Abstract
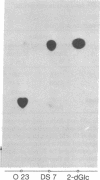
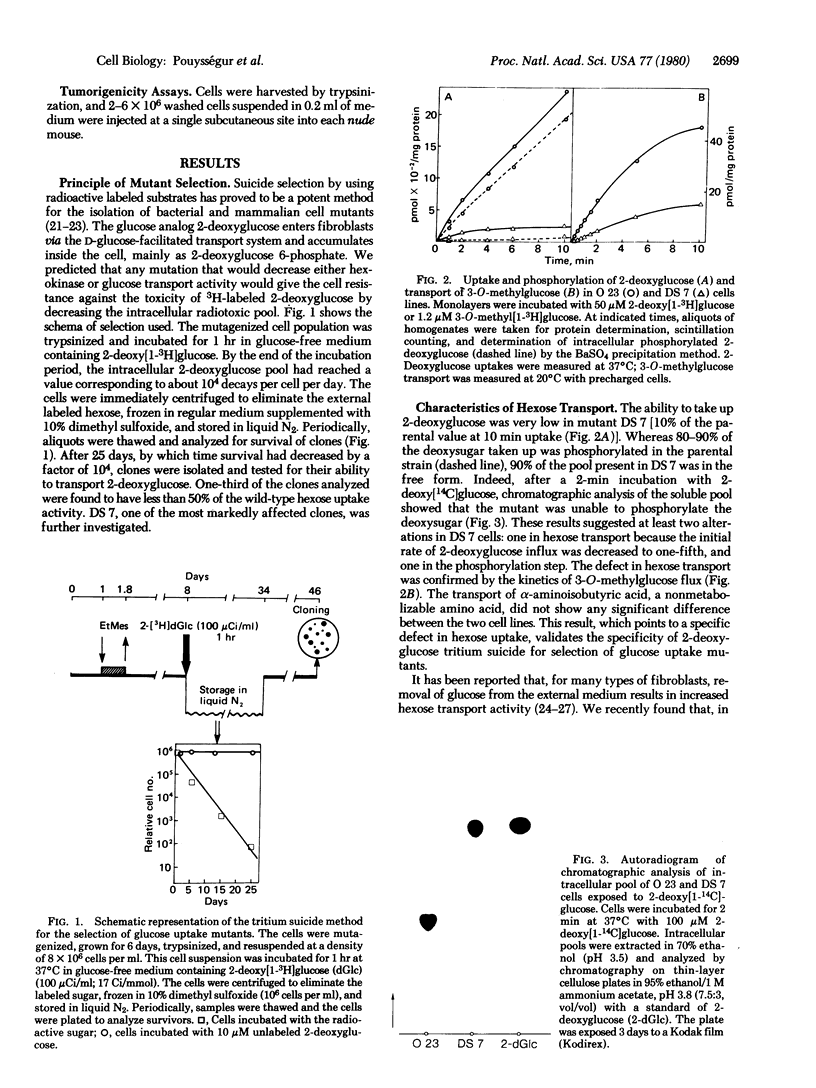
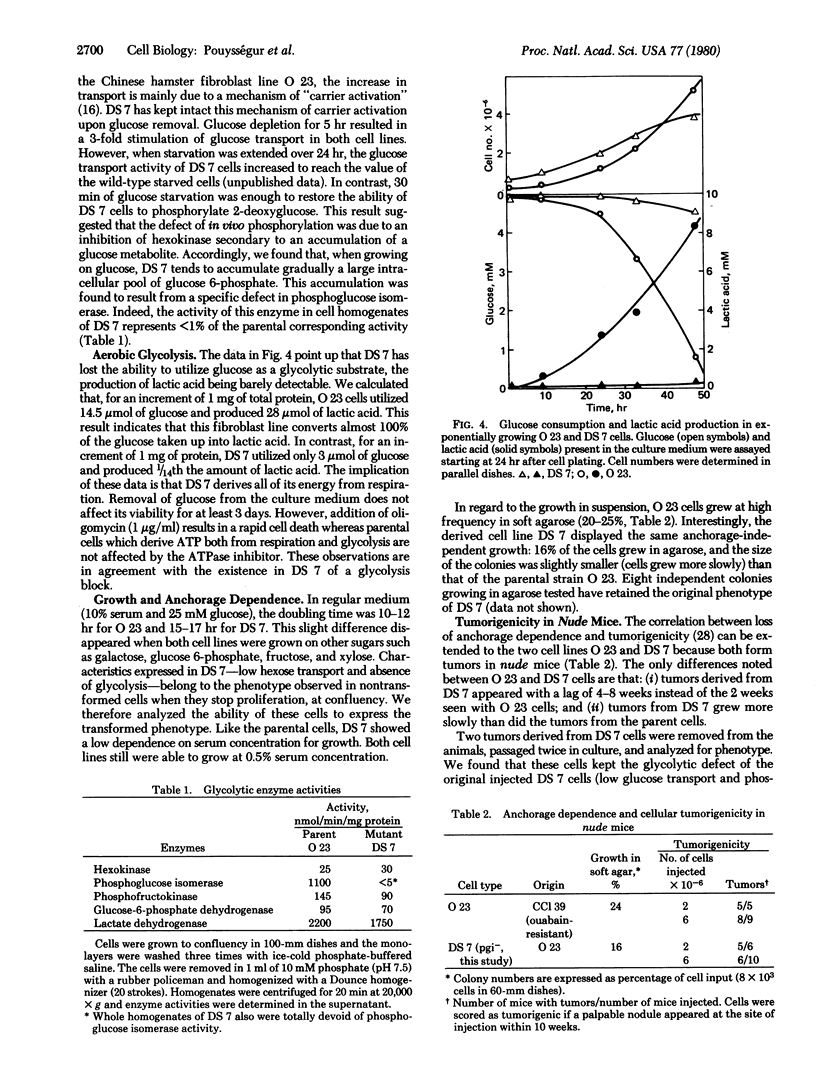
A procedure is described for the selection of glucose uptake mutants based upon radiation suicide of Chinese hamster fibroblasts by 2-deoxy[3H]glucose. In one of these mutants, DS 7, the ability to transport either 2-deoxyglucose or 3-O-methylglucose was decreased to one-fifth to one-fourth. Besides this defect, DS7 produces 1/14th the lactic acid produced by the parent when grown on 5 mM glucose. This block in aerobic glycolysis is due to a mutation that affects the expression of the phosphoglucose isomerase gene because no isomerase activity is detected in cell extracts of DS7. This glycolytic block makes that cell line dependent exclusively on respiration for its energy requirement. Consequently, DS7 survives well after removal of glucose but dies quickly in the presence of oligomycin. The parental line O23 (subclone of CCl39) grows at low serum concentration, is anchorage-independent, and is tumorigenic in nude mice. The derived glycolytic mutant DS7 has retained both the in vitro transformed phenotype (low serum dependence and loss of anchorage dependence) and the tumor-forming capability. The tumor cells derived from the injection of DS7 cells have kept the original glycolytic defect. This finding suggests that the transformed properties (high hexose transport and aerobic glycolysis) that can be uncoupled from abnormal growth control are not necessary for the expression of the malignant phenotype in fibroblasts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Carroll R. C., Ash J. F., Vogt P. K., Singer S. J. Reversion of transformed glycolysis to normal by inhibition of protein synthesis in rat kidney cells infected with temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5015–5019. doi: 10.1073/pnas.75.10.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M. C., Slayman C. W., Adelberg E. A. Tritium suicide selection of mammalian cell mutants defective in the transport of neutral amino acids. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4549–4551. doi: 10.1073/pnas.74.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Activation of phosphofructokinase by stimulants of cell multiplication. Nat New Biol. 1973 Dec 12;246(154):181–183. doi: 10.1038/newbio246181a0. [DOI] [PubMed] [Google Scholar]
- Franchi A., Silvestre P., Pouyssegur J. "Carrier activation" and glucose transport in Chinese hamster fibroblasts. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1526–1534. doi: 10.1016/0006-291x(78)91176-2. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- Hatanaka M. Sugar effects on murine sarcoma virus transformation. Proc Natl Acad Sci U S A. 1973 May;70(5):1364–1367. doi: 10.1073/pnas.70.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M., Christopher C. W., Ullrey D. Neoplastic potentials and regulation of uptake and nutrients. II. Inverse regulation of uptake of hexose and amino acid analogues in the neoplastic GIV line. J Cell Physiol. 