Abstract
Background
Although yoga and meditation have been used for stress reduction with reported improvement in inflammation, little is known about the biological mechanisms mediating such effects. The present study examined if a yogic meditation might alter the activity of inflammatory and antiviral transcription control pathways that shape immune cell gene expression.
Methods
Forty-five family dementia caregivers were randomized to either Kirtan Kriya Meditation (KKM) or Relaxing Music (RM) listening for 12 minutes daily for 8 weeks and 39 caregivers completed the study. Genome-wide transcriptional profiles were collected from peripheral blood leukocytes sampled at baseline and 8-week follow-up. Promoter-based bioinformatics analyses tested the hypothesis that observed transcriptional alterations were structured by reduced activity of the pro-inflammatory nuclear factor (NF)-κB family of transcription factors and increased activity of Interferon Response Factors (IRF; i.e., reversal of patterns previously linked to stress).
Results
In response to KKM treatment, 68 genes were found to be differentially expressed (19 up-regulated, 49 down-regulated) after adjusting for potentially confounded differences in sex, illness burden, and BMI. Up-regulated genes included immunoglobulin-related transcripts. Down-regulated transcripts included pro-inflammatory cytokines and activation-related immediate-early genes. Transcript origin analyses identified plasmacytoid dendritic cells and B lymphocytes as the primary cellular context of these transcriptional alterations (both p < .001). Promoter-based bioinformatic analysis implicated reduced NF-κB signaling and increased activity of IRF1 in structuring those effects (both p < .05).
Conclusion
A brief daily yogic meditation intervention may reverse the pattern of increased NF-κB-related transcription of pro-inflammatory cytokines and decreased IRF1-related transcription of innate antiviral response genes previously observed in healthy individuals confronting a significant life stressor.
Keywords: Dementia caregiver, stress, Kirtan Kriya, yoga, meditation, relaxation, NF-κB, genomics, gene expression profiling, microarray
Caring for a frail or demented family member can be a significant life stressor. Older adult caregivers report higher levels of perceived stress and depression, and lower levels of satisfaction with life, joy, vigor, and content than healthy controls (Pinquart and Sörensen, 2003; Miller et al., 2008). Moreover, caregivers show heightened levels of biological markers of inflammation (Wu et al., 1999) and reduced levels of cellular immunity (Kiecolt-Glaser et al., 1991; Damjanovic et al., 2007; Epel et al., 2010; Lovell and Wetherell, 2011). Familial caregivers are often considered to be at risk of stress-related disease and general health decline (Gouin et al., 2008). Some research suggests that psychosocial interventions for dementia caregivers reduce adverse effects of caregiver stress on physical and mental health (Schulz et al., 2002). However, the pathways by which such psychosocial interventions differentially impact biological processes (e.g., inflammation) among caregivers remain poorly understood. Indeed, initial pilot studies suggest that psychosocial interventions delivered to caregivers may potentially improve immune function (Garand et al., 2002; Northouse et al., 2012).
Stress signals interpreted by the central nervous system (CNS) can modulate the expression of immune response genes via the effects of hormones and neurotransmitters on gene transcription control pathways (Irwin and Cole, 2011). Several previous studies have shown that diverse types of significant life adversity such as social isolation (Cole et al., 2007), imminent bereavement (Miller et al., 2008), post-traumatic stress disorder (O’Donovan et al., 2011), chronic loneliness (Cole et al., 2007; Cole et al., 2011), social threat (Cole et al., 2010), and low socioeconomic status (SES) (Miller et al., 2009; Chen et al., 2009; Chen et al., 2010) are associated with an up-regulation of inflammation-related genes under the control of the transcription factor Nuclear Factor-κB (NF-κB) and a complementary down-regulation of innate antiviral genes targeted by Interferon Response Factors (IRF) (Irwin and Cole, 2011).
In the context of caregiving stress, one previous genome-wide transcriptional profiling study has shown that monocytes from familial caregivers exhibit heightened expression of genes with response elements for NF-κB and reduced expression of genes with response elements for IRF relative to healthy controls (Miller et al., 2008). These caregivers also showed relative elevations in the inflammatory markers C-reactive protein and interleukin-1 receptor antagonist. This limited literature suggests that psychological stressors experienced by older adult caregivers may alter gene expression that could promote chronic inflammation and undermine antiviral defenses. However, it is unknown whether psychosocial interventions can reverse such changes. This randomized controlled trial is the first to test whether a daily yogic meditation intervention can potentially reverse a pattern of pro-inflammatory and anti-antiviral leukocyte transcriptional alterations that has previously been linked to chronic stress and adversity (Irwin and Cole, 2011).
