Abstract
In oncology, inflammation is generally regarded as a cancer-promoting process only. Here, we argue that this view may represent a misleading oversimplification. We present evidence from our own work and from the literature documenting cancer-suppressive aspects of inflammation. We propose that specific types of inflammation, in particular inflammation driven by tumor-specific Th1 cells, may repress rather than promote cancer. Th1 cells collaborate with tumor-infiltrating M1 macrophages to efficiently recognize and eliminate malignant cells. In a Th1 environment, pro-inflammatory cytokines such as interleukin (IL)-1α, IL-1β, IL-6 and tumor-necrosis factor α (TNFα) enhance anti-cancer immunity. Inducing Th1-type inflammation may significantly improve immunotherapeutic strategies against cancer.
Keywords: cancer, inflammation, M1 macrophages, proinflammatory cytokines, Th1 cells
Introduction
Inflammation is an immunological process which is essential for the elimination of invading pathogens as well as for wound healing. Inflammation may be considered to be the normal response of the body to harmful stimuli. At cellular and molecular levels, characteristic features of inflammation include increased movement of plasma and leukocytes from the blood into the injured tissue, and production of pro-inflammatory cytokines such as interleukin (IL)-1α, IL-1β, IL-6 and tumor-necrosis factor α (TNFα).
A number of studies have demonstrated a link between inflammation and cancer development (reviewed in refs. 1–4). For instance, high IL-6 serum levels have been associated with shorter survival in patients affected by multiple myeloma, lymphoma and lung cancer.5-7 Chronic inflammatory diseases like ulcerative colitis, gastritis and rheumatoid arthritis predispose to colorectal cancer, gastric cancer and lymphoma, respectively.8-10 Furthermore, circumstantial evidence suggests that individuals taking non-steroidal anti-inflammatory drugs such as aspirin may have a reduced risk for cancer.11-13 Based on these observations, inflammation is widely considered to be detrimental in terms of cancer occurrence, growth and metastasis. Accordingly, dampening inflammation has been suggested as a novel strategy to fight cancer.1-4
Here, we argue that the general view that inflammation promotes cancer may represent a misleading oversimplification. We review published evidence in support of cancer-suppressive properties of inflammatory cells and of the canonical pro-inflammatory cytokines IL-1α, IL-1β, IL-6 and TNFα. This leads us to propose a model in which certain types of inflammation, in particular inflammation driven by tumor-specific Th1 cells, may repress rather than promote cancer.
Th1-Driven Inflammation Protects Mice against B-Cell Cancer
We investigated the mechanisms of cancer prevention mediated by tumor-specific CD4+ T cells in mouse models for myeloma and B-cell lymphoma. We used T-cell receptor (TCR) transgenic mice in which all CD4+ T cells recognize a tumor-specific, immunoglobulin-derived antigen that is secreted by the MOPC315 myeloma as well as by the F9 B-cell lymphoma.14,15 Tumor-specific TCR-transgenic mice are resistant against subcutaneous challenge with MOPC315 or F9 cells. Protection was shown to be antigen-specific, CD4+ T-cell mediated and independent of B cells and CD8+ T cells.14,15 Successful cancer elimination required antigen secretion by malignant cells, and migration of effector CD4+ T cells from the draining lymph node to the incipient tumor site.16-18 To study the mechanisms of cancer rejection by TCR-transgenic mice, we developed a strategy consisting of embedding injected tumor cells in a collagen gel. This method allowed us to analyze infiltrating immune cells and to quantify the cytokines that were secreted locally during a primary antitumor immune response.16,19 Using this technique, we uncovered a common core of nine cytokines that were consistently present at higher levels at the incipient tumor site during successful cancer immunosurveillance (Fig. 1). Strikingly, this core included both pro-inflammatory (IL-1α, IL-1β and IL-6) and Th1-associated cytokines such as IL-12 and interferon γ (IFNγ). Cancer eradication was achieved by a collaboration between tumor-specific Th1 cells and tumor-infiltrating, antigen-presenting M1 macrophages (i.e., IFNγ-activated macrophages).20,21 Th1 cells induced secretion of IL-1β and IL-6 by inflammatory M1 macrophages. Th1-derived IFNγ was shown to render macrophages directly cytotoxic against cancer cells. Therefore, we concluded that successful immunosurveillance of B-cell cancer in mice consists of an inflammatory reaction driven by tumor-specific Th1 cells19 (Fig. 1).

Figure 1. Successful immunosurveillance of B-cell cancer in mice consists of an inflammatory reaction driven by tumor-specific Th1 cells. Eradication of myeloma and lymphoma in mice is achieved through a collaboration between tumor-specific Th1 cells and tumor-infiltrating, antigen-presenting M1 macrophages. During this process, nine cytokines are secreted locally by immune cells, including pro-inflammatory (IL-1α, IL-1β and IL-6) and Th1-associated cytokines (IL-12 and IFNγ). Th1 cells induce secretion of IL-1α, IL-1β, IL-6, CXCL-9 and CXCL-10 by inflammatory M1 macrophages. Th1-derived IFNγ renders macrophages directly cytotoxic to cancer cells. (Figure modified from ref. 19).
A High Density of Immune Cells in Human Primary Tumors Predicts Longer Patient Survival
Infiltration of a tissue by blood-derived leukocytes such as T cells and macrophages, the so-called inflammatory infiltrate, is a typical histological feature of inflammation. It is well known that solid tumors are infiltrated by various numbers of immune cells, which is consistent with an inflammatory reaction taking place within and around tumors. For many types of human cancer, an association has been demonstrated between dense leukocyte infiltrates in primary tumors and longer patient survival. Infiltration of the primary tumor by undefined inflammatory cells predicted longer survival of patients with skin, colorectal, bladder and breast cancer.22-25 Elevated numbers of intratumoral T cells were shown to correlate with longer survival in patients with ovarian, esophageal, lung, colorectal and breast cancer.26-30 The presence of both CD8+ and CD4+ T cells positively influenced survival, suggesting that both cell types cooperate to exert anticancer effects.27-30 High numbers of CD4+ T cells in lymph nodes predicted disease-free survival in breast cancer.31 Among CD4+ T cell subsets, Th1 cells seem to be particularly beneficial, as reported for colorectal, liver and breast cancer.29,32,33 Furthermore, a high density of tumor-infiltrating macrophages was associated with prolonged survival in prostate, colorectal and lung cancer.23,34-36 Thus, for various types of human cancer, stronger inflammation in the primary tumor, as defined by a high density of infiltrating T cells and macrophages, represents an independent predictor for longer patient survival.