1976 Dec;89(4):765–767. doi: 10.1002/jcp.1040890439. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Induction of sugar transport in chick embryo fibroblasts by hexose starvation. Evidence for transcriptional regulation of transport. J Biol Chem. 1975 Jan 25;250(2):593–600. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. I. A functional change in the plasma membrane associated with the rate of cell growth. J Biol Chem. 1974 Jun 10;249(11):3366–3374. [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Martineau R., Kohlbacher M., Shaw S. N., Amos H. Enhancement of hexose entry into chick fibroblasts by starvation: differential effect on galactose and glucose. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3407–3411. doi: 10.1073/pnas.69.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Pastan I. Mutants of mouse fibroblasts altered in the synthesis of cell surface glycoproteins. Preliminary evidence for a defect in the acetylation of glucosamine 6-phosphate. J Biol Chem. 1977 Mar 10;252(5):1639–1646. [PubMed] [Google Scholar]
- Pouysségur J., Willingham M., Pastan I. Role of cell surface carbohydrates and proteins in cell behavior: studies on the biochemical reversion of an N-acetylglucosamine-deficient fibroblast mutant. Proc Natl Acad Sci U S A. 1977 Jan;74(1):243–247. doi: 10.1073/pnas.74.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E. Why do tumor cells have a high aerobic glycolysis? J Cell Physiol. 1976 Dec;89(4):697–700. doi: 10.1002/jcp.1040890429. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Diamond I., Rozengurt E. Glycolysis of quiescent cultures of 3T3 cells. Addition of serum, epidermal growth factor, and insulin increases the activity of phosphofructokinase in a protein synthesis-independent manner. J Biol Chem. 1978 Feb 10;253(3):872–877. [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash C. R., Cunningham D. D. Dissociation of increased hexose transport from initiation of fibroblast proliferation. Nature. 1974 Nov 1;252(5478):45–47. doi: 10.1038/252045a0. [DOI] [PubMed] [Google Scholar]
- Ullrey D., Gammon M. T., Kalckar H. M. Uptake patterns and transport enhancements in cultures of hamster cells deprived of carbohydrates. Arch Biochem Biophys. 1975 Apr;167(2):410–416. doi: 10.1016/0003-9861(75)90481-6. [DOI] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wang T., Marquardt C., Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976 Jun 24;261(5562):702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada K. M., Yamada S. S., Pouysségur J., Pastan I. Microfilament bundles and cell shape are related to adhesiveness to substratum and are dissociable from growth control in cultured fibroblasts. Cell. 1977 Mar;10(3):375–380. doi: 10.1016/0092-8674(77)90024-1. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Pouyssegur J. Cell surface glycoproteins and malignant transformation. Biochimie. 1978;60(11-12):1221–1233. doi: 10.1016/s0300-9084(79)80439-3. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]