Meditation practices can lead to improvements in physical and mental health (Grossman et al., 2004; Black et al., 2009; Chiesa and Serretti, 2009). Moreover, initial pilot studies indicate that dementia caregivers who practice meditation show improvement in mental health indices such as depression and anxiety (Waelde et al., 2004; Van Puymbroeck et al., 2007; Pace et al., 2009), which are indices associated with inflammatory markers such as IL-6 (Irwin and Olmstead, 2011). Our recent study showed that meditation increased telomerase activity when compared to a relaxing activity, and it also modulated depression, mental health, and cognition in dementia caregivers (Lavretsky et al 2012).
Among healthy and depressed older adults, various forms of meditation with movement (e.g., Tai Chi) or without movement (e.g., breath-focused meditation) are shown to improve biological markers of immune function including reduced circulating levels of inflammatory markers (e.g., IL-6, CRP) (Irwin and Olmstead, 2011; Lavretsky et al., 2011, 2012), increased cell-mediated immune response (e.g., varicella-zoster virus (VZV) specific cell-mediated immunity) (Irwin et al., 2003; Irwin et al., 2007; Irwin and Olmstead, 2011), greater rise in antibody titers in response to influenza vaccine (Davidson et al., 2003), greater rise in VZV specific response to vaccine (Irwin et al. 2007) and greater immune cell telomerase activity (Jacobs et al., 2011; Lavretsky et al 2012). However, the effects of meditation practice on gene expression pathways regulating immune function have not been explored among dementia caregivers.
The present study utilizes an in vivo genomics-based strategy to identify genes that are differentially expressed in immune cells in response to a daily yogic Kirtan Kriya Meditation (KKM) vs. Relaxing Music (RM) listening intervention among family dementia caregivers. The objective is to define upstream transcription-control pathways that mediate the differences in immune cell gene expression profile between treatment groups. We tested the hypothesis that KKM relative to RM would reverse the pattern of increased NF-κB-related transcription of pro-inflammatory cytokines and decreased expression of genes driven by interferon-related transcription factors (i.e., IRF-1), as was previously observed in individuals confronting caregiving stress and other forms of significant life stressors (Cole et al., 2007; Miller et al., 2008; Cole, 2008; Miller et al., 2009; Cole et al., 2009). As such, we hypothesized pre- to post-intervention decreases in expression of NF-κB-related transcripts and increases in expression of IRF-1-related transcripts in KKM relative to RM.
Methods
Participants
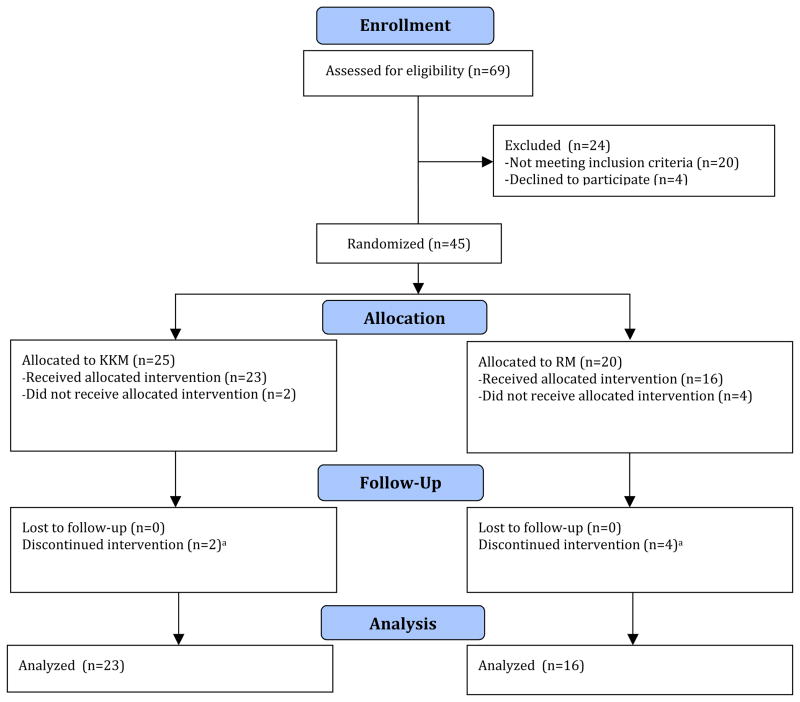
Over a 12-month study period, a total of 69 family dementia caregivers were screened. Of those screened, 49 were eligible to participate in the study, and four were not interested in participating. Forty-five caregivers were recruited and randomized to the treatment conditions, and 39 completed 8 weeks of the interventions (see Figure 1 for CONSORT-format flow of participants through the study). Criteria for study inclusion were: (1) being the primary caregivers of an elderly person, (2) being in contact with the dementia patient at least three days per week, and (3) agreeing to random assignment. Criteria for study exclusion were: (1) history of major depressive disorder, (2) history of any other psychiatric illness, (3) undergoing current depression treatment, (4) alcohol and/or substance abuse or dependence, (5) psychotropic drug use, (6) severe or acute medical illness, (7) acute suicidal or violent behavior, and (8) any other CNS disease or dementia. Exclusionary screening tools for mental health included the Structured Clinical Interview for the DSM-IVR (SCID) (First et al., 1995), the Hamilton Rating Scale for Depression (HAM-D>17) (Hamilton, 1960), and the Folstein Mini-Mental State Examination (MMSE≥26) (Folstein et al., 1975).
Figure 1. Flow diagram of participants through trial.
Notes. aDue to lack of interest in the intervention or inability to commit to study schedule
Procedures
The UCLA Institutional Review Board approved all study procedures (registered ClinicalTrials.gov trial # NCT01537679). Eligible recruits provided written informed consent prior to enrolling in the study. Using a computer-generated randomization table, a treatment-blinded statistician randomized participants to either KKM or RM practice for 12 minutes daily for eight weeks. All participants were seen for 6 visits with baseline visit and behavioral assessments every 2 weeks during 8-week intervention. As a supplement to both treatment conditions, all participants received an educational manual about the prognosis of dementia and maintaining good health. Treatment compliance and satisfaction were monitored at each visit by interpersonal interview and daily diary.
Intervention
Kirtan Kriya Meditation (KKM)
The protocol for KKM is standard for the Kundalini Yoga practice as taught by Yogi Bhajan, which has been discussed in previous studies conducted among older adults (Newberg et al., 2010) and described in detail elsewhere (ARPF - Alzheimer’s Research & Prevention Foundation, 2011). KKM is a 12-minute yogic meditation chanting practice guided by an audio CD that is performed at the same time each and every day for a total of eight weeks. KKM progresses through 1 minute of silently focusing inwards on the mind and body in the present moment, 11 minutes of mudras or repetitive finger movements while chanting “Saa, Taa, Naa, Maa,” meaning “Birth, Life, Death, and Rebirth” that are chanted first aloud, gradually softening into a whisper, and then silently. The meditation practice is completed with deep breathing and the visualization of light.
Relaxing Music (RM)
The RM protocol requested participants to relax in a quiet place with their eyes closed while listening to relaxing instrumental music provided to them on an audio CD for 12 minutes each and every day for eight weeks. Previous research indicates that music listening can induce a relaxation response both via self-report and biological measures (Nilsson, 2008). Given that KKM involves melodic chanting and focused attention that might induce a relaxation response, we used RM as an experimental control for the relaxation response and any other unknown psychosomatic responses elicited from rhythmic auditory sensation.
Analysis
General statistical analyses were performed in SPSS for Windows version 20 (SPSS Inc, Chicago, IL). Differences between groups at baseline on demographic, medical burden, and mental heath characteristics were assessed using t-tests for continuous measures and Chi-square tests for categorical measures.
