for the 266–296 and clear amide resin for the VEGF peptides (0.32 mmol/g) (Peptides International, Louisville, KY) were used for synthesis. The 266–296 cyclized epitope was assembled by choosing the regioselective side chain protector Trt on Cys residues 268 and 295,38 and in the VEGF peptides two cysteines were inserted between amino acid Gln79 and Gly92 and between Ile80 and Glu93. Peptides were cleaved from the resin using cleavage reagent B (trifluoroacetic acid: phenol: water: TIS, 90:4:4:2), and crude peptides purified by semi preparative reversed-phase-HPLC and characterized by electrospray ionization mass spectroscopy.39 Intramolecular disulphide bonds were formed using iodine oxidation as described40 and disulphide bridge formation was further confirmed by maleimide-PEO2-biotin reaction and subsequent analysis using electrospray ionization mass spectroscopy. All fractions were analyzed on analytical RP-HPLC and characterized by MALDI (Matrix Assisted Laser Desorption Ionization mass spectroscopy) at CCIC (Campus Chemical Instrumentation Center, The Ohio State University, Columbus, Ohio). RP-HPLC fractions showing same mass spectrum peak were pooled together and lyophilized. RP-HPLC pure peptides MVF-VEGF-P3, VEGF-P3 containing two Cys residues in each peptide were cyclized using acetic acid-iodine method, further purified on RP-HPLC and characterized by mass spectroscopy using established protocol as reported earlier.41 Amino acid sequences and molecular weight of all peptides and their molecular weights are shown in Figure 1.
Cardiotoxicity Studies
Groups of female mice (n = 5) that are heterozygous for the PyMT transgene were treated intravenously with 300 µg of either trastuzumab, paclitaxel, HER-2 L- amino acid peptide or the HER-2 D-amino acid peptide. Treatment was started from week four till week 11 and tumor sizes were measured twice weekly using calipers and tumor volume calculated using the formula Volume = Length X Width2/2. At the end of the treatment, blood was collected via retro-orbital bleeding and serum samples were used to measure the concentration of cardiac troponin I that was present after treatment. This was measured using the highly sensitive mouse cardiac troponin I ELISA kit from Life Diagnostics.
In vivo antitumor studies in transgenic mouse model
Animal breeding was performed in our facility following the institutional guidelines. Female Fvb/n mice that are heterozygous positive for the PyMT transgene were used for the studies. The mice were maintained in a sterile animal facility for the duration of the study. The mice were treated intravenously with the drugs or peptide or a combination of both starting at week four and weekly treatments were continued until week 11. The treatment included low dose paclitaxel alone (60 µg) or peptides alone (300 µg) or combination of both. In the combination studies groups of tumor bearing mice (n = 5) received a combination treatment of 60 µg of paclitaxel + 300 µg of peptide. All the mice were euthanized at 11 weeks of age or if the tumor burden becomes unbearable based on the evaluation of the university animal technician. Tumor sizes were measured twice weekly using calipers and tumor volume was calculated using the formula Volume = Length × Width2/2.
In vivo antitumor studies in transplantable mouse model
Female Balb/c wild type mice 5–6 weeks old were purchased from the Jackson laboratory. The mice were maintained in a sterile animal facility for the duration of the study. The mice were subcutaneously challenged with 1 × 105 TUBO cells. On the day of TUBO challenge, the mice were intravenously treated with 100 µg of either HER-2 or VEGF peptides or 20 µg of paclitaxel. In the combination studies groups of tumor bearing mice (n = 5) received a combination treatment of 20 µg of paclitaxel +100 µg of peptide. The mice were then treated weekly for a total of 6 weeks and palpable tumors were measured twice weekly. All the mice were euthanized at 7 weeks after TUBO challenge or if the tumor diameter reached 1 cm. Tumor sizes were measured using calipers and tumor volume was calculated using the formula Volume = Length × Width2/2.
Immunohistochemistry
In order to identify the different components of the tumors, serial sections were cut and stained for Ki-67 staining for actively dividing cells, CD31 for blood vessels and F4/80 for macrophages. The immunohistochemical detection protocol was performed as published.42 Total staining was analyzed using the NIH Image J software and the staining index was calculated as the percentage area occupied by the positive cells to the total area occupied by all the cells.
