Abstract
For many years, β-defensins were best known for their antimicrobial activity. However, β-defensins also exert immunomodulatory functions, such as the chemotactic recruitment of immune cells via chemokine receptors. We demonstrated that mouse β-defensin 14 recruits CCR6+ B cells into fibrosarcomas, resulting in enhanced angiogenesis and tumor development.
Keywords: angiogenesis, antimicrobial peptide, chemokine receptor 6, defensin, lymphotoxin β-receptor, tumor growth
The family of β defensins consists of small, cationic, cysteine-rich antimicrobial polypeptides. For a long time, β defensins were merely considered as parts of the antimicrobial innate immune response against a wide variety of pathogens.1 Interestingly, research over the past few years has revealed that - in addition to their direct antimicrobial activity - β defensins promote innate inflammatory and adaptive immune responses by interacting with CC-chemokine receptors like CCR2 and CCR6.2-4 Recently, we characterized mouse β defensin 14 (mBD14, Defb14), an ortholog of human β defensin 3 (hBD3, DEFB103). mBD14 is expressed in a variety of tissues, epithelial cells and CD11c+ dendritic cells (DCs). Furthermore, we described the chemotactic recruitment of CCR6-expressing cells through mBD14.5
Inflammation typically correlates with increased local expression of β defensins, but in severe cases β defensins can also be detected systemically. In relation to this expression pattern, it is interesting that inflammation is associated with an increased risk for cancer development, and that recent reports suggest a link between β defensin expression and tumor progression. For instance, epidermal growth factor (EGF)-enhanced expression of hBD3 has been reported in oral carcinoma lesions.6 Such an intratumoral hBD3 chemoattracts tumor-associated macrophages (TAMs) into carcinoma lesions in a CCR2-dependent manner and stimulates the production of tumor-promoting cytokines like interleukin-8 (IL-8), which is known to be a pro-angiogenic factor.7
Considering a potential relationship between β defensins and malignancy as well as our previous results demonstrating the pro-inflammatory properties of mBD14, we analyzed the expression pattern of mBD14 in fibrosarcoma tissues and its potential role during tumor development. Quantitative real-time PCR analysis of tumor biopsies revealed substantial expression of mBD14 in BFS-1 fibrosarcomas. Moreover, we identified tumor-infiltrating CD45+ hematopoietic host cells as a local source of mBD14. To analyze the influence of mBD14 expression on tumor growth we generated a BFS-1 cell line overexpressing the mature mBD14 protein fused to the Fc fragment of human IgG1 (mBD14:Ig). The mBD14:Ig fusion protein retains its biological activity (e.g., antimicrobial activity, chemotactic activity).5 Wild type, mBD14:Ig-expressing and control BFS-1 cells were intradermally inoculated into syngeneic C57BL/6 mice. Interestingly, overexpression of mBD14:Ig in BFS-1 cells resulted in significantly enhanced tumor growth in vivo, correlating with increased tumor vascularization, as demonstrated by immunohistochemical staining for the endothelial cell marker CD31 (PECAM-1). We found that while vascular-endothelial growth factor (VEGF) mRNA expression was not altered, CXCL2 mRNA expression was significantly increased in mBD14:Ig-expressing fibrosarcomas. These observations are in line with previous research showing that β defensins (e.g., hBD2) are implicated in the stimulation of endothelial cell migration and tube formation.8
The mechanism of β defensin-stimulated angiogenesis then comes into question. In our study, the monitoring of vascular cell sprouting during aortic ring assay experiments clearly demonstrated that stimulation with recombinant mBD14:Ig does not influence vascular outgrowth. Thus, we excluded direct effects of mBD14 on vascularization and hypothesized that there might be an angiogenesis-promoting cell population involved. To identify the cell population that might contribute to the mBD14:Ig-induced progression of tumor growth, we analyzed the cellular composition of tumor-infiltrating leukocytes (TILs). Surprisingly, the B220+/CD19+ cell population was the only cell compartment significantly increased in mBD14:Ig-expressing tumors, while no substantial difference in the frequency of CD4+, CD8+, NK1.1+, CD11c+, CD11b+ or F4/80+ TILs was detected. Taking into account that B220+/CD19+ cells express CCR6, we took advantage of the human IgG1-Fc tail fused to mBD14 and demonstrated specific binding of mBD14:Ig to CCR6+ B220+/CD19+ cells isolated from tumor samples (by cytofluorometric analysis using anti-human IgG1-specific antibody). To elucidate whether CCR6 is important for the mBD14:Ig-induced enhancement of tumor growth, we inoculated mBD14:Ig-expressing and control BFS-1 cells into Ccr6−/− and Ccr6+/− mice. Monitoring of tumor growth revealed that the pro-tumor effects of mBD14:Ig were abolished when mBD14:Ig-expressing tumors were innoculated into CCR6-deficient mice, demonstrating of the first time that a specific cell type is recruited in vivo to sites of β defensin expression in a CCR6-dependent manner. Consistently, the reduced recruitment of B220+/CD19+ cells into the tumor tissue of CCR6-deficient mice correlated with limited fibrosarcomatissue vascularization. Previously, we demonstrated that the activation of lymphotoxin β-receptor (LTβR) on fibrosarcoma cells by ligand-expressing activated T and B lymphocytes results in increased expression of pro-angiogenic CXCL2 (MIP-2), the functional mouse ortholog of human IL-8, and hence increased tumor growth.9 To prove this mechanism in the mBD14-dependent tumor model, we inoculated BFS-1 cells co-expressing mBD14:Ig and the soluble inhibitor of LTβR activation into C56BL/6 mice. Monitoring of tumor growth revealed that tumors co-expressing the LTβR inhibitor show reduced expression of CXCL2 mRNA and grow less than their control counterparts. Based on these observations, we propose a novel role for β defensins, like mBD14, in tumor vascularization and development. The model we propose predicts that mBD14 recruits activated B cells into tumors in a CCR6-dependent manner. These B cells, expressing LTβR-ligands, activate LTβR on fibrosarcoma cells, leading to CXCL2 expression, enhanced angiogenesis and increased tumor growth (Fig. 1).