Cancer-Suppressive Functions of IL-1α and IL-1β
IL-1α and IL-1β are two canonical pro-inflammatory cytokines that are typically secreted by activated macrophages. IL-1α and IL-1β have a number of important immunological functions, which are likely to contribute to successful antitumor immunity (Fig. 2). In line with their original name, namely lymphocyte activating factor, both IL-1α and IL-1β are potent stimulators for all leukocytes. For instance, IL-1α and IL-1β synergize with IL-2 to stimulate the proliferation of CD4+ T cells, CD8+ T cells and natural killer (NK) cells.37 Both IL-1α and IL-1β enhance the expansion and differentiation of CD4+ T cells.38,39 IL-1 also stimulates B-cell proliferation, generation of plasma cells and antibody production.40 Furthermore, IL-1α and IL-1β increase the expression of adhesion molecules on vascular endothelium, which may promote extravasation, i.e., the migration of leukocytes from the bloodstream into the inflamed tissue.41

Figure 2. Cancer-suppressive properties of IL-1α and IL-1β. IL-1α and IL-1β synergize with IL-2 to stimulate the proliferation of CD4+ T cells, CD8+ T cells and NK cells; enhance the expansion and differentiation of CD4+ T cells; stimulate B-cell proliferation, generation of plasma cells and antibody production; induce the expression of adhesion molecules on vascular endothelium, which promotes extravasation of leukocytes into the inflamed tissue; directly inhibit the proliferation of specific cancer cells; render human macrophages cytotoxic to some cancer cells; and they amplify antigen-presenting functions and cytokine production by dendritic cells. Ab, antibody; APC, antigen-presenting cell; B, B cell; CD4, CD4+ T cell; CD8, CD8+ T cell; DC, dendritic cell; EC, endothelial cell; MΦ, macrophage; NK, natural killer cell.
Although tumor-promoting effects of IL-1α and IL-1β are well documented, in particular during cancer metastasis and tumor angiogenesis,42,43 a number of studies provide experimental support for cancer-suppressive functions of these two cytokines (Fig. 2). IL-1 was shown to inhibit the proliferation of human A375 melanoma and mouse L929 fibroblast cell lines in vitro.44 IL-1 rendered human monocytes cytotoxic against a murine fibrosarcoma cell line.45 Intratumoral injection of IL-1α successfully cured mice from MethA sarcoma and B16 melanoma.46 IL-1α injected intramuscularly was effective in reducing the number of lung metastases in mice with Lewis lung carcinoma.46 Intraperitoneal injection of IL-1β caused complete regression of subcutaneous SA1 sarcoma and L5178Y lymphoma in mice.47 Because IL-1β lost its therapeutic effects in T-cell deficient mice, it was concluded that IL-1β functions by stimulating T-cell mediated antitumor immunity.47 Activated invasive RO1 T-lymphoma cells that displayed short-term IL-1α expression manifested reduced tumorigenicity and could be used to treat mice with lymphoma.48 Similarly, fibrosarcoma cells transfected with IL-1α became strongly immunogenic and failed to generate tumors in mice.49 Notably, IL-1β secretion is dependent on activation of the NLRP3 inflammasome, a cytosolic molecular complex responsible for generating active IL-1β by cleaving the inactive precursor. The NLRP3 inflammasome has been demonstrated to protect mice against colitis-associated cancer.50 Furthermore, in mouse models for cancer chemotherapy, activation of the NLRP3 inflammasome in dendritic cells appears to be essential for the induction of IL-1β-dependent adaptive antitumor immunity.51 In line with these experimental findings, a genetic analysis of multiple myeloma patients revealed that individuals with a polymorphism in the promoter region of the IL1B gene, leading to reduced IL-1β production, had significantly shorter survival after chemotherapy.52 Thus, antitumor effects for both IL-1α and IL-1β have been demonstrated in various mouse models, and several studies suggest that IL-1α and IL-1β can significantly enhance T-cell mediated antitumor immunity.
IL-1α and IL-1β are Natural Adjuvants for Antitumor Immunity
Although structurally quite different, mature IL-1α and IL-1β mediate their functions by binding to the same IL-1 receptor I (IL-1RI) which is present on the surface of most cells. Importantly, IL-1RI share with Toll-like receptors (TLRs) a common intracellular signaling pathway, which leads to activation of the nuclear factor κB (NFκB) and hence to the transcription of several pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-6 and TNFα (Fig. 3). Therefore, IL-1α and IL-1β function within positive feedback loops during inflammation. Because of the common intracellular signaling pathway, IL-1α and IL-1β operate as natural adjuvants, thus mimicking the detection of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) by TLRs (Fig. 3). Early work by Ralph Steinman and coworkers revealed that IL-1α can amplify the function of dendritic cells and thereby enhance T-cell dependent immunity.53 It was also shown that IL-1α can be used as an adjuvant to trigger the clonal expansion and differentiation of antigen-activated Th cells, as well as Th-mediated antibody production.54 Several studies investigated the potential of IL-1α and IL-1β as adjuvants for cancer vaccines. In a mouse model of lung cancer, a vaccine was made by combining irradiated cancer cells with IL-1α or IL-1β.55 IL-1β was successfully used as an adjuvant, together with sonicated cancer cells, to induce tumor-specific immunity against MOPC104E plasmacytoma and MethA sarcoma in mice.56 Finally, an IL-1RI-binding peptide derived from IL-1β was shown to augment antitumor immune responses induced by protein and DNA vaccines against 38C13 mouse B-cell lymphoma.57 Altogether, these data suggest that IL-1α and IL-1β, as whole proteins or biologically active fragments, may represent potent adjuvants for cancer vaccines.