Statistical analysis
Tumor growth over time was analyzed using Stata’s XTGEE (cross-sectional generalized estimating equations) model which fits general linear models that allow you to specify within animal correlation structure in data involving repeated measurements.43 For immunohistochemical analysis, a non-parametric Kruskal-Wallis test was used because of the small sample size and lack of normality in the distribution. The p values were adjusted using the Holm’s procedure to conserve the overall type I error rate at 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported partly by the National Institute of Health NCI Grant #84356 to P.T.P.K and OSU Peptide Research Fund
Glossary
Abbreviations:
- HER-2
human epidermal growth factor receptor 2
- hmAb
humanized monoclonal antibodies
- PyMT
polyma middle T oncoprotein
- VEGF
vascular endothelium growth factor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21057
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/S1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 3.Soffer SZ, Moore JT, Kim E, Huang J, Yokoi A, Manley C, et al. Combination antiangiogenic therapy: increased efficacy in a murine model of Wilms tumor. J Pediatr Surg. 2001;36:1177–81. doi: 10.1053/jpsu.2001.25747. [DOI] [PubMed] [Google Scholar]
- 4.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–80. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 5.Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;5:953–62. [PubMed] [Google Scholar]
- 6.Li B, Ogasawara AK, Yang R, Wei W, He GW, Zioncheck TF, et al. KDR (VEGF receptor 2) is the major mediator for the hypotensive effect of VEGF. Hypertension. 2002;39:1095–100. doi: 10.1161/01.HYP.0000018588.56950.7A. [DOI] [PubMed] [Google Scholar]
- 7.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006;42:3127–39. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Kaumaya PT, Foy KC, Garrett J, Rawale SV, Vicari D, Thurmond JM, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J Clin Oncol. 2009;27:5270–7. doi: 10.1200/JCO.2009.22.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vicari D, Foy KC, Liotta EM, Kaumaya PT. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J Biol Chem. 2011;286:13612–25. doi: 10.1074/jbc.M110.216812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foy KC, Liu Z, Phillips G, Miller M, Kaumaya PT. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J Biol Chem. 2011;286:13626–37. doi: 10.1074/jbc.M110.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy K, Miller M, Moldovan N, Carson WE, Kaumaya PTP. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. OncoImmunology. 2012;1 doi: 10.4161/onci.20708. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O’Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86. [PubMed] [Google Scholar]
- 13.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 14.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue D, Togami K, Shimoike N, Tamura R, Imai Y, Kimura T, et al. [Early diagnosis and successful treatment of catastrophic antiphospholipid syndrome complicated by multiple organ failure] Nihon Rinsho Meneki Gakkai Kaishi. 2010;33:24–30. doi: 10.2177/jsci.33.24. [DOI] [PubMed] [Google Scholar]
- 16.Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov. 2006;5:649–59. doi: 10.1038/nrd2089. [DOI] [PubMed] [Google Scholar]
- 17.Goodman M, Ro S, Yamazaki T, Spencer JR, Toy A, Huang Z, et al. Topochemical design of bioactive peptides and peptidomimetics. Bioorg Khim. 1992;18:1375–93. [PubMed] [Google Scholar]
- 18.Chorev M. The partial retro-inverso modification: a road traveled together. Biopolymers. 2005;80:67–84. doi: 10.1002/bip.20219. [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan M, Gienapp IE, Stuckman SS, Rogers CJ, Jewell SD, Kaumaya PT, et al. Suppression of experimental autoimmune encephalomyelitis using peptide mimics of CD28. J Immunol. 2002;169:2180–8. doi: 10.4049/jimmunol.169.4.2180. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, He B, Wang YJ, Fu Q. [Effect of herceptin combined with Doxorubicin on rat cardiotoxicity] Ai Zheng. 2004;23:367–71. [PubMed] [Google Scholar]
- 21.Rayson D, Richel D, Chia S, Jackisch C, van der Vegt S, Suter T. Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: current experience and future strategies. Ann Oncol. 2008;19:1530–9. doi: 10.1093/annonc/mdn292. [DOI] [PubMed] [Google Scholar]