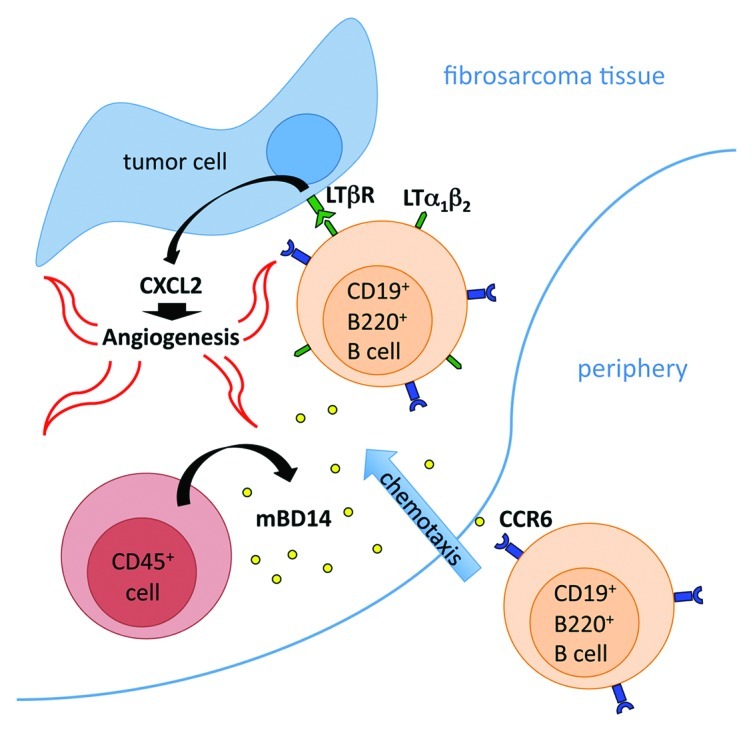
Figure 1. The model that we propose—based on our results—postulates that mBD14, expressed by host-derived CD45+ hematopoietic cells, recruits activated B220+/CD19+ B cells into tumors in a CCR6-dependent manner. These B cells, expressing LTβR-ligands, activate LTβR on tumor cells, in turn promoting CXCL2 expression, enhanced angiogenesis and increased tumor growth.
In summary, recent research from us and others suggest that antimicrobial peptides like β defensins play critical roles in the development and progression of numerous tumors.10 The detailed characterization of β defensin expression patterns during tumor development will provide further insights into the mechanisms of tumor progression. Importantly, β defensins appear to constitute a link between innate inflammatory immune reactions and the molecular and cellular mechanisms that promote tumor development.
Glossary
Abbreviations:
- hBD
human β defensin
- LTβR
lymphotoxin β receptor
- mBD
mouse β defensin
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/20825
References
- 1.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14:96–102. doi: 10.1016/S0952-7915(01)00303-X. [DOI] [PubMed] [Google Scholar]
- 2.Röhrl J, Yang D, Oppenheim JJ, Hehlgans T. Specific binding and chemotactic activity of mBD4 and its functional orthologue hBD2 to CCR6-expressing cells. J Biol Chem. 2010;285:7028–34. doi: 10.1074/jbc.M109.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 4.Röhrl J, Yang D, Oppenheim JJ, Hehlgans T. Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J Immunol. 2010;184:6688–94. doi: 10.4049/jimmunol.0903984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Röhrl J, Yang D, Oppenheim JJ, Hehlgans T. Identification and Biological Characterization of Mouse beta-defensin 14, the orthologue of human beta-defensin 3. J Biol Chem. 2008;283:5414–9. doi: 10.1074/jbc.M709103200. [DOI] [PubMed] [Google Scholar]
- 6.Kawsar HI, Weinberg A, Hirsch SA, Venizelos A, Howell S, Jiang B, et al. Overexpression of human beta-defensin-3 in oral dysplasia: potential role in macrophage trafficking. Oral Oncol. 2009;45:696–702. doi: 10.1016/j.oraloncology.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Jin G, Kawsar HI, Hirsch SA, Zeng C, Jia X, Feng Z, et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS One. 2010;5:e10993. doi: 10.1371/journal.pone.0010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baroni A, Donnarumma G, Paoletti I, Longanesi-Cattani I, Bifulco K, Tufano MA, et al. Antimicrobial human beta-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides. 2009;30:267–72. doi: 10.1016/j.peptides.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Daller B, Müsch W, Röhrl J, Tumanov AV, Nedospasov SA, Männel DN, et al. Lymphotoxin-β receptor activation by lymphotoxin-α(1)β(2) and LIGHT promotes tumor growth in an NFκB-dependent manner. Int J Cancer. 2011;128:1363–70. doi: 10.1002/ijc.25456. [DOI] [PubMed] [Google Scholar]
- 10.Coffelt SB, Scandurro AB. Tumors sound the alarmin(s) Cancer Res. 2008;68:6482–5. doi: 10.1158/0008-5472.CAN-08-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]