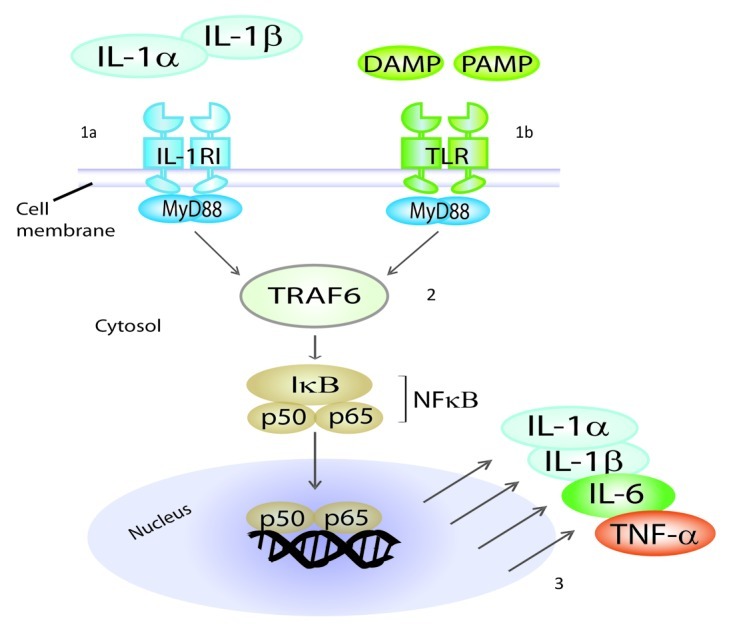
Figure 3. IL-1RI and TLRs share a common intracellular signaling pathway. Mature IL-1α and IL-1β mediate their functions by binding to the same IL-1 receptor I (IL-1RI) which is present on the surface of most cells (1a). Pathogen-associated molecular patterns (PAMP) and damage/danger-associated molecular patterns (DAMP) bind to Toll-like receptors (TLRs) (1b). IL-1RI and TLRs share a common intracellular signaling pathway (2), which leads to activation of NFκB and hence to the production of pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-6 and TNFα (3). Therefore, IL-1α and IL-1β function within positive feedback loops in inflammation. Because of this common signaling pathway, IL-1α and IL-1β operate as natural adjuvants, mimicking the detection of microbial products by TLRs. MyD88, Myeloid Differentiation primary response gene 88; TRAF6, TNF receptor associated factor 6.
Cancer-Suppressive Functions of IL-6
The pro-inflammatory cytokine IL-6 exerts multiple functions, including the stimulation of B and T cells as well as the production of acute-phase proteins by hepatocytes. IL-6 plays an essential role in antibacterial and antiviral immunity.58 IL-6 is a B-cell growth factor, which stimulates proliferation of normal and malignant B cells. In several types of human cancer, such as multiple myeloma, B-cell lymphoma and lung cancer, high IL-6 serum levels have been associated with short patient survival, supporting cancer-promoting effects for IL-6.5-7 However, a number of studies documented cancer-suppressive properties of IL-6 (Fig. 4). Treatment of mice with IL-6 induced the regression of established micrometastases in the liver and lungs of sarcoma and colon adenocarcinoma,59 in a process that required both CD4+ and CD8+ T cells.60 In mice inoculated with acute myeloid leukemia cells, IL-6 injections inhibited tumor development and increased survival.61 Murine B16 melanoma cells transfected with IL-6 became less tumorigenic, and mice with established melanoma were successfully treated with recombinant IL-6.62 Combined treatment with IL-6 and cyclophosphamide efficiently cured mice bearing advanced pulmonary metastases from fibrosarcoma.60 Immunization of mice with IL-6-transfected Lewis lung carcinoma cells induced high levels of tumor-specific cytotoxic T cells and functioned as an efficient prophylactic and therapeutic cancer vaccine.63 Fibrosarcoma cells transduced with IL-6 exhibited reduced tumorigenicity, increased immunogenicity and decreased metastatic potential.64 Similar findings were obtained in rats, in which IL-6-transduced glioma cells showed attenuated tumorigenicity and functioned as an efficient vaccine against intracranial glioma.65 In mice bearing B16F10 melanoma treated with the synthetic triacylated lipopeptide Pam3CSK4 (a TLR2 agonist), mast cell-derived IL-6 was essential for the inhibition of tumor growth.66 Endogenous IL-6 reportedly increases CD8+ T cell trafficking to the tumors in mice with B16 melanoma treated with systemic thermal therapy combined with adoptive T cell transfer.67 In humans, IL-6 has been shown to suppress the proliferation of acute myeloid leukemia and B-chronic lymphocytic leukemia cells, revealing an inhibitory effect of IL-6 on some human B-cell malignancies.61,68 Moreover, in vitro studies on human melanoma demonstrated that exposure to IL-6 inhibits the growth of early-stage but not advanced stage melanoma cells.69 Thus, IL-6 has been shown to inhibit in vitro proliferation of several types of human cancer cells, and cancer-suppressive effects of IL-6 have been demonstrated in vivo, in several animal models.

Figure 4. Cancer-suppressive properties of IL-6. IL-6 stimulates B-cell proliferation, differentiation as well as antibody production and it influences macrophage differentiation and activation. Treatment of mice with IL-6 may induce regression of established micrometastases in liver and lungs in a process which requires both CD4+ and CD8+ T cells. Immunization of mice with IL-6-transfected carcinoma cells may generate high levels of tumor-specific CD8+ T cells and functions as an efficient prophylactic and therapeutic cancer vaccine. Fibrosarcoma cells transduced with IL-6 exhibit reduced tumorigenicity, increased immunogenicity and decreased metastatic potential. IL-6 may increase CD8+ T cell trafficking to tumors by affecting endothelial cells and it inhibits proliferation of human acute myeloid leukemia, B-chronic lymphocytic leukemia and early-stage melanoma cells.