Gene Expression Profiling and Analysis
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation of antecubital venipuncture samples drawn between 10–11 am at baseline and 8 weeks later following the completion of intervention procedures. Genome-wide transcriptional profiling was carried out as previously described (Cole et al., 2011) in 39 individuals who completed the study. Briefly, total RNA was extracted (RNeasy; Qiagen, Valencia CA), tested for suitable mass (Nanodrop ND1000) and integrity (Agilent Bioanalyzer), and converted to fluorescent cRNA for hybridization to human HT-12 Bead Chips (Illumina, San Diego CA) following the manufacturer’s standard protocol in the UCLA Southern California Genotyping Consortium Core Laboratory. Quantile normalized gene expression values were transformed to log2 for genome-wide general linear model analysis. Effects of the intervention on the gene expression outcomes were evaluated in a 2 (Condition: KKM vs. RM) × 2 (Time:baseline to post-treatment, repeated measure) ANCOVA, controlling for sex, illness burden, and BMI (characteristics differing by chance across randomly assigned groups at p < .10). In both analyses, differentially expressed genes were identified by linear model parameter estimates exceeding a pre-specified effect-size cut-off (>1.2-fold difference across Conditions in the average magnitude of the Time effect). Substantively identical results emerged from preliminary differential expression analyses that did not control for any covariates (i.e., analyzing just the Condition x Time interaction and its constituent main effects). Differentially expressed genes were then analyzed by the TELiS transcription factor search engine (Cole et al., 2005) to assess the prevalence of transcription factor-binding motifs targeted by NF-κB (detected by TRANSFAC motif matrix V$CREL_01) and Interferon Response Factor 1 (IRF1; V$IRF1_01) in the promoter regions of genes that were relatively up-regulated over Time in KKM vs. RM, with results averaged over 9 parametric variations in motif scan stringency and promoter length. To identify the specific cell type mediating any observed difference in gene expression within the circulating leukocyte pool, Transcript Origin Analyses were carried out as previously described (Cole et al., 2011). In all bioinformatics analyses, the p< .05 level was used to test statistical significance.
Results
Table 1 presents the baseline demographic and clinical characteristics of intervention in 39 intervention completers. Groups were statistically equivalent at baseline across all measures assessed except for BMI; those in the RM vs. KKM condition had a higher mean BMI (M = 29.4 vs. M= 23.5, p = .001, respectively). As reported previously (Lavretsky et al 2012), the KKM group showed significantly lower levels of depressive symptoms and greater improvement in mental health compared with the RM group, following the intervention. In KKM, 65.2% of the participants showed 50% improvement on the Hamilton Depression Rating scale. Fifty-two percent of participants showed 50% improvement on the Mental Health Composite Summary score of the Short Form-36 scale. In RM, 31.2% of the participants showed 50% improvement on the Hamilton Depression Rating scale. Nineteen percent of the participants showed 50% improvement on the Mental Health Composite Summary score of the Short Form-36 scale. These proportions were significantly different across condition (p<0.05).
Table 1.
Baseline characteristics by treatment group
| Variable | KKM (N = 23) | RM (N = 16) | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | N (%) | Mean (SD) | N (%) | torchi-square | p | |
| Age (years) | 60.5 (28.2) | 60.6 (12.5) | 0.03 | 0.9 | ||
| Sex (female) | 23 (100 %) | 14 (88 %) | 3.0 | 0.08 | ||
| Education (years) | 16.1 (2.1) | 15.1 (2.8) | 1.2 | 0.2 | ||
| Years of caregiving | 4.7 (2.4) | 4.2 (2.9) | 0.6 | 0.6 | ||
| Hours of care/week | 47.8 (35.8) | 63.3 (36.2) | 1.3 | 0.2 | ||
| Medical Burden | ||||||
| CIRS | 3.0 (2.3) | 4.6 (3.1) | 1.8 | 0.08 | ||
| CVRF | 5.2 (3.7) | 7.4 (6.4) | 1.4 | 0.2 | ||
| BMI | 23.5 (2.5) | 29.4 (6.9) | 3.8 | 0.001 | ||
| Health Functioning | ||||||
| SF-36 MCS | 34.4 (10.2) | 37.3 (11.0) | 0.8 | 0.4 | ||
| SF-36 PCS | 58.7 (6.7) | 55.4 (8.3) | 1.4 | 0.2 | ||
| Distress | ||||||
| HAM-D | 11.9 (4.1) | 11.4 (4.0) | 0.4 | 0.7 | ||
| Cognition | ||||||
| MMSE | 29.5 (1.0) | 29.6 (0.6) | 0.3 | 0.6 |
Notes: KKM = Kirtan Kriya Meditation; RM = Relaxing Music; SF-36 MCS – Mental health composite summary scores of SF-36; SF-36 PCS – Physical health composite summary scores of SF-36; MMSE - Mini-Mental State Examination; HAM-D - Hamilton Depression Rating Scale; CIRS – Cumulative Illness Rating Scale; CVRF - Cerebrovascular Risk Factors; BMI = body mass index
To determine whether KKM vs. RM might reverse the social stressor-related pattern of increased pro-inflammatory gene expression and decreased antiviral gene expression, covariate-adjusted analyses compared the magnitude of pre-post change in gene expression across treatment conditions. Table 2 lists the set of 68 genes showing a >1.2 fold difference in change over time between conditions (49 genes relatively down-regulated and 19 genes relatively up-regulated in KKM vs. RM). Up-regulated target genes found in KKM relative to RM included immunoglobulin-related transcripts (e.g., IGJ, IGLL3) and multiple un-named genes of unknown function (LOC genes), whereas down-regulated transcripts included pro-inflammatory cytokines (e.g., IL8) and activation-related immediate-early genes (JUN, FOSB). Based on a priori hypothesis, we also examined 3 other prototypical pro-inflammatory cytokines in addition to IL8 and found that 2 (IL1B and IL6) showed .94-fold reductions in KKM vs. RM, whereas one (TNF) showed a 1.07-fold increase.