- 22.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–42. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 24.Fox WD, Higgins B, Maiese KM, Drobnjak M, Cordon-Cardo C, Scher HI, et al. Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer. Clin Cancer Res. 2002;8:3226–31. [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Vermorken JB. The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs. 1998;55:5–30. doi: 10.2165/00003495-199855010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–72. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepard HM, Lewis GD, Sarup JC, Fendly BM, Maneval D, Mordenti J, et al. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol. 1991;11:117–27. doi: 10.1007/BF00918679. [DOI] [PubMed] [Google Scholar]
- 28.Ryan AJ, Wedge SR. ZD6474--a novel inhibitor of VEGFR and EGFR tyrosine kinase activity. Br J Cancer. 2005;92(Suppl 1):S6–13. doi: 10.1038/sj.bjc.6602603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grothey A. Recognizing and managing toxicities of molecular targeted therapies for colorectal cancer. Oncology. 2006;20(Suppl 10):21–8. [PubMed] [Google Scholar]
- 30.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor EM, Otero DA, Banks WA, O’Brien JS. Retro-inverso prosaptide peptides retain bioactivity, are stable In vivo, and are blood-brain barrier permeable. J Pharmacol Exp Ther. 2000;295:190–4. [PubMed] [Google Scholar]
- 32.Fischer PM. The design, synthesis and application of stereochemical and directional peptide isomers: a critical review. Curr Protein Pept Sci. 2003;4:339–56. doi: 10.2174/1389203033487054. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher MD, Campbell MM. Partially Modified Retro-Inverso Peptides: Development, Synthesis, and Conformational Behavior. Chem Rev. 1998;98:763–96. doi: 10.1021/cr970468t. [DOI] [PubMed] [Google Scholar]
- 34.Allen SD, Rawale SV, Whitacre CC, Kaumaya PT. Therapeutic peptidomimetic strategies for autoimmune diseases: costimulation blockade. J Pept Res. 2005;65:591–604. doi: 10.1111/j.1399-3011.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 35.Damyanov C, Radoslavova M, Gavrilov V, Stoeva D. Low dose chemotherapy in combination with insulin for the treatment of advanced metastatic tumors. Preliminary experience. J BUON. 2009;14:711–5. [PubMed] [Google Scholar]
- 36.Nagy B, Molnár J, Rovó L, Paczona R, Thurzó L. [Radiotherapy in combination with low-dose chemotherapy in locally advanced head and neck cancer] Magy Onkol. 2004;48:145–9. [PubMed] [Google Scholar]
- 37.Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/ metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol. 2002;13:12–5. doi: 10.1093/annonc/mdf093. [DOI] [PubMed] [Google Scholar]
- 38.Allen SD, Garrett JT, Rawale SV, Jones AL, Phillips G, Forni G, et al. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol. 2007;179:472–82. doi: 10.4049/jimmunol.179.1.472. [DOI] [PubMed] [Google Scholar]
- 39.Sundaram R, Lynch MP, Rawale SV, Sun Y, Kazanji M, Kaumaya PT. De novo design of peptide immunogens that mimic the coiled coil region of human T-cell leukemia virus type-1 glycoprotein 21 transmembrane subunit for induction of native protein reactive neutralizing antibodies. J Biol Chem. 2004;279:24141–51. doi: 10.1074/jbc.M313210200. [DOI] [PubMed] [Google Scholar]
- 40.Söll R, Beck-Sickinger AG. On the synthesis of orexin A: a novel one-step procedure to obtain peptides with two intramolecular disulphide bonds. J Pept Sci. 2000;6:387–97. doi: 10.1002/1099-1387(200008)6:8<387::AID-PSC267>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Dakappagari NK, Lute KD, Rawale S, Steele JT, Allen SD, Phillips G, et al. Conformational HER-2/neu B-cell epitope peptide vaccine designed to incorporate two native disulfide bonds enhances tumor cell binding and antitumor activities. J Biol Chem. 2005;280:54–63. doi: 10.1074/jbc.M411020200. [DOI] [PubMed] [Google Scholar]
- 42.Chiesa-Vottero AG, Malpica A, Deavers MT, Broaddus R, Nuovo GJ, Silva EG. Immunohistochemical overexpression of p16 and p53 in uterine serous carcinoma and ovarian high-grade serous carcinoma. Int J Gynecol Pathol. 2007;26:328–33. doi: 10.1097/01.pgp.0000235065.31301.3e. [DOI] [PubMed] [Google Scholar]
- 43.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. doi: 10.2307/2531734. [DOI] [PubMed] [Google Scholar]