Cancer-Suppressive Functions of TNFα
TNFα is a pleiotropic pro-inflammatory cytokine typically produced by activated macrophages. The effects of TNFα are mediated by two distinct receptors: TNFα receptor 1 (TNFR1) and TNFR2. TNFR1 is present on most cell types and accounts for the majority of pro-inflammatory (through induction of NFκB) and apoptotic effects of TNFα.70 TNFR2 is present on the surface of lymphocytes and promotes activation and proliferation. TNFα has an impact on all aspects of T-cell biology including T-cell proliferation, co-stimulation and survival (reviewed in ref. 71). A number of studies documented tumor-promoting effects of TNFα (reviewed in ref. 72). For example, TNFα-deficient mice were less susceptible to carcinogen-induced papilloma, a benign epithelial tumor, suggesting that TNFα may promote skin cancer development.73 In chronic lymphocytic leukemia, patients with high plasma levels of TNFα had a significantly shorter survival rate.74 However, a large body of literature has revealed the cancer-suppressive properties of TNFα (Fig. 5). TNFα was first identified as an endotoxin-induced serum factor produced by macrophages that caused hemorrhagic necrosis of MethA sarcoma and other solid tumors.75 The hemorrhagic reaction apparently resulted from the destruction of tumor blood vessels.76 Experiments with T-cell deficient mice revealed that TNFα does not solely damage tumor blood vessels, but it also stimulates tumor-specific T cells.76 Furthermore, in vitro studies demonstrated that TNFα is directly cytotoxic for various human cancer cell lines such as melanoma, breast, cervix and colon carcinoma.77,78 TNFα rendered human monocytes cytotoxic against a murine fibrosarcoma cell line in vitro.45 Synergistic in vivo antitumor effects against the murine MCA-106 sarcoma were reported for the combined administration of TNFα and IL-2.79 J558L myeloma cells transfected with TNFα were efficiently eliminated in mice, and it was proposed that TNFα would function by recruiting and activating macrophages.80 In a follow-up study, rejection of TNFα-transfected J558L myeloma cells was shown to be partly dependent on CD8+ T cells.81 In a mouse model for T-cell lymphoma, an opposing effect of TNFα on tumor growth was reported: TNFα induced the rejection of solid tumors but promoted the formation of hepatic metastases.82 TNFα was essential for recruitment of NK cells to the peritoneum to eliminate MHC Class I-negative RMA-S lymphoma cells.83 TNFα-deficient mice were more susceptible than wild-type mice to sarcoma induction with the carcinogen methylcholanthrene.84 TNFα was critical for the prevention of pancreatic tumors in a mouse model of cancer immunosurveillance mediated by tumor-specific CD8+ T cells.85 In this model, TNFα was important for the priming, proliferation and recruitment of tumor-specific CD8+ T cells.85 Importantly, this study identified the critical roles of TNFR1 on antigen-presenting cells and TNFR2 on T cells for successful antitumor immunity.85

Figure 5. Cancer-suppressive properties of TNFα. TNFα stimulates T-cell activation, proliferation and recruitment; causes hemorrhagic necrosis of solid tumors through destruction of tumor blood vessels; is directly cytotoxic to several human cancer cell lines; and it renders human macrophages cytotoxic to some cancer cells. TNFα-transfected myeloma cells are eliminated in mice through recruitment and activation of macrophages. TNFα mediates recruitment of NK cells to the peritoneum to eliminate MHC Class I-negative cancer cells; is important for priming, proliferation and recruitment of tumor-specific CD8+ T cells; stimulates antigen-presenting cell functions and cytokine production; and it increases vascular permeability leading to improved penetration of chemotherapeutics in the tumor tissue.
Using TNFα for Cancer Immunotherapy in Humans
On the basis of the experimental evidence described above, TNFα is currently being used for human cancer immunotherapy (reviewed in ref. 86). A main limitation for clinical application of TNFα has been the toxicity that is associated with systemic administration. To circumvent this problem, isolated limb perfusion was performed and successful treatment of advanced melanoma and soft tissue sarcoma was achieved.87,88 During isolated limb perfusion, TNFα has a double effect on the tumor-associated vasculature: (1) TNFα increases vascular permeability leading to improved penetration of chemotherapy within the tumor tissue, and (2) TNFα selectively kills angiogenic endothelial cells resulting in tumor vessel destruction.86 Several strategies are being developed to allow for the systemic administration of TNFα while limiting toxicity to the patient, in particular by that targeted delivery of TNFα to tumors.89-91
Pro-Inflammatory Cytokines Synergize With Other Cytokines to Fight Cancer
The biological effects of a given cytokine are strongly dependent on the cytokine milieu. In cancer immunology, it is well documented that pro-inflammatory cytokines may synergize with other cytokines against neoplastic cells. For example, TNFα and IFNγ acted in synergy to block the in vitro proliferation of murine Lewis lung carcinoma and B16 melanoma cells.92 Similarly, treatment of mice bearing B16 melanoma with both TNFα and IFNγ was more efficient than the administration of either cytokine alone.92 Combined treatment with IL-1β and IL-2 generated a synergistic T-cell dependent antitumor response against highly metastatic Friend leukemia in mice.93 Mice with established subcutaneous MCA-106 sarcoma tumors were cured by the combinatorial treatment with TNFα and IL-6, revealing a synergistic antitumor effect of these two pro-inflammatory cytokines.59 In vitro studies demonstrated that a combination of IFNγ and IL-1β induced necrosis of murine L929 fibrosarcoma cells.94 Combined treatment of human papillary thyroid carcinoma cell lines with IL-1β and IFNγ efficiently inhibited proliferation and invasiveness.95 TNFα and IFNγ synergized to mediate in vitro cytotoxicity against a large number of human cell lines including breast, cervix and colon carcinoma cells.78 Finally, TNFα and IFNγ were shown to synergize to induce the tumoricidal activity of murine macrophages.96 Thus, although proinflammatory cytokines may be directly cytotoxic to cancer cells, synergistic interactions with other cytokines are likely to be very important for successful antitumor immunity. Importantly, the combination of pro-inflammatory cytokines with Th1 cytokines like IL-2 and IFNγ seems to be particularly beneficial for cancer therapy.