Table 2.
Fold difference>1.2 across treatments in gene expression.
| Gene Symbol | Up-regulated | Gene Symbol | Down-regulated |
|---|---|---|---|
| Fold Difference | Fold Difference | ||
| TNFRSF17 | 1.648 | USP49 | .833 |
| LOC649923 | 1.616 | PRO0628 | .833 |
| LOC647506 | 1.478 | HBG2 | .833 |
| LOC652493 | 1.471 | BCYRN1 | .831 |
| IGJ | 1.445 | unnamed gene | .831 |
| MGC29506 | 1.410 | C5ORF28 | .831 |
| LOC401845 | 1.407 | unnamed gene | .831 |
| LOC647450 | 1.397 | HDC | .829 |
| LOC652775 | 1.352 | SLC25A37 | .829 |
| LOC652694 | 1.329 | ATHL1 | .829 |
| LOC652102 | 1.318 | MGC16384 | .829 |
| TXNDC5 | 1.309 | TMEM17 | .829 |
| LOC642113 | 1.270 | LOC1001128098 | .826 |
| LOC100133565 | 1.267 | LOC100132585 | .826 |
| IGLL3 | 1.226 | LOC728809 | .825 |
| GLDC | 1.213 | FKBP14 | .825 |
| HIST1H3F | 1.207 | CD83 | .825 |
| TRA1P2 | 1.204 | KLF4 | .825 |
| ABCB9 | 1.201 | IL8 | .823 |
| RNU4-1 | .822 | ||
| TMEM137 | .822 | ||
| FAM175A | .822 | ||
| CLC | .821 | ||
| unnamed gene | .821 | ||
| LOC90586 | .821 | ||
| DUSP19 | .820 | ||
| JUN | .819 | ||
| SGK1 | .819 | ||
| CCBE1 | .818 | ||
| LOC100133516 | .817 | ||
| FLJ36131 | .817 | ||
| HBA2 | .815 | ||
| TCN1 | .812 | ||
| LOC100190986 | .810 | ||
| ZNF14 | .808 | ||
| LOC100133840 | .805 | ||
| G0S2 | .804 | ||
| LOC732450 | .803 | ||
| LOC389765 | .801 | ||
| MS4A3 | .800 | ||
| RNU1-5 | .797 | ||
| DEFA1B | .794 | ||
| unnamed gene | .794 | ||
| GPR1 | .793 | ||
| LOC100130229 | .756 | ||
| DEFA1 | .752 | ||
| HBA1 | .743 | ||
| DEFA3 | .734 | ||
| FOSB | .661 |
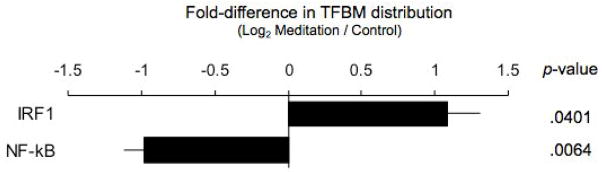
Results from promoter-based bioinformatic analysis (see Figure 2) indicate that KKM vs. RM treated participants showed reduced expression of genes bearing NF-κB-response elements (p = .006) and increased expression of genes bearing IRF1 response elements (p = .040). Transcript origin analysis identified differentially expressed genes as deriving particularly from plasmacytoid dendritic cells (p = .002) and B lymphocytes (p = .002).