A Model for How Inflammation May Either Suppress or Promote Cancer
From the literature overviewed above, it is apparent that inflammatory cells and pro-inflammatory cytokines not only can promote but also may suppress cancer in various settings. Therefore, cancer-related inflammation cannot be generalized as solely detrimental or beneficial. To reconcile conflicting data, we propose that, among all the existing types of inflammation, certain types are cancer-suppressive while others may be cancer-promoting. Based on our own findings and evidence from the literature, we suggest that inflammation driven by tumor-specific Th1 cells may be particularly beneficial against cancer (Fig. 6, left). During Th1-driven cancer-suppressive inflammation, tumor-specific Th1 cells collaborate with tumor-infiltrating M1 macrophages to efficiently recognize and eliminate malignant cells. M1 macrophages function as efficient antigen-presenting cells that process and present cancer antigens to tumor-specific CD4+ and CD8+ T cells in the tumor. Th1 cells are characterized by the production of IFNγ97 and experiments with gene-targeted mice demonstrated that IFNγ is critical for cancer immunosurveillance.19,98,99 IFNγ is a potent macrophage-activating factor, stimulating their tumoricidal activity and the acquisition of a M1 phenotype.100-102 M1 macrophages are cancer-suppressive in vitro100-102 and in vivo,16,19 and produce pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNFα), the Th1-polarizing cytokine IL-12, as well as IFNγ-induced chemokines (CXCL9/MIG, CXCL10/IP-10 and CXCL11/I-TAC).21 In a Th1 environment, pro-inflammatory cytokines may play essential roles in cancer elimination, e.g., by recruiting T cells and macrophages from the circulation and by stimulating leukocyte tumoricidal functions (Fig. 6, left). Pro-inflammatory cytokines synergize with Th1-derived cytokines (IL-2 and IFNγ) to fight cancer.78,92-96 CXCL9, CXCL10 and CXCL11 may participate in antitumor immunity by blocking tumor angiogenesis19,103-105 and by recruiting Th1 cells, CD8+ T cells and NK cells to the tumor site.106,107 In contrast to Th1-driven cancer-suppressive inflammation, other types of inflammation may instead promote cancer development (reviewed in refs. 1–4). In the absence of sufficient numbers of tumor-specific Th1 cells, pro-inflammatory cytokines (Il-1α, IL-1β, IL-6, TNFα) may participate in cancer development, progression and metastasis, for instance by stimulating angiogenesis and cancer cell growth, and by increasing vascular permeability1-4 (Fig. 6, right).
Figure 6. A model for how inflammation may either suppress or promote cancer. (Left) Cancer-suppressive inflammation driven by tumor-specific Th1 cells. Tumor-specific Th1 cells collaborate with tumor-infiltrating M1 macrophages to efficiently recognize and eliminate malignant cells. M1 macrophages function as efficient antigen-presenting cells that process and present cancer antigens to tumor-specific CD4+ and CD8+ T cells in the tumor. Th1 cells are characterized by the production of IFNγ, which is a potent macrophage-activating factor, inducing their tumoricidal activity. IFNγ-induced M1 macrophages are cancer-suppressive in vitro and in vivo, and produce pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNFα) as well as the Th1-polarizing cytokine IL-12. In a Th1 environment, pro-inflammatory cytokines play essential roles in cancer elimination by stimulating various aspects of the antitumor immune response. (Right) Cancer-promoting inflammation. In the absence of sufficient numbers of tumor-specific Th1 cells, tumor-infiltrating macrophages do not differentiate into a cancer-suppressive M1 phenotype. In this setting, pro-inflammatory cytokines may contribute to cancer development, progression and metastasis, for instance by stimulating angiogenesis and cancer cell growth and/or by increasing vascular permeability.
Inducing Inflammation to Treat Cancer
On the basis of the model described above, we propose that immunotherapy protocols that induce or promote an inflammatory reaction driven by tumor-specific Th1 cells may be particularly efficient at eradicating cancer. PAMPs tend to induce Th1-type inflammatory immune responses upon binding to receptors such as TLRs on immune cells. Several reports document successful cancer immunotherapy protocols based on inducing inflammation with PAMPs. Early studies in mice revealed that treatment with double-stranded RNA or lipopolysaccharide induced lymphocyte-mediated rejection of L5178Y lymphoma and FS6 fibrosarcoma.108 More recently, mice with C26 colon carcinoma and B16F0 melanoma have been successfully treated with a combination of anti-IL-10-receptor antibodies and the intratumoral injection with CpG, a TLR-9 ligand.109 This treatment has been show to stimulate the production of IL-12 by tumor-infiltrating dendritic cells, thereby enhancing T-cell mediated antitumor immunity.109 In humans, the intravesical application of the bacterium Mycobacterium bovis Bacillus Calmette-Guerin (BCG) is an efficient and widely-used treatment for superficial bladder cancer (reviewed in ref. 110). Moreover, imiquimod, an imidazoquinoline compound which activates immune cells via TLR7,111 is an effective treatment for several human malignancies such as superficial basal cell carcinoma, actinic keratinosis and vulvar intraepithelial neoplasia.112-114 Collectively, these data suggest that inducing Th1-type inflammation may represent an efficient strategy to achieve successful cancer immunotherapy.
Concluding Remarks
Treating cancer with anti-inflammatory drugs
This new therapeutic approach is based on the well-documented cancer-promoting aspects of inflammation.1-4 The model that we present here suggests that caution should be taken. Anti-inflammatory drugs that dampen ongoing cancer-suppressive Th1 inflammation in patients may potentially exacerbate, rather than cure, established malignancies.
Anti-inflammatory drugs and cancer risk
Novel anti-inflammatory drugs that specifically block key inflammatory molecules, e.g., pro-inflammatory cytokines, have been developed. These drugs have revolutionized the treatment of several chronic inflammatory diseases such as rheumatoid arthritis. Moreover, evidence suggests that some non-steroidal anti-inflammatory drugs, e.g., aspirin, may lead to a reduced risk for cancer.11-13 However, anti-inflammatory drugs may potentially weaken cancer immunosurveillance. Therefore, it is advisable that patients receiving long-term treatment with novel anti-inflammatory drugs should be followed up for cancer development.
Acute vs. chronic inflammation
It has been suggested that acute inflammation protects against cancer, whereas chronic inflammation promotes cancer. This hypothesis is based on the observation that acute inflammation may cure cancer,108-110,112-114 whereas several chronic inflammatory diseases predispose to cancer.8-10 However, there are indications that chronic inflammation may not always be detrimental. For several types of human cancer, a strong inflammatory response in the primary tumor, as defined by a high density of infiltrating T cells and macrophages, has been shown to represent an independent predictor for longer patient survival.22-30,32,34-36 Because a detectable tumor takes a long time to develop, tumor-associated inflammation, unless treatment-induced, should arguably be defined as chronic inflammation. This implies that a strong chronic inflammation in tumors may repress rather that promote cancer, therefore representing an example of beneficial chronic inflammation against cancer.
Synergy between chemotherapy and immunotherapy
Although chemotherapy is generally considered immunosuppressive, several reports revealed that it may improve cancer immunotherapy. For instance, treatment of mice with AB1 mesothelioma with the cytidine analog gemcitabine significantly enhanced immunotherapy with a viral cancer vaccine or with anti-CD40 monoclonal antibodies.115,116 In humans, chemotherapy of melanoma patients with the DNA alkylating agent dacarbazine was shown to improve the CD8+ T-cell response to a cancer vaccine.117 Mouse studies revealed that inflammatory mediators induced by chemotherapy may stimulate antitumor immunity.51,118 Thus, induction of a strong inflammation in tumors is likely to be a main component of the observed synergy between chemotherapy and immunotherapy.
Acknowledgments
We thank Kristina Berg Lorvik, Inger Øynebråten, Ranveig Braathen and Peter O. Hofgaard for critical reading of the manuscript. This work was supported by grants from Helse Sør-Øst, the Research Council of Norway, the Norwegian Cancer Society, Andrine og Hans Gysler Berg fund and S. G. Sønneland foundation.
Author contributions
A.C. wrote the manuscript. O.A.W.H. and B.B. contributed in writing the manuscript. O.A.W.H. prepared the figures.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21542
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelliniemi TT, Irjala K, Mattila K, Pulkki K, Rajamäki A, Tienhaara A, et al. Finnish Leukemia Group Immunoreactive interleukin-6 and acute phase proteins as prognostic factors in multiple myeloma. Blood. 1995;85:765–71. [PubMed] [Google Scholar]
- 6.Preti HA, Cabanillas F, Talpaz M, Tucker SL, Seymour JF, Kurzrock R. Prognostic value of serum interleukin-6 in diffuse large-cell lymphoma. Ann Intern Med. 1997;127:186–94. doi: 10.7326/0003-4819-127-3-199708010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Martín F, Santolaria F, Batista N, Milena A, González-Reimers E, Brito MJ, et al. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine. 1999;11:80–6. doi: 10.1006/cyto.1998.0398. [DOI] [PubMed] [Google Scholar]
- 8.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polk DB, Peek RM., Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–14. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekström K, Hjalgrim H, Brandt L, Baecklund E, Klareskog L, Ekbom A, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48:963–70. doi: 10.1002/art.10939. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–12. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 13.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–27. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 14.Lauritzsen GF, Weiss S, Dembic Z, Bogen B. Naive idiotype-specific CD4+ T cells and immunosurveillance of B-cell tumors. Proc Natl Acad Sci USA. 1994;91:5700–4. doi: 10.1073/pnas.91.12.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogen B, Munthe L, Sollien A, Hofgaard P, Omholt H, Dagnaes F, et al. Naive CD4+ T cells confer idiotype-specific tumor resistance in the absence of antibodies. Eur J Immunol. 1995;25:3079–86. doi: 10.1002/eji.1830251114. [DOI] [PubMed] [Google Scholar]
- 16.Corthay A, Skovseth DK, Lundin KU, Røsjø E, Omholt H, Hofgaard PO, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–83. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Corthay A, Lundin KU, Lorvik KB, Hofgaard PO, Bogen B. Secretion of tumor-specific antigen by myeloma cells is required for cancer immunosurveillance by CD4+ T cells. Cancer Res. 2009;69:5901–7. doi: 10.1158/0008-5472.CAN-08-4816. [DOI] [PubMed] [Google Scholar]
- 18.Lorvik KB, Bogen B, Corthay A. Fingolimod blocks immunosurveillance of myeloma and B-cell lymphoma resulting in cancer development in mice. Blood. 2012;119:2176–7. doi: 10.1182/blood-2011-10-388892. [DOI] [PubMed] [Google Scholar]
- 19.Haabeth OA, Lorvik KB, Hammarström C, Donaldson IM, Haraldsen G, Bogen B, et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. doi: 10.1038/ncomms1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 22.Clark WH, Jr., Elder DE, Guerry D, 4th, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 23.Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645–54. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Cai T, Nesi G, Boddi V, Mazzoli S, Dal Canto M, Bartoletti R. Prognostic role of the tumor-associated tissue inflammatory reaction in transitional bladder cell carcinoma. Oncol Rep. 2006;16:329–34. [PubMed] [Google Scholar]
- 25.Lee AH, Gillett CE, Ryder K, Fentiman IS, Miles DW, Millis RR. Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology. 2006;48:692–701. doi: 10.1111/j.1365-2559.2006.02410.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 27.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–9. [PubMed] [Google Scholar]
- 28.Yoshida N, Abe H, Ohkuri T, Wakita D, Sato M, Noguchi D, et al. Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens and T cell infiltration in non-small cell lung carcinoma and their prognostic significance. Int J Oncol. 2006;28:1089–98. [PubMed] [Google Scholar]
- 29.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 31.Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci USA. 2012;109:2802–7. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, Thompson TC. Reduced infiltration of tumor-associated macrophages in human prostate cancer: association with cancer progression. Cancer Res. 2000;60:5857–61. [PubMed] [Google Scholar]
- 35.Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23:8959–67. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 36.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–9. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 37.Ben Aribia MH, Leroy E, Lantz O, Métivier D, Autran B, Charpentier B, et al. rIL 2-induced proliferation of human circulating NK cells and T lymphocytes: synergistic effects of IL 1 and IL 2. J Immunol. 1987;139:443–51. [PubMed] [Google Scholar]
- 38.Von Stebut E, Ehrchen JM, Belkaid Y, Kostka SL, Molle K, Knop J, et al. Interleukin 1alpha promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med. 2003;198:191–9. doi: 10.1084/jem.20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106:7119–24. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipsky PE, Thompson PA, Rosenwasser LJ, Dinarello CA. The role of interleukin 1 in human B cell activation: inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocytic pyrogen. J Immunol. 1983;130:2708–14. [PubMed] [Google Scholar]
- 41.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–54. [PubMed] [Google Scholar]
- 42.Bani MR, Garofalo A, Scanziani E, Giavazzi R. Effect of interleukin-1-beta on metastasis formation in different tumor systems. J Natl Cancer Inst. 1991;83:119–23. doi: 10.1093/jnci/83.2.119. [DOI] [PubMed] [Google Scholar]
- 43.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onozaki K, Matsushima K, Aggarwal BB, Oppenheim JJ. Human interleukin 1 is a cytocidal factor for several tumor cell lines. J Immunol. 1985;135:3962–8. [PubMed] [Google Scholar]
- 45.Philip R, Epstein LB. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986;323:86–9. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura S, Nakata K, Kashimoto S, Yoshida H, Yamada M. Antitumor effect of recombinant human interleukin 1 alpha against murine syngeneic tumors. Jpn J Cancer Res. 1986;77:767–73. [PubMed] [Google Scholar]