Figure 2. Promoter-based bioinformatic analysis.
Notes. Data represent the log2-transformed mean (+/− standard error) fold-difference in prevalence of transcription factor-binding motifs within promoters of genes up-regulated in KKM vs. RM conditions (genes listed in Table 2); NF- κB = nuclear factor-κB; IRF1 = Interferon Response Factor 1
Discussion
The results of this randomized controlled intervention study show that 8 weeks of structured daily yogic meditation can reverse the pattern of increased expression of NF-κB-associated pro-inflammatory genes and decreased expression of IRF1-associated genes, which were previously observed in healthy individuals confronting caregiving stress and other forms of significant life stressors/adversity (Cole et al., 2007; Cole, 2008; Miller et al., 2008; Miller et al., 2009; Cole et al., 2009).Given that relatively few genes showed differential expression at even moderate effect sizes (>1.2 fold difference in average expression change across groups), our findings are not strikingly robust when viewed at the level of individual genes. However, the general pattern of results across the spectrum of small effects is highly non-random, and quite consistent with emerging themes in the social genomics literature regarding the general biological characteristics of gene modules regulated in association with social adversity (i.e., up-regulation of pro-inflammatory signaling pathways and down-regulation of antiviral pathways) (Schultz et al., 2007; Rohleder et al., 2009; Irwin and Cole, 2011).
Also consistent with previous social genomics research (Cole et al., 2011), our Transcript Origin Analyses identified plasmacytoid dendritic cells and B lymphocytes as candidate cellular mediators of the gene expression dynamics impacted by 8 weeks of yogic meditation. The present findings parallel another recent analysis of a randomized controlled cognitive behavioral stress management intervention for breast cancer patients in showing that positive psychological interventions can causally alter gene expression profiles in circulating immune cells toward a more salutary profile (i.e., less pro-inflammatory gene expression and greater expression of innate anti-viral response genes) (Antoni et al., 2011).
Caregivers are known to report poorer mental health status (Pinquart and Sörensen, 2003; Miller et al., 2008; Lavretsky et al 2010; 2012) and show more compromised biological immune profiles (Kiecolt-Glaser et al., 1991; Damjanovic et al., 2007; Epel et al., 2010; Lovell and Wetherell, 2011) than controls. Various forms of meditation practice have been shown to improve general health status (Grossman et al., 2004; Black et al., 2009; Chiesa and Serretti, 2009). Our findings provide new insights into the gene regulatory mechanisms that may possibly underlie the effects of psychosocial interventions on health of dementia caregivers (Schulz et al., 2002), suggesting potential beneficial change in gene expression and immune profiles associated with chronic disease and aging (Irwin et al., 2003; Davidson et al., 2003; Dusek et al., 2008; Jacobs et al., 2011; Irwin and Olmstead, 2011;).
In particular, the effects of Kaman suppressing expression of inflammation-related genes and up-regulating expression of genes involved in antiviral and immunoglobulin responses in this sample might potentially counteract the adverse effects of caregiving on inflammation, antibody responses to vaccination, and resistance to viral infections documented in previous studies of caregiving (Glaser et al., 1998; Kiecolt-Glaseret al., 2003; Lavretsky, 2005; Damjanovic et al., 2007; Rohleder et al., 2009). The current study advances this research by providing early evidence of the specific molecular pathways that might potentially contribute to differential health outcomes. However, future intervention research is needed to replicate these results and add direct measures of immune function, mediating cell types, transcription factor activation, and health outcomes to supplement the gene regulatory profiles and promoter-based bioinformatics analyses utilized here.