- 47.North RJ, Neubauer RH, Huang JJ, Newton RC, Loveless SE. Interleukin 1-induced, T cell-mediated regression of immunogenic murine tumors. Requirement for an adequate level of already acquired host concomitant immunity. J Exp Med. 1988;168:2031–43. doi: 10.1084/jem.168.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voronov E, Weinstein Y, Benharroch D, Cagnano E, Ofir R, Dobkin M, et al. Antitumor and immunotherapeutic effects of activated invasive T lymphoma cells that display short-term interleukin 1alpha expression. Cancer Res. 1999;59:1029–35. [PubMed] [Google Scholar]
- 49.Song X, Voronov E, Dvorkin T, Fima E, Cagnano E, Benharroch D, et al. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol. 2003;171:6448–56. doi: 10.4049/jimmunol.171.12.6448. [DOI] [PubMed] [Google Scholar]
- 50.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–56. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 52.Vangsted AJ, Klausen TW, Abildgaard N, Andersen NF, Gimsing P, Gregersen H, et al. Single nucleotide polymorphisms in the promoter region of the IL1B gene influence outcome in multiple myeloma patients treated with high-dose chemotherapy independently of relapse treatment with thalidomide and bortezomib. Ann Hematol. 2011;90:1173–81. doi: 10.1007/s00277-011-1194-3. [DOI] [PubMed] [Google Scholar]
- 53.Koide SL, Inaba K, Steinman RM. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987;165:515–30. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–8. [PubMed] [Google Scholar]
- 55.McCune CS, Marquis DM. Interleukin 1 as an adjuvant for active specific immunotherapy in a murine tumor model. Cancer Res. 1990;50:1212–5. [PubMed] [Google Scholar]
- 56.Moriguchi Y, Kan N, Okino T, Harada T, Yamasaki S, Ichinose Y, et al. A new model of active specific immunotherapy using interleukin-1 and sonicated tumor supernatant in murine tumor system. J Surg Oncol. 1996;62:78–85. doi: 10.1002/(SICI)1096-9098(199606)62:2<78::AID-JSO2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 57.Hakim I, Levy S, Levy R. A nine-amino acid peptide from IL-1beta augments antitumor immune responses induced by protein and DNA vaccines. J Immunol. 1996;157:5503–11. [PubMed] [Google Scholar]
- 58.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 59.Mulé JJ, McIntosh JK, Jablons DM, Rosenberg SA. Antitumor activity of recombinant interleukin 6 in mice. J Exp Med. 1990;171:629–36. doi: 10.1084/jem.171.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mulé JJ, Custer MC, Travis WD, Rosenberg SA. Cellular mechanisms of the antitumor activity of recombinant IL-6 in mice. J Immunol. 1992;148:2622–9. [PubMed] [Google Scholar]
- 61.Givon T, Slavin S, Haran-Ghera N, Michalevicz R, Revel M. Antitumor effects of human recombinant interleukin-6 on acute myeloid leukemia in mice and in cell cultures. Blood. 1992;79:2392–8. [PubMed] [Google Scholar]
- 62.Sun WH, Kreisle RA, Phillips AW, Ershler WB. In vivo and in vitro characteristics of interleukin 6-transfected B16 melanoma cells. Cancer Res. 1992;52:5412–5. [PubMed] [Google Scholar]
- 63.Porgador A, Tzehoval E, Katz A, Vadai E, Revel M, Feldman M, et al. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992;52:3679–86. [PubMed] [Google Scholar]
- 64.Mullen CA, Coale MM, Levy AT, Stetler-Stevenson WG, Liotta LA, Brandt S, et al. Fibrosarcoma cells transduced with the IL-6 gene exhibited reduced tumorigenicity, increased immunogenicity, and decreased metastatic potential. Cancer Res. 1992;52:6020–4. [PubMed] [Google Scholar]
- 65.Graf MR, Merchant RE. Interleukin-6 transduction of a rat T9 glioma clone results in attenuated tumorigenicity and induces glioma immunity in Fischer F344 rats. J Neurooncol. 1999;45:209–18. doi: 10.1023/A:1006357424124. [DOI] [PubMed] [Google Scholar]
- 66.Oldford SA, Haidl ID, Howatt MA, Leiva CA, Johnston B, Marshall JS. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J Immunol. 2010;185:7067–76. doi: 10.4049/jimmunol.1001137. [DOI] [PubMed] [Google Scholar]
- 67.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121:3846–59. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aderka D, Maor Y, Novick D, Engelmann H, Kahn Y, Levo Y, et al. Interleukin-6 inhibits the proliferation of B-chronic lymphocytic leukemia cells that is induced by tumor necrosis factor-alpha or -beta. Blood. 1993;81:2076–84. [PubMed] [Google Scholar]
- 69.Lu C, Vickers MF, Kerbel RS. Interleukin 6: a fibroblast-derived growth inhibitor of human melanoma cells from early but not advanced stages of tumor progression. Proc Natl Acad Sci USA. 1992;89:9215–9. doi: 10.1073/pnas.89.19.9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–5. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 71.Chatzidakis I, Mamalaki C. T cells as sources and targets of TNF: implications for immunity and autoimmunity. Curr Dir Autoimmun. 2010;11:105–18. doi: 10.1159/000289200. [DOI] [PubMed] [Google Scholar]
- 72.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 73.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–31. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 74.Ferrajoli A, Keating MJ, Manshouri T, Giles FJ, Dey A, Estrov Z, et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100:1215–9. [PubMed] [Google Scholar]
- 75.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Havell EA, Fiers W, North RJ. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med. 1988;167:1067–85. doi: 10.1084/jem.167.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helson L, Green S, Carswell E, Old LJ. Effect of tumour necrosis factor on cultured human melanoma cells. Nature. 1975;258:731–2. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- 78.Fransen L, Van der Heyden J, Ruysschaert R, Fiers W. Recombinant tumor necrosis factor: its effect and its synergism with interferon-gamma on a variety of normal and transformed human cell lines. Eur J Cancer Clin Oncol. 1986;22:419–26. doi: 10.1016/0277-5379(86)90107-0. [DOI] [PubMed] [Google Scholar]
- 79.McIntosh JK, Mulé JJ, Merino MJ, Rosenberg SA. Synergistic antitumor effects of immunotherapy with recombinant interleukin-2 and recombinant tumor necrosis factor-alpha. Cancer Res. 1988;48:4011–7. [PubMed] [Google Scholar]
- 80.Blankenstein T, Qin ZH, Uberla K, Müller W, Rosen H, Volk HD, et al. Tumor suppression after tumor cell-targeted tumor necrosis factor alpha gene transfer. J Exp Med. 1991;173:1047–52. doi: 10.1084/jem.173.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hock H, Dorsch M, Kunzendorf U, Qin Z, Diamantstein T, Blankenstein T. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci USA. 1993;90:2774–8. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin Z, Krüger-Krasagakes S, Kunzendorf U, Hock H, Diamantstein T, Blankenstein T. Expression of tumor necrosis factor by different tumor cell lines results either in tumor suppression or augmented metastasis. J Exp Med. 1993;178:355–60. doi: 10.1084/jem.178.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smyth MJ, Kelly JM, Baxter AG, Körner H, Sedgwick JD. An essential role for tumor necrosis factor in natural killer cell-mediated tumor rejection in the peritoneum. J Exp Med. 1998;188:1611–9. doi: 10.1084/jem.188.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105:652–6. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calzascia T, Pellegrini M, Hall H, Sabbagh L, Ono N, Elford AR, et al. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117:3833–45. doi: 10.1172/JCI32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lejeune FJ, Liénard D, Matter M, Rüegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6:6. [PubMed] [Google Scholar]
- 87.Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 88.Eggermont AM, Schraffordt Koops H, Klausner JM, Kroon BB, Schlag PM, Liénard D, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg. 1996;224:756–64, discussion 764-5. doi: 10.1097/00000658-199612000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santoro A, Pressiani T, Citterio G, Rossoni G, Donadoni G, Pozzi F, et al. Activity and safety of NGR-hTNF, a selective vascular-targeting agent, in previously treated patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;103:837–44. doi: 10.1038/sj.bjc.6605858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jung M, Dimtchev A, Velena A, Dritschilo A. Combining radiation therapy with interstitial radiation-inducible TNF-α expression for locoregional cancer treatment. Cancer Gene Ther. 2011;18:189–95. doi: 10.1038/cgt.2010.69. [DOI] [PubMed] [Google Scholar]
- 91.Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci USA. 2012;109:7841–6. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Talmadge JE, Tribble HR, Pennington RW, Phillips H, Wiltrout RH. Immunomodulatory and immunotherapeutic properties of recombinant gamma-interferon and recombinant tumor necrosis factor in mice. Cancer Res. 1987;47:2563–70. [PubMed] [Google Scholar]
- 93.Belardelli F, Ciolli V, Testa U, Montesoro E, Bulgarini D, Proietti E, et al. Anti-tumor effects of interleukin-2 and interleukin-1 in mice transplanted with different syngeneic tumors. Int J Cancer. 1989;44:1108–16. doi: 10.1002/ijc.2910440629. [DOI] [PubMed] [Google Scholar]
- 94.Vercammen E, Staal J, Van Den Broeke A, Haegman M, Vereecke L, Schotte P, et al. Prolonged exposure to IL-1beta and IFNgamma induces necrosis of L929 tumor cells via a p38MAPK/NF-kappaB/NO-dependent mechanism. Oncogene. 2008;27:3780–8. doi: 10.1038/onc.2008.4. [DOI] [PubMed] [Google Scholar]
- 95.Yip I, Pang XP, Berg L, Hershman JM. Antitumor actions of interferon-gamma and interleukin-1 beta on human papillary thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1995;80:1664–9. doi: 10.1210/jc.80.5.1664. [DOI] [PubMed] [Google Scholar]
- 96.Hori K, Ehrke MJ, Mace K, Mihich E. Effect of recombinant tumor necrosis factor on tumoricidal activation of murine macrophages: synergism between tumor necrosis factor and gamma-interferon. Cancer Res. 1987;47:5868–74. [PubMed] [Google Scholar]
- 97.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 98.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 100.Schreiber RD, Pace JL, Russell SW, Altman A, Katz DH. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983;131:826–32. [PubMed] [Google Scholar]
- 101.Pace JL, Russell SW, Schreiber RD, Altman A, Katz DH. Macrophage activation: priming activity from a T-cell hybridoma is attributable to interferon-gamma. Proc Natl Acad Sci USA. 1983;80:3782–6. doi: 10.1073/pnas.80.12.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schultz RM, Kleinschmidt WJ. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983;305:239–40. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- 103.Sgadari C, Angiolillo AL, Cherney BW, Pike SE, Farber JM, Koniaris LG, et al. Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo. Proc Natl Acad Sci USA. 1996;93:13791–6. doi: 10.1073/pnas.93.24.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, et al. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981–92. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sgadari C, Farber JM, Angiolillo AL, Liao F, Teruya-Feldstein J, Burd PR, et al. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood. 1997;89:2635–43. [PubMed] [Google Scholar]
- 106.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parr I, Wheeler E, Alexander P. Similarities of the anti-tumour actions of endotoxin, lipid A and double-stranded RNA. Br J Cancer. 1973;27:370–89. doi: 10.1038/bjc.1973.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vicari AP, Chiodoni C, Vaure C, Aït-Yahia S, Dercamp C, Matsos F, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541–9. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexandroff AB, Nicholson S, Patel PM, Jackson AM. Recent advances in bacillus Calmette-Guerin immunotherapy in bladder cancer. Immunotherapy. 2010;2:551–60. doi: 10.2217/imt.10.32. [DOI] [PubMed] [Google Scholar]
- 111.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 112.Geisse JK, Rich P, Pandya A, Gross K, Andres K, Ginkel A, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: a double-blind, randomized, vehicle-controlled study. J Am Acad Dermatol. 2002;47:390–8. doi: 10.1067/mjd.2002.126215. [DOI] [PubMed] [Google Scholar]
- 113.Lebwohl M, Dinehart S, Whiting D, Lee PK, Tawfik N, Jorizzo J, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol. 2004;50:714–21. doi: 10.1016/j.jaad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 114.van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358:1465–73. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 115.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 116.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–6. [PubMed] [Google Scholar]
- 117.Nisticò P, Capone I, Palermo B, Del Bello D, Ferraresi V, Moschella F, et al. Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int J Cancer. 2009;124:130–9. doi: 10.1002/ijc.23886. [DOI] [PubMed] [Google Scholar]
- 118.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]