Limitations of this study include a small sample size with results based on a completer analysis, which may account for the relatively low number of significantly up-regulated and down-regulated genes. Although, on average, caregivers in our study scored the same or higher on the distress measure (RMBP-distress) compared with other studies of caregiver stress, we excluded those with major depression. Our sample of caregivers was restricted to those with mild-to- moderate depressive symptoms that may be not generalizable to caregivers with major depression. Our results need to be replicated in a larger sample to document the effects of meditation on inflammation, antiviral responses, immunoglobulin production, and underlying gene expression. Moreover, the genomic effects found were mainly small when considered individually; however, when grouped, the biological themes that emerged across those individual changes suggest that the meditation intervention may have reversed some of the pro-inflammatory and anti-antiviral transcriptional bias previously associated with chronic stress (Irwin and Cole, 2011). Thus, the pattern of differential gene expression observed here is consistent with that emerging from other analyses of social adversity and its reduction by psychological interventions. Although BMI values significantly differed at baseline, BMI was not related to variables of interest at baseline and BMI did not change during the short period of the intervention. Nevertheless, we explored any potential role of baseline BMI by statistically controlling for group differences. Finally, due to the group differences in changes in gene expression in our study, we were able to discern differences between the effects of KKM compared to a relaxation response in the RM comparison group. However, it remains difficult to pinpoint the specific components of KKM (e.g., chanting, breath-focused meditation, or mudras) that are responsible for these group differences. Although these limitations are important to consider in the interpretation of the results presented and in future research, this study is among the first to show that daily meditation practice might reverse the known detrimental effects of stress/adversity on the general transcription control pathways driving gene expression.
Acknowledgments
This work was supported by the Alzheimer’s Research and Prevention Foundation grant; and the NIH grants MH077650, MH086481 and AT003480 to H.L., and NIH grants T32-MH19925, HL 079955, AG 026364, CA 10014152, CA116778, RR00827, P30-AG028748, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core to M.R.I.
The role of funding source:
This work was supported by the Alzheimer’s Research and Prevention Foundation grant. Despite contributing training CD audio for Kirtan Kriya the Foundation and Dr Dharma Khlasa did not influence the development of the study design or implementation, or data analyses.
Footnotes
Contributors:
David Black - Managed the literature searches, assisted with interpretation of Results, and took the lead role in writing the manuscript
Steve Cole: Performed statistical and genomic analyses, and assisted in interpretation of results and writing of the manuscript
Michael Irwin: Supervised laboratory analyses, and assisted in interpretation of results and writing of the manuscript
Elizabeth Breen - Provided assistance with interpretation and writing of the Result section
Natalie M. St. Cyr - Study coordinator and psychomethrist, and recruited and assessed participants
Nora Nazarian - Study coordinator, database manager, and recruited and assessed participants
Dharma S. Khalsa - Provided training program in Kirtan Kriya
Helen Lavretsky - Served as PI and designed and executed the study, was responsible for all aspects of the study and reporting
All authors contributed to and have approved the final manuscript
Conflicts of interest:
H.L. received grant funding from the Forest Research Institute and is a consultant to the Lilly and Dey Pharmaceutical Companies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, et al. Cognitive-Behavioral Stress Management Reverses Anxiety-Related Leukocyte Transcriptional Dynamics. Biol Psychiatry. 2011;15:366–72. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Research & Prevention Foundation. ARPF 2011 Kirtan Kriya. Retrieved Mar 27, 2012 from http://www.alzheimersprevention.org/kirtan_kriya.htm.
- Benson H, Beary JF, Carol MP. The relaxation response. Psychiatry. 1974;37:37–46. doi: 10.1080/00332747.1974.11023785. [DOI] [PubMed] [Google Scholar]
- Black DS, Milam J, Sussman S. Sitting-Meditation Interventions Among Youth: A Review of Treatment Efficacy. Pediatrics. 2009;124:532–41. doi: 10.1542/peds.2008-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Dusek JA, Benson H. Psychobiological changes from relaxation response elicitation: long-term practitioners vs. novices. Psychosomatics. 2011;52:550–9. doi: 10.1016/j.psym.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2010;16:729–37. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Walker HA, Arevalo JM, Sung CY, Cole SW. Genome-wide transcriptional profiling linked to social class in asthma. Thorax. 2009;64:38–43. doi: 10.1136/thx.2007.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness-Based Stress Reduction for Stress Management in Healthy People: A Review and Meta-Analysis. J Altern Complement Med. 2009;15:593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- Cole SW. Social regulation of leukocyte homeostasis: the role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–55. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107:5681–6. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci. 2011;108:3080–5. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Mendoza SP, Capitanio JP. Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosom Med. 2009;71:591–97. doi: 10.1097/PSY.0b013e3181aa95a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics. 2005;21:803–10. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65:564–70. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Otu HH, Wohlhueter AL, Bhasin M, Zerbini LF, Joseph MG, et al. Genomic counter-stress changes induced by the relaxation response. PLoS ONE. 2008;3:e2576. doi: 10.1371/journal.pone.0002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24:531–39. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein-Lubow G, McBee L, Darling E, Armey M, Miller I. A pilot investigation of Mindfulness-Based Stress Reduction for caregivers of frail elderly. Mindfulness. 2011;2:95–102. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part I: Description. J Pers Disord. 1995;9:83–91. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garand L, Buckwalter KC, Lubaroff DM, Tripp-Reimer T, Frantz RA, Ansley TN. A pilot study of immune and mood outcomes of a community-based intervention for dementia caregivers: the PLST intervention. Arch Psychiatr Nurs. 2002;16:156–67. doi: 10.1053/apnu.2002.34392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Malarkey WB, Sheridan JF. The influence of psychological stress on the immune response to vaccines. Annals of the New York Academy of Sciences. 1998;840:649. doi: 10.1111/j.1749-6632.1998.tb09603.x. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 2008;15:251–59. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neuro Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R. Mitigating Cellular Inflammation in Older Adults: A Randomized Controlled Trial of Tai Chi Chih. Am J of Geriatr Psychiatry. 2011 doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55:511–17. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Pike JL, Cole JC, Oxman MN. Effects of a behavioral intervention, Tai Chi Chih, on varicella-zoster virus specific immunity and health functioning in older adults. Psychosom Med. 2003;65:824–830. doi: 10.1097/01.psy.0000088591.86103.8f. [DOI] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, Zanesco AP, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–81. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–62. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci. 2003;100 (15):9090. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H. Stress and depression in informal family caregivers of patients with Alzheimer’s disease. Aging Health. 2005;1(1):117–33. [Google Scholar]
- Lavretsky H, Siddarth P, Irwin MR. Improving depression and enhancing resilience in family dementia caregivers: a pilot randomized placebo-controlled trial of escitalopram. Am J Geriatr Psychiatry. 2010;18:154–62. doi: 10.1097/JGP.0b013e3181beab1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, Lin J, Blackburn E, Irwin MR. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.3790. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, Cyr NS, Irwin MR. Complementary use of Tai Chi Chih augments Escitalopram treatment of geriatric depression: a randomized controlled trial. Am J of Geriatr Psychiatry. 2011;19:839–50. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell B, Wetherell MA. The cost of caregiving: Endocrine and immune implications in elderly and non-elderly caregivers. Neurosci Biobehav Rev. 2011;35:1342–52. doi: 10.1016/j.neubiorev.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JMG, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappa B signaling. Biol Psychiatry. 2008;64:266–72. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg AB, Wintering N, Khalsa DS, Roggenkamp H, Waldman MR. Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: a preliminary study. J Alzheimers Dis. 2010;20:517–26. doi: 10.3233/JAD-2010-1391. [DOI] [PubMed] [Google Scholar]
- Nilsson U. The anxiety-and pain-reducing effects of music interventions: a systematic review. AORN. 2008;87:780–7. doi: 10.1016/j.aorn.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Northouse L, Williams AL, Given B, McCorkle R. Psychosocial care for family caregivers of patients with cancer. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.39.5798. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011;30:123–32. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychol Aging. 2003;18:250–67. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Marin TJ, Ma R, Miller GE. Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J Clin Oncol. 2009;20;27:2909–15. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’Brien A, Czaja S, Ory M, Norris R, Martire LM, et al. Dementia caregiver intervention research: In search of clinical significance. Gerontologist. 2002;42:589–602. doi: 10.1093/geront/42.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz PE, Haberman M, Nichols J, Daratha K, Blank SA. Down-regulated lymphocyte NF-kB activation in breast cancer survivors following yoga participation. The FASEB Journal. 2007;21:765.7. [Google Scholar]
- Van Puymbroeck M, Payne LL, Hsieh PC. A phase I feasibility study of yoga on the physical health and coping of informal caregivers. Evid Based Complement Alternat Med. 2007;4:519–29. doi: 10.1093/ecam/nem075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60:677–87. doi: 10.1002/jclp.10259. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang J, Cacioppo JT, Glaser R, Kiecolt-Glaser JK, Malarkey WB. Chronic stress associated with spousal caregiving of patients with Alzheimer’s dementia is associated with downregulation of B-lymphocyte GH mRNA. J Gerontol A Biol Sci Med Sci. 1999;54:M 212–5. doi: 10.1093/gerona/54.4.m212. [DOI] [PubMed] [Google Scholar]